Oxygen–Glucose Deprivation Increases NR4A1 Expression and Promotes Its Extranuclear Translocation in Mouse Astrocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Preparation of Astrocyte-Enriched Cultures
2.3. Oxygen–Glucose Deprivation
2.4. Quantitative Real-Time PCR
2.5. Immunocytochemistry
2.6. Quantitative Image Analyses
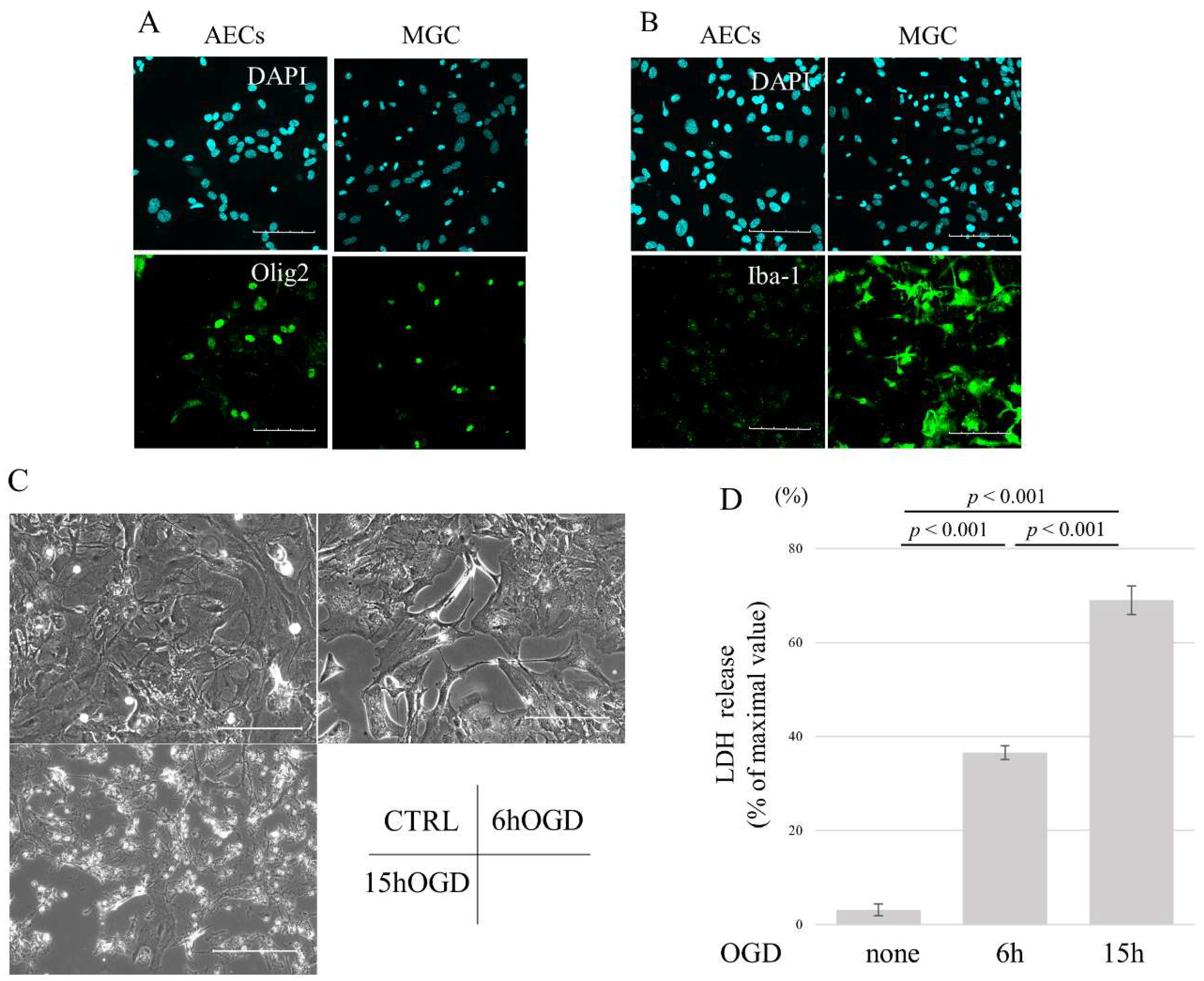
2.7. LDH Assay
2.8. Transcriptome Analysis
2.9. siRNA-Mediated Knockdown
2.10. Statistical Analysis
3. Results
3.1. OGD Induces Cell Death in AECs in a Time-Dependent Manner
3.2. OGD Promotes HIF-1α Nuclear Accumulation Leading to the Upregulation of Its Downstream Target Genes in AECs
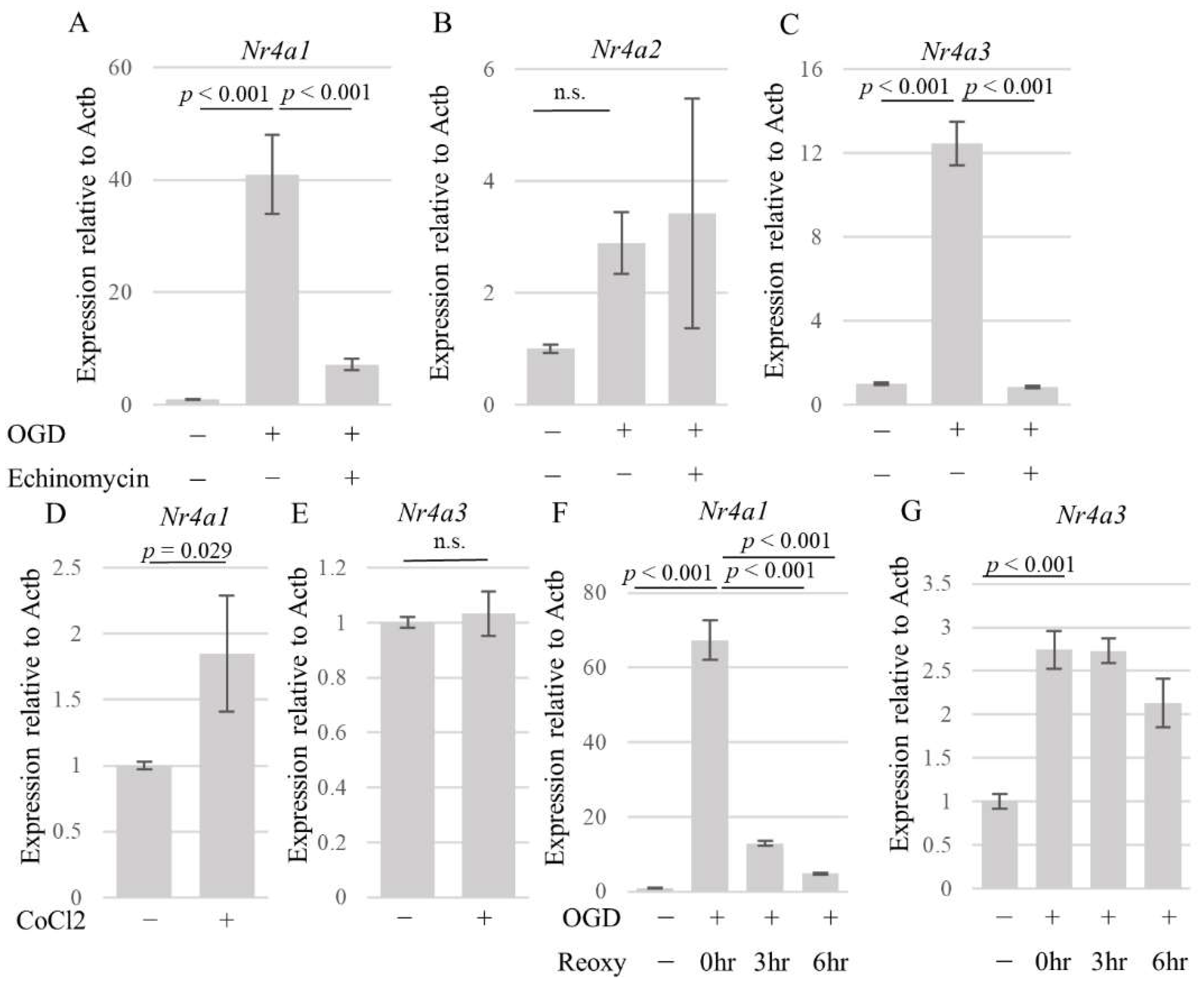
3.3. DNA Microarray Identified NR4A1 and NR4A3 as OGD-Responsive Factors
3.4. HIF-1α Partly Regulates Nr4a1 and Nr4a3 Expression
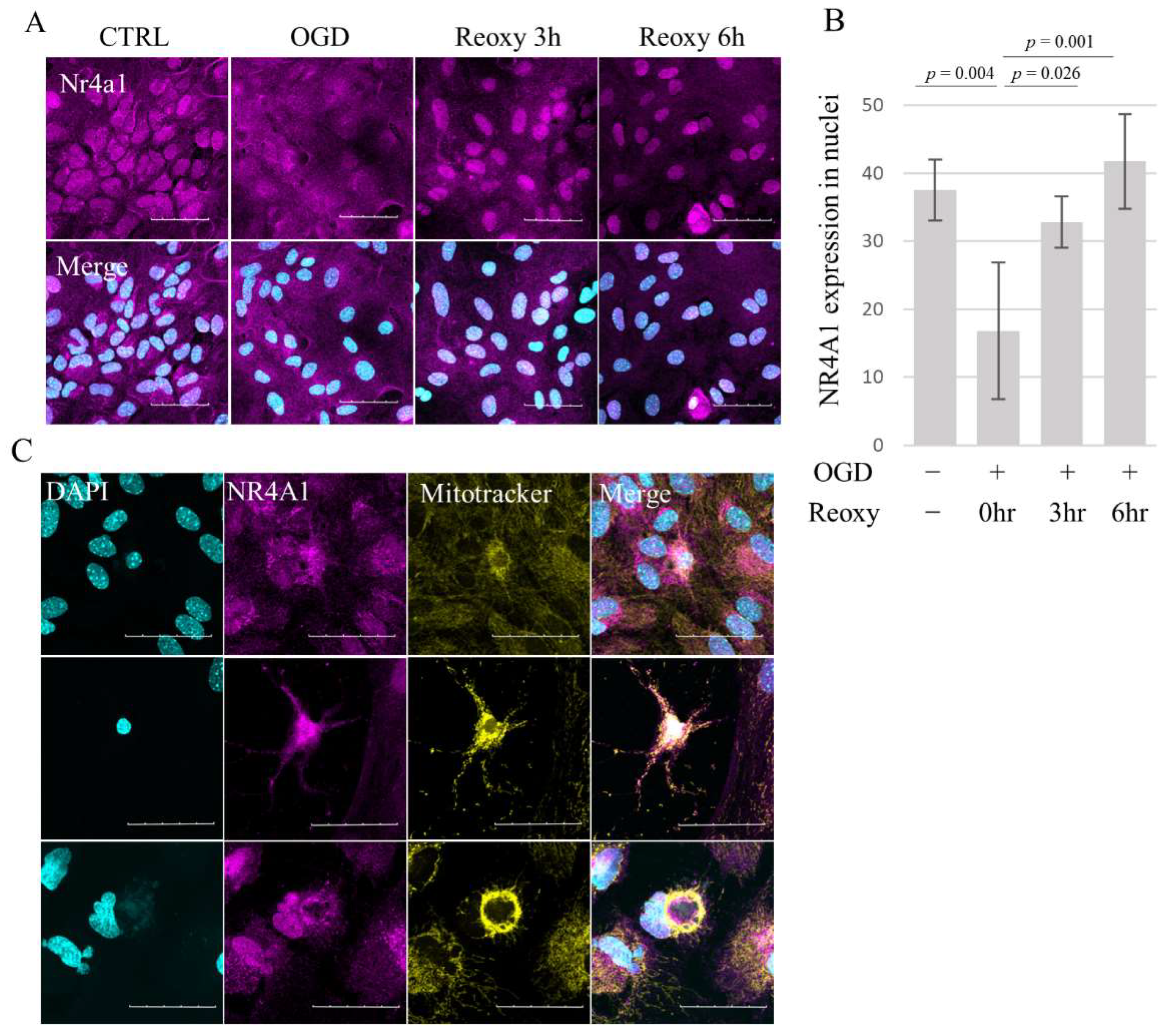
3.5. Nr4a1 Translocates from Nucleus to Cytosol following OGD
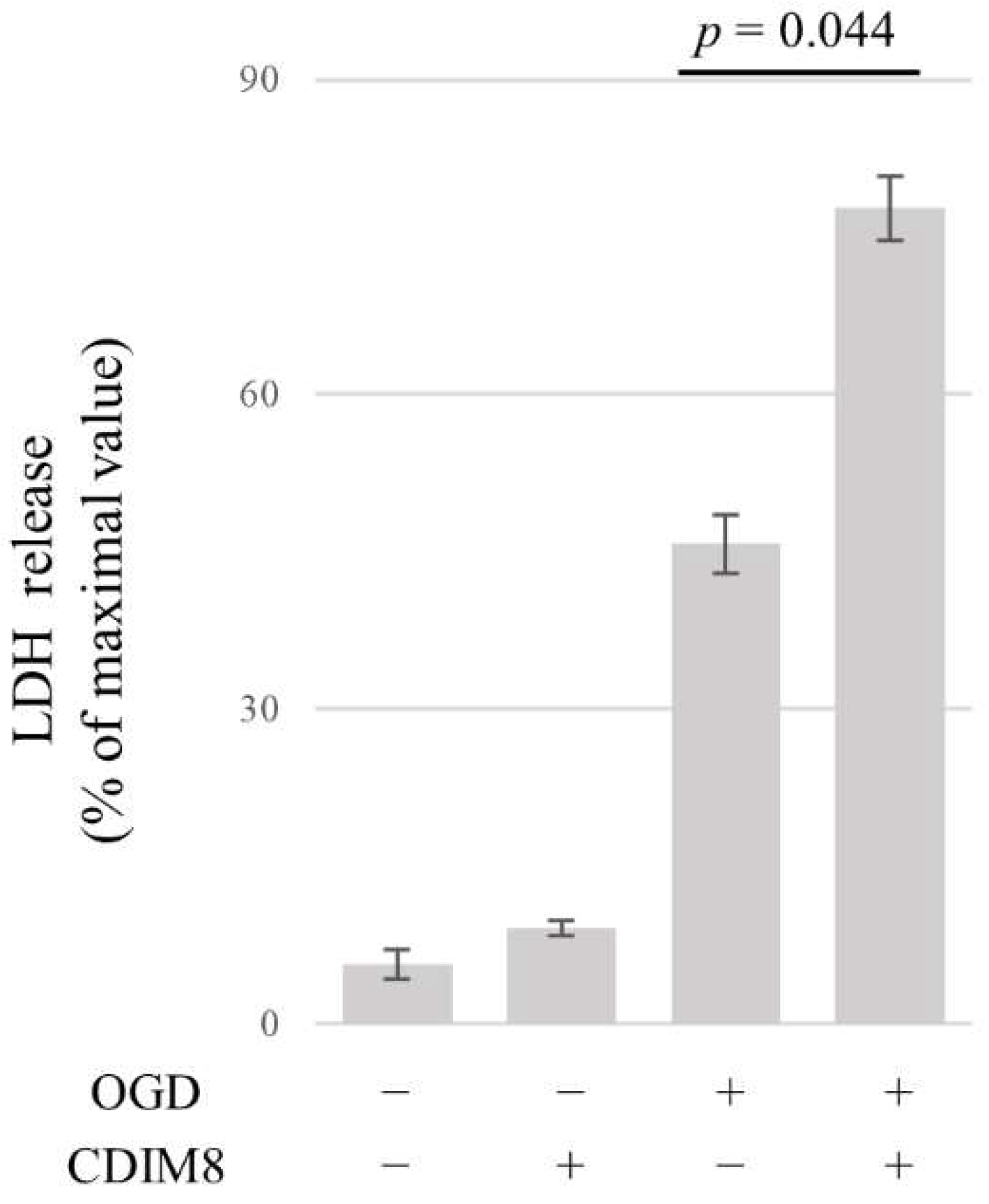
3.6. NR4A1 Protects AECs from OGD-Induced Cell Death
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Graham, E.M.; Ruis, K.A.; Hartman, A.L.; Northington, F.J.; Fox, H.E. A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 2008, 199, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Solevag, A.L.; Schmolzer, G.M.; Cheung, P.Y. Novel interventions to reduce oxidative-stress related brain injury in neonatal asphyxia. Free Radic. Biol. Med. 2019, 142, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.J.; Kuzniewicz, M.; Niki, H.; Wu, Y.W. Antenatal and Intrapartum Risk Factors for Hypoxic-Ischemic Encephalopathy in a US Birth Cohort. J. Pediatr. 2018, 203, 163–169. [Google Scholar] [CrossRef]
