Clinical Potential of Transcranial Focused Ultrasound for Neurorehabilitation in Pediatric Cancer Survivors
Abstract
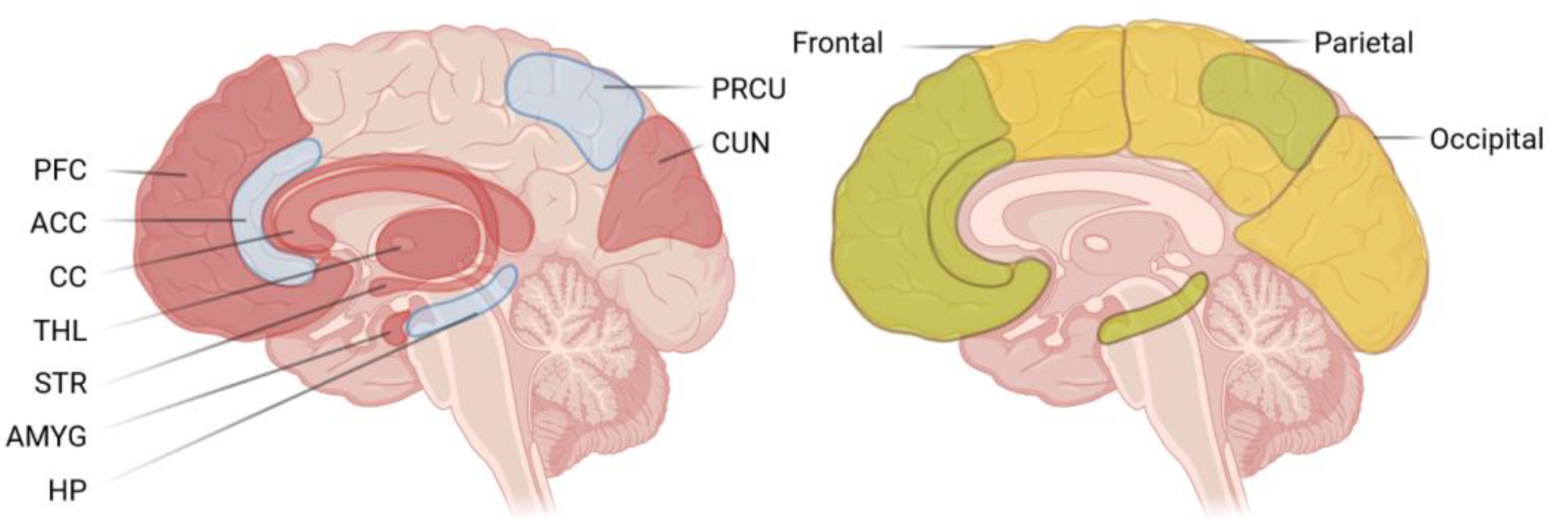
1. Introduction
2. Cognitive Impairment in Pediatric Cancers
3. Existing Therapeutic Interventions for Cognitive Impairment
3.1. Cognitive Training Programs
3.2. Therapeutic Potential for Non-Invasive Electrical Neuromodulation
3.2.1. Transcranial Electrical Stimulation
3.2.2. Behavioral Effects of Electrical Stimulation
3.3. Pairing Neuromodulation with Cognitive Training in Cancer Survivorship
3.4. Limitations of Electrical Neuromodulation
4. Transcranial Focused Ultrasound
4.1. Results from Animal Studies
4.1.1. Neurophysiological Measures
4.1.2. Sensory or Behavioral Responses
4.2. Results from Human Studies
4.2.1. Cortical Areas
4.2.2. Subcortical Areas
4.3. Translational and Clinical Approaches
5. On the Promising Potential of Multimodal tFUS
5.1. Outlook in Cancer Survivorship
5.2. Pairing tFUS with Neuroimaging
5.3. Safety and Considerations
Author Contributions
Funding
Conflicts of Interest
References
- Gibson, T.M.; Mostoufi-Moab, S.; Stratton, K.L.; Leisenring, W.M.; Barnea, D.; Chow, E.J.; Donaldson, S.S.; Howell, R.M.; Hudson, M.M.; Mahajan, A.; et al. Temporal Patterns in the Risk of Chronic Health Conditions in Survivors of Childhood Cancer Diagnosed 1970–99: A Report from the Childhood Cancer Survivor Study Cohort. Lancet Oncol. 2018, 19, 1590–1601. [Google Scholar] [CrossRef]
- Bhakta, N.; Liu, Q.; Ness, K.K.; Baassiri, M.; Eissa, H.; Yeo, F.; Chemaitilly, W.; Ehrhardt, M.J.; Bass, J.; Bishop, M.W.; et al. The Cumulative Burden of Surviving Childhood Cancer: An Initial Report from the St Jude Lifetime Cohort Study (SJLIFE). Lancet 2017, 390, 2569–2582. [Google Scholar] [CrossRef] [PubMed]
- Mulrooney, D.A.; Hyun, G.; Ness, K.K.; Bhakta, N.; Pui, C.H.; Ehrhardt, M.J.; Krull, K.R.; Crom, D.B.; Chemaitilly, W.; Srivastava, D.K.; et al. The Changing Burden of Long-Term Health Outcomes in Survivors of Childhood Acute Lymphoblastic Leukaemia: A Retrospective Analysis of the St Jude Lifetime Cohort Study. Lancet Haematol. 2019, 6, e306–e316. [Google Scholar] [CrossRef] [PubMed]
- Korai, S.A.; Ranieri, F.; Di Lazzaro, V.; Papa, M.; Cirillo, G. Neurobiological after-Effects of Low Intensity Transcranial Electric Stimulation of the Human Nervous System: From Basic Mechanisms to Metaplasticity. Front. Neurol. 2021, 12, 587771. [Google Scholar] [CrossRef]
- Pasquinelli, C.; Hanson, L.G.; Siebner, H.R.; Lee, H.J.; Thielscher, A. Safety of Transcranial Focused Ultrasound Stimulation: A Systematic Review of the State of Knowledge from Both Human and Animal Studies. Brain Stimul. 2019, 12, 1367–1380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pan, N.; Wang, Y.; Liu, C.; Hu, S. Transcranial Focused Ultrasound Neuromodulation: A Review of the Excitatory and Inhibitory Effects on Brain Activity in Human and Animals. Front. Hum. Neurosci. 2021, 15, 749162. [Google Scholar] [CrossRef] [PubMed]
- Foster, R.; Zheng, D.J.; Netson-Amore, K.L.; Kadan-Lottick, N.S. Cognitive Impairment in Survivors of Pediatric Extracranial Solid Tumors and Lymphomas. J. Clin. Oncol. 2021, 39, 1727–1740. [Google Scholar] [CrossRef]
- Oyefiade, A.; Paltin, I.; De Luca, C.R.; Hardy, K.K.; Grosshans, D.R.; Chintagumpala, M.; Mabbott, D.J.; Kahalley, L.S. Cognitive Risk in Survivors of Pediatric Brain Tumors. J. Clin. Oncol. 2021, 39, 1718. [Google Scholar] [CrossRef] [PubMed]
- Schuitema, I.; Alexander, T.; Hudson, M.M.; Krull, K.R.; Edelstein, K. Aging in Adult Survivors of Childhood Cancer: Implications for Future Care. J. Clin. Oncol. 2021, 39, 1741. [Google Scholar] [CrossRef]
- Dijkshoorn, A.B.C.; van Stralen, H.E.; Sloots, M.; Schagen, S.B.; Visser-Meily, J.M.A.; Schepers, V.P.M. Prevalence of Cognitive Impairment and Change in Patients with Breast Cancer: A Systematic Review of Longitudinal Studies. Psychooncology 2021, 30, 635–648. [Google Scholar] [CrossRef]
- Dixon, S.B.; Chen, Y.; Yasui, Y.; Pui, C.H.; Hunger, S.P.; Silverman, L.B.; Ness, K.K.; Green, D.M.; Howell, R.M.; Leisenring, W.M.; et al. Reduced Morbidity and Mortality in Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2020, 38, 3418–3429. [Google Scholar] [CrossRef] [PubMed]
- Maris, J.M.; Hogarty, M.D.; Bagatell, R.; Cohn, S.L. Neuroblastoma. Lancet 2007, 369, 2106–2120. [Google Scholar] [CrossRef]
- Millard, N.E.; De Braganca, K.C. Medulloblastoma. J. Child Neurol. 2016, 31, 1341–1353. [Google Scholar] [CrossRef]