- Douglas-Escobar, M.; Weiss, M.D. Hypoxic-ischemic encephalopathy: A review for the clinician. JAMA Pediatr. 2015, 169, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Sabir, H.; Scull-Brown, E.; Liu, X.; Thoresen, M. Immediate hypothermia is not neuroprotective after severe hypoxia-ischemia and is deleterious when delayed by 12 hours in neonatal rats. Stroke 2012, 43, 3364–3370. [Google Scholar] [CrossRef]
- Li, D.; Shao, Z.; Vanden Hoek, T.L.; Brorson, J.R. Reperfusion accelerates acute neuronal death induced by simulated ischemia. Exp. Neurol. 2007, 206, 280–287. [Google Scholar] [CrossRef]
- Higgins, R.D.; Raju, T.; Edwards, A.D.; Azzopardi, D.V.; Bose, C.L.; Clark, R.H.; Ferriero, D.M.; Guillet, R.; Gunn, A.J.; Hagberg, H.; et al. Hypothermia and other treatment options for neonatal encephalopathy: An executive summary of the Eunice Kennedy Shriver NICHD workshop. J. Pediatr. 2011, 159, 851–858.e851. [Google Scholar] [CrossRef]
- Kleuskens, D.G.; Goncalves Costa, F.; Annink, K.V.; van den Hoogen, A.; Alderliesten, T.; Groenendaal, F.; Benders, M.J.N.; Dudink, J. Pathophysiology of Cerebral Hyperperfusion in Term Neonates with Hypoxic-Ischemic Encephalopathy: A Systematic Review for Future Research. Front. Pediatr. 2021, 9, 631258. [Google Scholar] [CrossRef]
- Stobart, J.L.; Anderson, C.M. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front. Cell Neurosci. 2013, 7, 38. [Google Scholar] [CrossRef]