- Otte, A.; Muller, H.L. Childhood-Onset Craniopharyngioma. J. Clin. Endocrinol. Metab. 2021, 106, e3820–e3836. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Altekruse, S.F.; Adamson, P.C.; Reaman, G.H.; Seibel, N.L. Declining Childhood and Adolescent Cancer Mortality. Cancer 2014, 120, 2497–2506. [Google Scholar] [CrossRef] [PubMed]
- Hudson, M.M.; Ness, K.K.; Gurney, J.G.; Mulrooney, D.A.; Chemaitilly, W.; Krull, K.R.; Green, D.M.; Armstrong, G.T.; Nottage, K.A.; Jones, K.E.; et al. Clinical Ascertainment of Health Outcomes among Adults Treated for Childhood Cancer. JAMA 2013, 309, 2371–2381. [Google Scholar] [CrossRef]
- Palladino, M.M. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. Arch. Argent. Pediatr. 2016, 114, e273–e274. [Google Scholar]
- Klages, K.L.; Berlin, K.S.; Cook, J.L.; Merchant, T.E.; Wise, M.S.; Mandrell, B.N.; Conklin, H.M.; Crabtree, V.M.L. Health-Related Quality of Life, Obesity, Fragmented Sleep, Fatigue, and Psychosocial Problems among Youth with Craniopharyngioma. Psychooncology 2022, 31, 779–787. [Google Scholar] [CrossRef]
- Özyurt, J.; Müller, H.L.; Thiel, C.M. A Systematic Review of Cognitive Performance in Patients with Childhood Craniopharyngioma. J. Neurooncol. 2015, 125, 9–21. [Google Scholar] [CrossRef]
- Schreiber, J.E.; Palmer, S.L.; Conklin, H.M.; Mabbott, D.J.; Swain, M.A.; Bonner, M.J.; Chapieski, M.L.; Huang, L.; Zhang, H.; Gajjar, A. Posterior Fossa Syndrome and Long-Term Neuropsychological Outcomes among Children Treated for Medulloblastoma on a Multi-Institutional, Prospective Study. Neuro Oncol. 2017, 19, 1673–1682. [Google Scholar] [CrossRef]
- Tonning Olsson, I.; Brinkman, T.M.; Wang, M.; Ehrhardt, M.J.; Banerjee, P.; Mulrooney, D.A.; Huang, I.C.; Ness, K.K.; Bishop, M.W.; Srivastava, D.; et al. Neurocognitive and Psychosocial Outcomes in Adult Survivors of Childhood Soft-Tissue Sarcoma: A Report from the St. Jude Lifetime Cohort. Cancer 2020, 126, 1576–1584. [Google Scholar] [CrossRef]
- Yeung, V.; Gabriel, M.; Padhye, B.D. Late Effects and Treatment Related Morbidity Associated with Treatment of Neuroblastoma Patients in a Tertiary Paediatric Centre. Cancer Rep. 2023, 6, e1738. [Google Scholar] [CrossRef]
- Krull, K.R.; Okcu, M.F.; Potter, B.; Jain, N.; Dreyer, Z.A.; Kamdar, K.; Brouwers, P. Screening for Neurocognitive Impairment in Pediatric Cancer Long-Term Survivors. J. Clin. Oncol. 2008, 26, 4138–4143. [Google Scholar] [CrossRef]
- Krull, K.R.; Brinkman, T.M.; Li, C.; Armstrong, G.T.; Ness, K.K.; Kumar Srivastava, D.; Gurney, J.G.; Kimberg, C.; Krasin, M.J.; Pui, C.H.; et al. Neurocognitive Outcomes Decades after Treatment for Childhood Acute Lymphoblastic Leukemia: A Report from the St Jude Lifetime Cohort Study. J. Clin. Oncol. 2013, 31, 4407–4415. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Krull, K.R. Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia Treated on Contemporary Treatment Protocols: A Systematic Review. Neurosci. Biobehav. Rev. 2015, 53, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Krull, K.R.; Cheung, Y.T.; Liu, W.; Fellah, S.; Reddick, W.E.; Brinkman, T.M.; Kimberg, C.; Ogg, R.; Srivastava, D.; Pui, C.H.; et al. Chemotherapy Pharmacodynamics and Neuroimaging and Neurocognitive Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2016, 34, 2644–2653. [Google Scholar] [CrossRef]
- van der Plas, E.; Modi, A.J.; Li, C.K.; Krull, K.R.; Cheung, Y.T. Cognitive Impairment in Survivors of Pediatric Acute Lymphoblastic Leukemia Treated with Chemotherapy Only. J. Clin. Oncol. 2021, 39, 1705–1717. [Google Scholar] [CrossRef] [PubMed]
- Kadan-Lottick, N.S.; Zeltzer, L.K.; Liu, Q.; Yasui, Y.; Ellenberg, L.; Gioia, G.; Robison, L.L.; Krull, K.R. Neurocognitive Functioning in Adult Survivors of Childhood Non-Central Nervous System Cancers. J. Natl. Cancer Inst. 2010, 102, 881–893. [Google Scholar] [CrossRef]
- Kimberg, C.I.; Klosky, J.L.; Zhang, N.; Brinkman, T.M.; Ness, K.K.; Srivastava, D.K.; Robison, L.L.; Hudson, M.M.; Krull, K.R. Predictors of Health Care Utilization in Adult Survivors of Childhood Cancer Exposed to Central Nervous System-Directed Therapy. Cancer 2015, 121, 774–782. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Brinkman, T.M.; Mulrooney, D.A.; Mzayek, Y.; Liu, W.; Banerjee, P.; Panoskaltsis-Mortari, A.; Srivastava, D.; Pui, C.H.; Robison, L.L.; et al. Impact of Sleep, Fatigue, and Systemic Inflammation on Neurocognitive and Behavioral Outcomes in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Cancer 2017, 123, 3410–3419. [Google Scholar] [CrossRef]
- Iijima, M.; Liu, W.; Panetta, J.C.; Hudson, M.M.; Pui, C.H.; Srivastava, D.K.; Krull, K.R.; Inaba, H. Association between Obesity and Neurocognitive Function in Survivors of Childhood Acute Lymphoblastic Leukemia Treated Only with Chemotherapy. Cancer 2021, 127, 3202–3213. [Google Scholar] [CrossRef]
- Phillips, N.S.; Cheung, Y.T.; Glass, J.O.; Scoggins, M.A.; Liu, W.; Ogg, R.J.; Mulrooney, D.A.; Pui, C.H.; Robison, L.L.; Reddick, W.E.; et al. Neuroanatomical Abnormalities Related to Dexamethasone Exposure in Survivors of Childhood Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2020, 67, e27968. [Google Scholar] [CrossRef]
- Kesler, S.R.; Gugel, M.; Huston-Warren, E.; Watson, C. Atypical Structural Connectome Organization and Cognitive Impairment in Young Survivors of Acute Lymphoblastic Leukemia. Brain Connect. 2016, 6, 273–282. [Google Scholar] [CrossRef]
- Reddick, W.E.; Shan, Z.Y.; Glass, J.O.; Helton, S.; Xiong, X.; Wu, S.; Bonner, M.J.; Howard, S.C.; Christensen, R.; Khan, R.B.; et al. Smaller White-Matter Volumes Are Associated with Larger Deficits in Attention and Learning among Long-Term Survivors of Acute Lymphoblastic Leukemia. Cancer 2006, 106, 941–949. [Google Scholar] [CrossRef]
- Reddick, W.E.; Taghipour, D.J.; Glass, J.O.; Ashford, J.; Xiong, X.; Wu, S.; Bonner, M.; Khan, R.B.; Conklin, H.M. Prognostic Factors That Increase the Risk for Reduced White Matter Volumes and Deficits in Attention and Learning for Survivors of Childhood Cancers. Pediatr. Blood Cancer 2014, 61, 1074–1079. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, E.; Schachar, R.J.; Hitzler, J.; Crosbie, J.; Guger, S.L.; Spiegler, B.J.; Ito, S.; Nieman, B.J. Brain Structure, Working Memory and Response Inhibition in Childhood Leukemia Survivors. Brain Behav. 2017, 7, e00621. [Google Scholar] [CrossRef]
- Morioka, S.; Morimoto, M.; Yamada, K.; Hasegawa, T.; Morita, T.; Moroto, M.; Isoda, K.; Chiyonobu, T.; Imamura, T.; Nishimura, A.; et al. Effects of Chemotherapy on the Brain in Childhood: Diffusion Tensor Imaging of Subtle White Matter Damage. Neuroradiology 2013, 55, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Tamnes, C.K.; Zeller, B.; Amlien, I.K.; Kanellopoulos, A.; Andersson, S.; Due-Tønnessen, P.; Ruud, E.; Walhovd, K.B.; Fjell, A.M. Cortical Surface Area and Thickness in Adult Survivors of Pediatric Acute Lymphoblastic Leukemia. Pediatr. Blood Cancer 2015, 62, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Gandy, K.; Scoggins, M.A.; Phillips, N.S.; van der Plas, E.; Fellah, S.; Jacola, L.M.; Pui, C.-H.; Hudson, M.M.; Reddick, W.E.; Sitaram, R.; et al. Sex-Based Differences in Functional Brain Activity During Working Memory in Survivors of Pediatric Acute Lymphoblastic Leukemia. JNCI Cancer Spectr. 2022, 6, pkac026. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.W. Attentional Processes and Their Remediation in Childhood Cancer. Med. Pediatr. Oncol. 1998, 30, 75–78. [Google Scholar] [CrossRef]
- Butler, R.W.; Copeland, D.R. Attentional Processes and Their Remediation in Children Treated for Cancer: A Literature Review and the Development of a Therapeutic Approach. J. Int. Neuropsychol. Soc. 2002, 8, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sohlberg, M.M.; Mateer, C.A. Effectiveness of an Attention-Training Program. J. Clin. Exp. Neuropsychol. 1987, 9, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Astle, D.E.; Barnes, J.J.; Baker, K.; Colclough, G.L.; Woolrich, M.W. Cognitive Training Enhances Intrinsic Brain Connectivity in Childhood. J. Neurosci. 2015, 35, 6277–6283. [Google Scholar] [CrossRef]
- Wass, S.V. Applying Cognitive Training to Target Executive Functions during Early Development. Child Neuropsychol. 2015, 21, 150–166. [Google Scholar] [CrossRef]
- Butler, R.W.; Copeland, D.R.; Fairclough, D.L.; Mulhern, R.K.; Katz, E.R.; Kazak, A.E.; Noll, R.B.; Patel, S.K.; Sahler, O.J.Z. A Multicenter, Randomized Clinical Trial of a Cognitive Remediation Program for Childhood Survivors of a Pediatric Malignancy. J. Consult. Clin. Psychol. 2008, 76, 367–378. [Google Scholar] [CrossRef]
- Patel, S.K.; Katz, E.R.; Richardson, R.; Rimmer, M.; Kilian, S. Cognitive and Problem Solving Training in Children with Cancer: A Pilot Project. J. Pediatr. Hematol. Oncol. 2009, 31, 670–677. [Google Scholar] [CrossRef]
- Akel, B.S.; Şahin, S.; Huri, M.; Akyüz, C. Cognitive Rehabilitation Is Advantageous in Terms of Fatigue and Independence in Pediatric Cancer Treatment: A Randomized-Controlled Study. Int. J. Rehabil. Res. 2019, 42, 145–151. [Google Scholar] [CrossRef]