- Beard, E.; Lengacher, S.; Dias, S.; Magistretti, P.J.; Finsterwald, C. Astrocytes as Key Regulators of Brain Energy Metabolism: New Therapeutic Perspectives. Front. Physiol. 2021, 12, 825816. [Google Scholar] [CrossRef]
- Belanger, M.; Allaman, I.; Magistretti, P.J. Brain energy metabolism: Focus on astrocyte-neuron metabolic cooperation. Cell Metab. 2011, 14, 724–738. [Google Scholar] [CrossRef]
- Lee, J.A.; Hall, B.; Allsop, J.; Alqarni, R.; Allen, S.P. Lipid metabolism in astrocytic structure and function. Semin. Cell Dev. Biol. 2021, 112, 123–136. [Google Scholar] [CrossRef]
- Hirayama, Y.; Ikeda-Matsuo, Y.; Notomi, S.; Enaida, H.; Kinouchi, H.; Koizumi, S. Astrocyte-mediated ischemic tolerance. J. Neurosci. 2015, 35, 3794–3805. [Google Scholar] [CrossRef]
- Revuelta, M.; Elicegui, A.; Moreno-Cugnon, L.; Buhrer, C.; Matheu, A.; Schmitz, T. Ischemic stroke in neonatal and adult astrocytes. Mech. Ageing Dev. 2019, 183, 111147. [Google Scholar] [CrossRef]
- Turner, D.A.; Adamson, D.C. Neuronal-astrocyte metabolic interactions: Understanding the transition into abnormal astrocytoma metabolism. J. Neuropathol. Exp. Neurol. 2011, 70, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Horino-Shimizu, A.; Moriyama, K.; Mori, T.; Kohyama, K.; Nishito, Y.; Sakuma, H. Lipocalin-2 production by astrocytes in response to high concentrations of glutamate. Brain Res. 2023, 1815, 148463. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Campos-Melo, D.; Galleguillos, D.; Sanchez, N.; Gysling, K.; Andres, M.E. Nur transcription factors in stress and addiction. Front. Mol. Neurosci. 2013, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Milbrandt, J. Nerve growth factor induces a gene homologous to the glucocorticoid receptor gene. Neuron 1988, 1, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Fan, F.; Wu, L.; Zhao, Y. The nuclear receptor 4A family members: Mediators in human disease and autophagy. Cell Mol. Biol. Lett. 2020, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, T.; Kashiwagi, I.; Yoshida, R.; Fukaya, T.; Morita, R.; Kimura, A.; Ichinose, H.; Metzger, D.; Chambon, P.; Yoshimura, A. Nr4a receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 2013, 14, 230–237. [Google Scholar] [CrossRef]
- Sekiya, T.; Kasahara, H.; Takemura, R.; Fujita, S.; Kato, J.; Doki, N.; Katayama, Y.; Ozawa, Y.; Takada, S.; Eto, T.; et al. Essential Roles of the Transcription Factor NR4A1 in Regulatory T Cell Differentiation under the Influence of Immunosuppressants. J. Immunol. 2022, 208, 2122–2130. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Chen, B.; Huo, J.; Liu, X. Therapeutic potential of NR4A1 in cancer: Focus on metabolism. Front. Oncol. 2022, 12, 972984. [Google Scholar] [CrossRef] [PubMed]
- Mullican, S.E.; Zhang, S.; Konopleva, M.; Ruvolo, V.; Andreeff, M.; Milbrandt, J.; Conneely, O.M. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nat. Med. 2007, 13, 730–735. [Google Scholar] [CrossRef] [PubMed]
- Kaelin, W.G., Jr.; Ratcliffe, P.J. Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol. Cell 2008, 30, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Expression of hypoxia-inducible factor 1: Mechanisms and consequences. Biochem. Pharmacol. 2000, 59, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. Hypoxia-inducible factor 1: Oxygen homeostasis and disease pathophysiology. Trends Mol. Med. 2001, 7, 345–350. [Google Scholar] [CrossRef]