- Moore Ki, I.M.; Hockenberry, M.J.; Anhalt, C.; Mccarthy, K.; Krull, K.R. Mathematics Intervention for Prevention of Neurocognitive Deficits in Childhood Leukemia. Pediatr. Blood Cancer 2012, 59, 278–284. [Google Scholar] [CrossRef]
- Hardy, K.K.; Willard, V.W.; Bonner, M.J. Computerized Cognitive Training in Survivors of Childhood Cancer: A Pilot Study. J. Pediatr. Oncol. Nurs. 2011, 28, 27–33. [Google Scholar] [CrossRef]
- Hardy, K.K.; Willard, V.W.; Allen, T.M.; Bonner, M.J. Working Memory Training in Survivors of Pediatric Cancer: A Randomized Pilot Study. Psychooncology 2013, 22, 1856–1865. [Google Scholar] [CrossRef]
- Conklin, H.M.; Ogg, R.J.; Ashford, J.M.; Scoggins, M.A.; Zou, P.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Jeha, S.; et al. Computerized Cognitive Training for Amelioration of Cognitive Late Effects among Childhood Cancer Survivors: A Randomized Controlled Trial. J. Clin. Oncol. 2015, 33, 3894–3902. [Google Scholar] [CrossRef]
- Conklin, H.M.; Ashford, J.M.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Ogg, R.J.; Jeha, S.; Huang, L.; Zhang, H. Long-Term Efficacy of Computerized Cognitive Training among Survivors of Childhood Cancer: A Single-Blind Randomized Controlled Trial. J. Pediatr. Psychol. 2017, 42, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Zucchella, C.; Capone, A.; Codella, V.; De Nunzio, A.M.; Vecchione, C.; Sandrini, G.; Pace, A.; Pierelli, F.; Bartolo, M. Cognitive Rehabilitation for Early Post-Surgery Inpatients Affected by Primary Brain Tumor: A Randomized, Controlled Trial. J. Neurooncol. 2013, 114, 93–100. [Google Scholar] [CrossRef]
- Yang, S.; Chun, M.H.; Son, Y.R. Effect of Virtual Reality on Cognitive Dysfunction in Patients with Brain Tumor. Ann. Rehabil. Med. 2014, 38, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Holmes, J.; Gathercole, S.E.; Dunning, D.L. Adaptive Training Leads to Sustained Enhancement of Poor Working Memory in Children. Dev. Sci. 2009, 12, F9–F15. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.E.; Ashford, J.M.; Clark, K.N.; Martin-Elbahesh, K.; Hardy, K.K.; Merchant, T.E.; Ogg, R.J.; Jeha, S.; Willard, V.W.; Huang, L.; et al. Feasibility and Acceptability of a Remotely Administered Computerized Intervention to Address Cognitive Late Effects among Childhood Cancer Survivors. Neuro-Oncol. Pract. 2015, 2, 78–87. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, K.; Robertson, J.; Ramos, J.; Gella, S. Computer-Based Cognitive Retraining for Adults with Chronic Acquired Brain Injury: A Pilot Study. Occup. Ther. Health Care 2013, 27, 333–344. [Google Scholar] [CrossRef]
- Shipstead, Z.; Redick, T.S.; Engle, R.W. Does Working Memory Training Generalize? Psychol. Belg. 2010, 50, 245–276. [Google Scholar] [CrossRef]
- Zou, P.; Conklin, H.M.; Scoggins, M.A.; Li, Y.; Li, X.; Jones, M.M.; Palmer, S.L.; Gajjar, A.; Ogg, R.J. Functional MRI in Medulloblastoma Survivors Supports Prophylactic Reading Intervention during Tumor Treatment. Brain Imaging Behav. 2016, 10, 258–271. [Google Scholar] [CrossRef]
- Kirchhoff, B.A.; Anderson, B.A.; Smith, S.E.; Barch, D.M.; Jacoby, L.L. Cognitive Training-Related Changes in Hippocampal Activity Associated with Recollection in Older Adults. Neuroimage 2012, 62, 1956–1964. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rosen, A.C.; Sugiura, L.; Kramer, J.H.; Whitfield-Gabrieli, S.; Gabrieli, J.D. Cognitive Training Changes Hippocampal Function in Mild Cognitive Impairment: A Pilot Study. J. Alzheimer’s Dis. 2011, 26, 349. [Google Scholar] [CrossRef]
- Olesen, P.J.; Westerberg, H.; Klingberg, T. Increased Prefrontal and Parietal Activity after Training of Working Memory. Nat. Neurosci. 2004, 7, 75–79. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Ekhtiari, H.; Antal, A.; Auvichayapat, P.; Baeken, C.; Benseñor, I.M.; Bikson, M.; Boggio, P.; Borroni, B.; Brighina, F.; et al. Digitalized Transcranial Electrical Stimulation: A Consensus Statement. Clin. Neurophysiol. 2022, 143, 154–165. [Google Scholar] [CrossRef]
- Zewdie, E.; Ciechanski, P.; Kuo, H.C.; Giuffre, A.; Kahl, C.; King, R.; Cole, L.; Godfrey, H.; Seeger, T.; Swansburg, R.; et al. Safety and Tolerability of Transcranial Magnetic and Direct Current Stimulation in Children: Prospective Single Center Evidence from 3.5 Million Stimulations. Brain Stimul. 2020, 13, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W. Transcranial Electrical Stimulation (TES-TDCS; TRNS, TACS) Methods. Neuropsychol. Rehabil. 2011, 21, 602–617. [Google Scholar] [CrossRef] [PubMed]
- Reed, T.; Cohen Kadosh, R. Transcranial Electrical Stimulation (TES) Mechanisms and Its Effects on Cortical Excitability and Connectivity. J. Inherit. Metab. Dis. 2018, 41, 1123. [Google Scholar] [CrossRef] [PubMed]
- van Boekholdt, L.; Kerstens, S.; Khatoun, A.; Asamoah, B.; Mc Laughlin, M. TDCS Peripheral Nerve Stimulation: A Neglected Mode of Action? Mol. Psychiatry 2021, 26, 456–461. [Google Scholar] [CrossRef] [PubMed]
- De Cicco, V.; Tramonti Fantozzi, M.P.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2018, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Hulsey, D.R.; Riley, J.R.; Loerwald, K.W.; Rennaker, R.L.; Kilgard, M.P.; Hays, S.A. Parametric Characterization of Neural Activity in the Locus Coeruleus in Response to Vagus Nerve Stimulation. Exp. Neurol. 2017, 289, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Yakunina, N.; Kim, S.S.; Nam, E.-C. Optimization of Transcutaneous Vagus Nerve Stimulation Using Functional MRI. Neuromodulation Technol. Neural Interface 2017, 20, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Ruffoli, R.; Giorgi, F.S.; Pizzanelli, C.; Murri, L.; Paparelli, A.; Fornai, F. The Chemical Neuroanatomy of Vagus Nerve Stimulation. J. Chem. Neuroanat. 2011, 42, 288–296. [Google Scholar] [CrossRef]
- Bunai, T.; Hirosawa, T.; Kikuchi, M.; Fukai, M.; Yokokura, M.; Ito, S.; Takata, Y.; Terada, T.; Ouchi, Y. TDCS-Induced Modulation of GABA Concentration and Dopamine Release in the Human Brain: A Combination Study of Magnetic Resonance Spectroscopy and Positron Emission Tomography. Brain Stimul. 2021, 14, 154–160. [Google Scholar] [CrossRef]
- Lea-Carnall, C.A.; Williams, S.R.; Sanaei-Nezhad, F.; Trujillo-Barreto, N.J.; Montemurro, M.A.; El-Deredy, W.; Parkes, L.M. GABA Modulates Frequency-Dependent Plasticity in Humans. iScience 2020, 23, 101657. [Google Scholar] [CrossRef]
- Pearson-Fuhrhop, K.M.; Kleim, J.A.; Cramer, S.C. Brain Plasticity and Genetic Factors. Top. Stroke Rehabil. 2009, 16, 282–299. [Google Scholar] [CrossRef]
- Colzato, L.S.; Vonck, K. Transcutaneous Vagus and Trigeminal Nerve Stimulation. In Theory-Driven Approaches to Cognitive Enhancement; Springer: Cham, Switzerland, 2017; pp. 116–127. [Google Scholar]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus Coeruleus: A New Look at the Blue Spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Follesa, P.; Biggio, F.; Gorini, G.; Caria, S.; Talani, G.; Dazzi, L.; Puligheddu, M.; Marrosu, F.; Biggio, G. Vagus Nerve Stimulation Increases Norepinephrine Concentration and the Gene Expression of BDNF and BFGF in the Rat Brain. Brain Res. 2007, 1179, 28–34. [Google Scholar] [CrossRef]
- Hulsey, D.R.; Shedd, C.M.; Sarker, S.F.; Kilgard, M.P.; Hays, S.A. Norepinephrine and Serotonin Are Required for Vagus Nerve Stimulation Directed Cortical Plasticity. Exp. Neurol. 2019, 320, 112975. [Google Scholar] [CrossRef]
- Chen, M.J.; Nguyen, T.V.; Pike, C.J.; Russo-Neustadt, A.A. Norepinephrine Induces BDNF and Activates the PI-3K and MAPK Cascades in Embryonic Hippocampal Neurons. Cell Signal. 2007, 19, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Tully, K.; Bolshakov, V.Y. Emotional Enhancement of Memory: How Norepinephrine Enables Synaptic Plasticity. Mol. Brain 2010, 3, 15. [Google Scholar] [CrossRef]
- Boggio, P.S.; Nunes, A.; Rigonatti, S.P.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. Repeated Sessions of Noninvasive Brain DC Stimulation Is Associated with Motor Function Improvement in Stroke Patients. Restor. Neurol. Neurosci. 2007, 25, 123–129. [Google Scholar]
- Hummel, F.C.; Voller, B.; Celnik, P.; Floel, A.; Giraux, P.; Gerloff, C.; Cohen, L.G. Effects of Brain Polarization on Reaction Times and Pinch Force in Chronic Stroke. BMC Neurosci. 2006, 7, 73. [Google Scholar] [CrossRef]
- Buell, E.; Loerwald, K.; Engineer, C.; Borland, M.; Buell, J.; Kelly, C.; Khan, I.; Hays, S.; Kilgard, M. Cortical map plasticity as a function of vagus nerve stimulation rate. Brain Stimul. 2018, 11, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Darrow, M.J.; Mian, T.M.; Torres, M.; Haider, Z.; Danaphongse, T.; Rennaker, R.L., Jr.; Kilgard, M.P.; Hays, S.A. Restoration of Somatosensory Function by Pairing Vagus Nerve Stimulation with Tactile Rehabilitation. Ann. Neurol. 2020, 87, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Khaleghi, A.; Zarafshan, H.; Vand, S.R.; Mohammadi, M.R. Effects of Non-Invasive Neurostimulation on Autism Spectrum Disorder: A Systematic Review. Clin. Psychopharmacol. Neurosci. 2020, 18, 527. [Google Scholar] [CrossRef] [PubMed]