- Dirnagl, U.; Becker, K.; Meisel, A. Preconditioning and tolerance against cerebral ischaemia: From experimental strategies to clinical use. Lancet Neurol. 2009, 8, 398–412. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1alpha pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef]
- Choi, J.W.; Park, S.C.; Kang, G.H.; Liu, J.O.; Youn, H.D. Nur77 activated by hypoxia-inducible factor-1alpha overproduces proopiomelanocortin in von Hippel-Lindau-mutated renal cell carcinoma. Cancer Res. 2004, 64, 35–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yoo, Y.G.; Yeo, M.G.; Kim, D.K.; Park, H.; Lee, M.O. Novel function of orphan nuclear receptor Nur77 in stabilizing hypoxia-inducible factor-1alpha. J. Biol. Chem. 2004, 279, 53365–53373. [Google Scholar] [CrossRef]
- Martorell, L.; Gentile, M.; Rius, J.; Rodriguez, C.; Crespo, J.; Badimon, L.; Martinez-Gonzalez, J. The hypoxia-inducible factor 1/NOR-1 axis regulates the survival response of endothelial cells to hypoxia. Mol. Cell Biol. 2009, 29, 5828–5842. [Google Scholar] [CrossRef]
- Li, H.; Kolluri, S.K.; Gu, J.; Dawson, M.I.; Cao, X.; Hobbs, P.D.; Lin, B.; Chen, G.; Lu, J.; Lin, F.; et al. Cytochrome c release and apoptosis induced by mitochondrial targeting of nuclear orphan receptor TR3. Science 2000, 289, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Kolluri, S.K.; Lin, F.; Liu, W.; Han, Y.H.; Cao, X.; Dawson, M.I.; Reed, J.C.; Zhang, X.K. Conversion of Bcl-2 from protector to killer by interaction with nuclear orphan receptor Nur77/TR3. Cell 2004, 116, 527–540. [Google Scholar] [CrossRef]