- Westwood, S.J.; Radua, J.; Rubia, K. Noninvasive Brain Stimulation in Children and Adults with Attention-Deficit/Hyperactivity Disorder: A Systematic Review and Meta-Analysis. J. Psychiatry Neurosci. 2021, 46, E14–E33. [Google Scholar] [CrossRef]
- Loo, S.K.; Salgari, G.C.; Ellis, A.; Cowen, J.; Dillon, A.; McGough, J.J. Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder: Cognitive and Electroencephalographic Predictors of Treatment Response. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 856–864. [Google Scholar] [CrossRef] [PubMed]
- McGough, J.J.; Loo, S.K.; Sturm, A.; Cowen, J.; Leuchter, A.F.; Cook, I.A. An Eight-Week, Open-Trial, Pilot Feasibility Study of Trigeminal Nerve Stimulation in Youth with Attention-Deficit/Hyperactivity Disorder. Brain Stimul. 2015, 8, 299–304. [Google Scholar] [CrossRef]
- McGough, J.J.; Sturm, A.; Cowen, J.; Tung, K.; Salgari, G.C.; Leuchter, A.F.; Cook, I.A.; Sugar, C.A.; Loo, S.K. Double-Blind, Sham-Controlled, Pilot Study of Trigeminal Nerve Stimulation for Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 403–411. [Google Scholar] [CrossRef]
- Gao, Z.B.; Zhang, W.J.; Tuo, R.; Xiao, X.; Cao, W.J. Transcranial Direct Current Stimulation in the Treatment of Anxiety and Depression in Patients with Oral Cancer during Perioperative Period. Medicine 2022, 101, E30220. [Google Scholar] [CrossRef]
- Gaynor, A.M.; Pergolizzi, D.; Alici, Y.; Ryan, E.; McNeal, K.; Ahles, T.A.; Root, J.C. Impact of Transcranial Direct Current Stimulation on Sustained Attention in Breast Cancer Survivors: Evidence for Feasibility, Tolerability, and Initial Efficacy. Brain Stimul. 2020, 13, 1108–1116. [Google Scholar] [CrossRef]
- Knotkova, H.; Malamud, S.C.; Cruciani, R.A. Transcranial Direct Current Stimulation (TDCS) Improved Cognitive Outcomes in a Cancer Survivor with Chemotherapy-Induced Cognitive Difficulties. Brain Stimul. 2014, 7, 767–768. [Google Scholar] [CrossRef]
- Oldrati, V.; Colombo, B.; Antonietti, A. Combination of a Short Cognitive Training and TDCS to Enhance Visuospatial Skills: A Comparison between Online and Offline Neuromodulation. Brain Res. 2018, 1678, 32–39. [Google Scholar] [CrossRef]
- Agarwal, S.; Pawlak, N.; Cucca, A.; Sharma, K.; Dobbs, B.; Shaw, M.; Charvet, L.; Biagioni, M. Remotely-Supervised Transcranial Direct Current Stimulation Paired with Cognitive Training in Parkinson’s Disease: An Open-Label Study. J. Clin. Neurosci. 2018, 57, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Boroda, E.; Krueger, A.M.; Bansal, P.; Schumacher, M.J.; Roy, A.V.; Boys, C.J.; Lim, K.O.; Wozniak, J.R. A Randomized Controlled Trial of Transcranial Direct-Current Stimulation and Cognitive Training in Children with Fetal Alcohol Spectrum Disorder. Brain Stimul. 2020, 13, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Berger, I.; Dakwar-Kawar, O.; Grossman, E.S.; Nahum, M.; Cohen Kadosh, R. Scaffolding the Attention-Deficit/Hyperactivity Disorder Brain Using Transcranial Direct Current and Random Noise Stimulation: A Randomized Controlled Trial. Clin. Neurophysiol. 2021, 132, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Rocke, M.; Knochenhauer, E.; Thams, F.; Antonenko, D.; Fromm, A.E.; Jansen, N.; Grittner, U.; Schmidt, S.; Brakemeier, E.-L.; Flöel, A. Neuromodulation through Brain Stimulation-Assisted Cognitive Training in Patients with Post-Chemotherapy Cognitive Impairment (Neuromod-PCCI): Study Protocol of a Randomized Controlled Trial. medRxiv 2022. [Google Scholar] [CrossRef]
- Nguyen, J.P.; Gaillard, H.; Suarez, A.; Terzidis-Mallat, É.; Constant-David, D.; Van Langhenhove, A.; Evin, A.; Malineau, C.; Tan, S.V.O.; Mhalla, A.; et al. Bicentre, Randomized, Parallel-Arm, Sham-Controlled Trial of Transcranial Direct-Current Stimulation (TDCS) in the Treatment of Palliative Care Patients with Refractory Cancer Pain. BMC Palliat. Care 2023, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Mortimer, G.; Nadine, H.; Nina, T.; Kirsten, S.; Anke, R.S. Effect of Transcutaneous Auricular Vagal Nerve Stimulation on the Fatigue Syndrome in Patients with Gastrointestinal Cancers—FATIVA: A Randomized, Placebo-Controlled Pilot Study Protocol. Pilot Feasibility Stud. 2023, 9, 66. [Google Scholar]
- Study Details | Neurostimulation in Adult Survivors of Childhood Acute Lymphoblastic Leukemia (ALL) | ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT04007601 (accessed on 10 October 2023).