- Lee, S.O.; Abdelrahim, M.; Yoon, K.; Chintharlapalli, S.; Papineni, S.; Kim, K.; Wang, H.; Safe, S. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Res. 2010, 70, 6824–6836. [Google Scholar] [CrossRef] [PubMed]
- Safe, S.; Karki, K. The Paradoxical Roles of Orphan Nuclear Receptor 4A (NR4A) in Cancer. Mol. Cancer Res. 2021, 19, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, E.; Lee, S.O.; Kim, G.; Abdelrahim, M.; Jin, U.H.; Safe, S.; Abudayyeh, A. Nuclear Receptor 4A1 (NR4A1) as a Drug Target for Renal Cell Adenocarcinoma. PLoS ONE 2015, 10, e0128308. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Song, J.R.; Zhao, M.J. NR4A1 regulates cerebral ischemia-induced brain injury by regulating neuroinflammation through interaction with NF-kappaB/p65. Biochem. Biophys. Res. Commun. 2019, 518, 59–65. [Google Scholar] [CrossRef]


| Gene Symbol Name | Fold Change | p Value (×10−5) |
|---|---|---|
| Nfil3 | 13.3 | 0.005 |
| Vegfa | 11.7 | 0.010 |
| Runx2 | 10.9 | 0.020 |
| Ero1a | 22.0 | 0.030 |
| Lrrfip1 | 14.1 | 0.039 |
| Gdf15 | 11.4 | 0.043 |
| Errfi1 | 11.1 | 0.065 |
| Adm | 23.1 | 0.072 |
| Hk2 | 11.5 | 0.074 |
| Trib3 | 20.3 | 0.088 |
| Ube2ql1 | 33.0 | 0.095 |
| Csf2 | 19.2 | 0.107 |
| Areg | 17.6 | 0.107 |
| Ncoa6 | 22.9 | 0.109 |
| Kpna7 | 10.6 | 0.109 |
| Pvr | 12.2 | 0.109 |
| Prdm10 | 10.7 | 0.112 |
| Flt1 | 11.9 | 0.118 |
| Tbl2 | 17.9 | 0.123 |
| Chac1 | 22.7 | 0.143 |
| Ankrd33b | 29.9 | 0.151 |
| Smurf1 | 10.5 | 0.155 |
| Fgf21 | 240.6 | 0.164 |
| Prok2 | 73.4 | 0.165 |
| Nr4a1 | 58.4 | 0.167 |