- Adair, D.; Truong, D.; Esmaeilpour, Z.; Gebodh, N.; Borges, H.; Ho, L.; Douglas Bremner, J.; Badran, B.W.; Napadow, V.; Clark, V.P.; et al. Electrical Stimulation of Cranial Nerves in Cognition and Disease. Brain Stimul. 2020, 13, 717–750. [Google Scholar] [CrossRef]
- Dubreuil-Vall, L.; Chau, P.; Ruffini, G.; Widge, A.S.; Camprodon, J.A. TDCS to the Left DLPFC Modulates Cognitive and Physiological of Executive Function in a State-Dependent Manner. Brain Stimul. 2019, 12, 1456. [Google Scholar] [CrossRef]
- Krause, B.; Kadosh, R.C. Not All Brains Are Created Equal: The Relevance of Individual Differences in Responsiveness to Transcranial Electrical Stimulation. Front. Syst. Neurosci. 2014, 8, 25. [Google Scholar] [CrossRef]
- ter Haar, G. Therapeutic Applications of Ultrasound. Prog. Biophys. Mol. Biol. 2007, 93, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Izadifar, Z.; Izadifar, Z.; Chapman, D.; Babyn, P. An Introduction to High Intensity Focused Ultrasound: Systematic Review on Principles, Devices, and Clinical Applications. J. Clin. Med. 2020, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Timbie, K.F.; Mead, B.P.; Price, R.J. Drug and Gene Delivery across the Blood-Brain Barrier with Focused Ultrasound. J. Control. Release 2015, 219, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-F. High Intensity Focused Ultrasound in Clinical Tumor Ablation. World J. Clin. Oncol. 2011, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Fry, F.J.; Ades, H.W.; Fry, W.J. Production of Reversible Changes in the Central Nervous System by Ultrasound. Science 1958, 127, 83–84. [Google Scholar] [CrossRef] [PubMed]
- Tufail, Y.; Yoshihiro, A.; Pati, S.; Li, M.M.; Tyler, W.J. Ultrasonic Neuromodulation by Brain Stimulation with Transcranial Ultrasound. Nat. Protoc. 2011, 6, 1453–1470. [Google Scholar] [CrossRef] [PubMed]
- Tufail, Y.; Matyushov, A.; Baldwin, N.; Tauchmann, M.L.; Georges, J.; Yoshihiro, A.; Helms-Tillery, S.I.; Tyler, W.J. Transcranial Pulsed Ultrasound Stimulates Intact Brain Circuits. Neuron 2010, 66, 681–694. [Google Scholar] [CrossRef]
- Tyler, W.J.; Tufail, Y.; Finsterwald, M.; Tauchmann, M.L.; Olson, E.J.; Majestic, C. Remote Excitation of Neuronal Circuits Using Low-Intensity, Low-Frequency Ultrasound. PLoS ONE 2008, 3, e3511. [Google Scholar] [CrossRef]
- Yoo, S.S.; Bystritsky, A.; Lee, J.H.; Zhang, Y.; Fischer, K.; Min, B.K.; McDannold, N.J.; Pascual-Leone, A.; Jolesz, F.A. Focused Ultrasound Modulates Region-Specific Brain Activity. Neuroimage 2011, 56, 1267–1275. [Google Scholar] [CrossRef]
- Kamimura, H.A.S.; Wang, S.; Chen, H.; Wang, Q.; Aurup, C.; Acosta, C.; Carneiro, A.A.O.; Konofagou, E.E. Focused Ultrasound Neuromodulation of Cortical and Subcortical Brain Structures Using 1.9 MHz. Med. Phys. 2016, 43, 5730–5735. [Google Scholar] [CrossRef]
- Lee, W.; Lee, S.D.; Park, M.Y.; Foley, L.; Purcell-Estabrook, E.; Kim, H.; Fischer, K.; Maeng, L.S.; Yoo, S.S. Image-Guided Focused Ultrasound-Mediated Regional Brain Stimulation in Sheep. Ultrasound Med. Biol. 2016, 42, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Mehić, E.; Xu, J.M.; Caler, C.J.; Coulson, N.K.; Moritz, C.T.; Mourad, P.D. Increased Anatomical Specificity of Neuromodulation via Modulated Focused Ultrasound. PLoS ONE 2014, 9, e86939. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.C.; Jones, J.P.; Reines, F.; Price, L.R.R. Modification by Focused Ultrasound Pulses of Electrically Evoked Responses from an in Vitro Hippocampal Preparation. Brain Res. 1991, 558, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Bachtold, M.R.; Rinaldi, P.C.; Jones, J.P.; Reines, F.; Price, L.R. Focused Ultrasound Modifications of Neural Circuit Activity in a Mammalian Brain. Ultrasound Med. Biol. 1998, 24, 557–565. [Google Scholar] [CrossRef]
- Khraiche, M.L.; Phillips, W.B.; Jackson, N.; Muthuswamy, J. Ultrasound Induced Increase in Excitability of Single Neurons. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; IEEE: Piscataway, NJ, USA, 2008; pp. 4246–4249. [Google Scholar]
- Yang, P.S.; Kim, H.; Lee, W.; Bohlke, M.; Park, S.; Maher, T.J.; Yoo, S.S. Transcranial Focused Ultrasound to the Thalamus Is Associated with Reduced Rxtracellular GABA Levels in Rats. Neuropsychobiology 2012, 65, 153–160. [Google Scholar] [CrossRef]
- Min, B.K.; Yang, P.S.; Bohlke, M.; Park, S.; S. vago, D.; Maher, T.J.; Yoo, S.S. Focused Ultrasound Modulates the Level of Cortical Neurotransmitters: Potential as a New Functional Brain Mapping Technique. Int. J. Imaging Syst. Technol. 2011, 21, 232–240. [Google Scholar] [CrossRef]
- Kim, H.; Park, M.Y.; Lee, S.D.; Lee, W.; Chiu, A.; Yoo, S.S. Suppression of EEG Visual-Evoked Potentials in Rats through Neuromodulatory Focused Ultrasound. Neuroreport 2015, 26, 211–215. [Google Scholar] [CrossRef]
- Daniels, D.; Sharabi, S.; Last, D.; Guez, D.; Salomon, S.; Zivli, Z.; Castel, D.; Volovick, A.; Grinfeld, J.; Rachmilevich, I.; et al. Focused Ultrasound-Induced Suppression of Auditory Evoked Potentials In Vivo. Ultrasound Med. Biol. 2018, 44, 1022–1030. [Google Scholar] [CrossRef]