| Tram2 | 12.8 | 0.237 |
| Rassf8 | 14.7 | 0.277 |
| Egr4 | 136.0 | 0.286 |
| Ptgs2 | 27.1 | 0.296 |
| Pdxk | 11.1 | 0.302 |
| Amotl1 | 10.7 | 0.307 |
| Wdfy1 | 66.9 | 0.323 |
| Ereg | 11.1 | 0.336 |
| Skil | 22.9 | 0.340 |
| Tnfaip3 | 17.0 | 0.349 |
| Csrnp1 | 23.1 | 0.372 |
| Ankrd33b | 41.6 | 0.373 |
| Ern1 | 27.7 | 0.375 |
| Atf3 | 13.3 | 0.385 |
| Prkd3 | 44.1 | 0.391 |
| Adm2 | 58.8 | 0.395 |
| Nr4a3 | 167.6 | 0.398 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moriyama, K.; Horino, A.; Kohyama, K.; Nishito, Y.; Morio, T.; Sakuma, H. Oxygen–Glucose Deprivation Increases NR4A1 Expression and Promotes Its Extranuclear Translocation in Mouse Astrocytes. Brain Sci. 2024, 14, 244. https://doi.org/10.3390/brainsci14030244
Moriyama K, Horino A, Kohyama K, Nishito Y, Morio T, Sakuma H. Oxygen–Glucose Deprivation Increases NR4A1 Expression and Promotes Its Extranuclear Translocation in Mouse Astrocytes. Brain Sciences. 2024; 14(3):244. https://doi.org/10.3390/brainsci14030244
Chicago/Turabian StyleMoriyama, Kengo, Asako Horino, Kuniko Kohyama, Yasumasa Nishito, Tomohiro Morio, and Hiroshi Sakuma. 2024. "Oxygen–Glucose Deprivation Increases NR4A1 Expression and Promotes Its Extranuclear Translocation in Mouse Astrocytes" Brain Sciences 14, no. 3: 244. https://doi.org/10.3390/brainsci14030244
APA StyleMoriyama, K., Horino, A., Kohyama, K., Nishito, Y., Morio, T., & Sakuma, H. (2024). Oxygen–Glucose Deprivation Increases NR4A1 Expression and Promotes Its Extranuclear Translocation in Mouse Astrocytes. Brain Sciences, 14(3), 244. https://doi.org/10.3390/brainsci14030244