- Lu, G.; Qian, X.; Castillo, J.; Li, R.; Jiang, L.; Lu, H.; Shung, K.K.; Humayun, M.S.; Thomas, B.B.; Zhou, Q. Transcranial Focused Ultrasound for Non-Invasive Neuromodulation of the Visual Cortex. IEEE Int. Ultrason. Symp. IUS 2020, 68, 21–28. [Google Scholar] [CrossRef]
- Wattiez, N.; Constans, C.; Deffieux, T.; Daye, P.M.; Tanter, M.; Aubry, J.F.; Pouget, P. Transcranial Ultrasonic Stimulation Modulates Single-Neuron Discharge in Macaques Performing an Antisaccade Task. Brain Stimul. 2017, 10, 1024–1031. [Google Scholar] [CrossRef]
- Yuan, Y.; Yan, J.; Ma, Z.; Li, X. Noninvasive Focused Ultrasound Stimulation Can Modulate Phase-Amplitude Coupling between Neuronal Oscillations in the Rat Hippocampus. Front. Neurosci. 2016, 10, 348. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, C.B.; Li, X.L. Effect of Focused Ultrasound Stimulation at Different Ultrasonic Power Levels on the Local Field Potential Power Spectrum. Chin. Phys. B 2015, 24, 088704. [Google Scholar] [CrossRef]
- Yu, K.; Sohrabpour, A.; He, B. Electrophysiological Source Imaging of Brain Networks Perturbed by Low-Intensity Transcranial Focused Ultrasound. IEEE Trans. Biomed. Eng. 2016, 63, 1787–1794. [Google Scholar] [CrossRef]
- Yu, K.; Niu, X.; Krook-Magnuson, E.; He, B. Intrinsic Functional Neuron-Type Selectivity of Transcranial Focused Ultrasound Neuromodulation. Nat. Commun. 2021, 12, 2519. [Google Scholar] [CrossRef]
- Kim, E.; Anguluan, E.; Kim, J.G. Monitoring Cerebral Hemodynamic Change during Transcranial Ultrasound Stimulation Using Optical Intrinsic Signal Imaging. Sci. Rep. 2017, 7, 13148. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, Y.; Jia, H.; Liu, M.; Hu, S.; Li, Y.; Li, X. Cortical Hemodynamic Responses Under Focused Ultrasound Stimulation Using Real-Time Laser Speckle Contrast Imaging. Front. Neurosci. 2018, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Folloni, D.; Verhagen, L.; Mars, R.B.; Fouragnan, E.; Constans, C.; Aubry, J.-F.; Rushworth, M.F.S.; Sallet, J. Manipulation of Subcortical and Deep Cortical Activity in the Primate Brain Using Transcranial Focused Ultrasound Stimulation. Neuron 2019, 101, 1109–1116.e5. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, L.; Gallea, C.; Folloni, D.; Constans, C.; Jensen, D.E.A.; Ahnine, H.; Roumazeilles, L.; Santin, M.; Ahmed, B.; Lehericy, S.; et al. Offline Impact of Transcranial Focused Ultrasound on Cortical Activation in Primates. Elife 2019, 8, e40541. [Google Scholar] [CrossRef] [PubMed]
- Fouragnan, E.F.; Chau, B.K.H.; Folloni, D.; Kolling, N.; Verhagen, L.; Klein-Flügge, M.; Tankelevitch, L.; Papageorgiou, G.K.; Aubry, J.F.; Sallet, J.; et al. The Macaque Anterior Cingulate Cortex Translates Counterfactual Choice Value into Actual Behavioral Change. Nat. Neurosci. 2019, 22, 797–808. [Google Scholar] [CrossRef]
- Yang, P.F.; Phipps, M.A.; Newton, A.T.; Chaplin, V.; Gore, J.C.; Caskey, C.F.; Chen, L.M. Neuromodulation of Sensory Networks in Monkey Brain by Focused Ultrasound with MRI Guidance and Detection. Sci. Rep. 2018, 8, 7993. [Google Scholar] [CrossRef]
- Biswal, B.; Zerrin Yetkin, F.; Haughton, V.M.; Hyde, J.S. Functional Connectivity in the Motor Cortex of Resting Human Brain Using Echo-planar Mri. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A Default Mode of Brain Function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [PubMed]
- McAfee, S.S.; Liu, Y.; Sillitoe, R.V.; Heck, D.H. Cerebellar Coordination of Neuronal Communication in Cerebral Cortex. Front. Syst. Neurosci. 2022, 15, 165. [Google Scholar] [CrossRef] [PubMed]
- Passingham, R.E.; Stephan, K.E.; Kötter, R. The Anatomical Basis of Functional Localization in the Cortex. Nat. Rev. Neurosci. 2002, 3, 606–616. [Google Scholar] [CrossRef]
- van den Bosch, G.E.; El Marroun, H.; Schmidt, M.N.; Tibboel, D.; Manoach, D.S.; Calhoun, V.D.; White, T.J.H. Brain Connectivity during Verbal Working Memory in Children and Adolescents. Hum. Brain Mapp. 2014, 35, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Phillips, N.S.; Kesler, S.R.; Scoggins, M.A.; Glass, J.O.; Cheung, Y.T.; Liu, W.; Banerjee, P.; Ogg, R.J.; Srivastava, D.; Pui, C.H.; et al. Connectivity of the Cerebello-Thalamo-Cortical Pathway in Survivors of Childhood Leukemia Treated with Chemotherapy Only. JAMA Netw. Open 2020, 3, e2025839. [Google Scholar] [CrossRef] [PubMed]
- Colombatti, R.; Lucchetta, M.; Montanaro, M.; Rampazzo, P.; Ermani, M.; Talenti, G.; Baracchini, C.; Favero, A.; Basso, G.; Manara, R.; et al. Cognition and the Default Mode Network in Children with Sickle Cell Disease: A Resting State Functional MRI Study. PLoS ONE 2016, 11, e0157090. [Google Scholar] [CrossRef]
- Dillen, K.N.H.; Jacobs, H.I.L.; Kukolja, J.; von Reutern, B.; Richter, N.; Onur, Ö.A.; Dronse, J.; Langen, K.J.; Fink, G.R. Aberrant Functional Connectivity Differentiates Retrosplenial Cortex from Posterior Cingulate Cortex in Prodromal Alzheimer’s Disease. Neurobiol. Aging 2016, 44, 114–126. [Google Scholar] [CrossRef]
- Kesler, S.R.; Ogg, R.; Reddick, W.E.; Phillips, N.S.; Scoggins, M.; Glass, J.O.; Cheung, Y.T.; Pui, C.H.; Robison, L.L.; Hudson, M.M.; et al. Brain Network Connectivity and Executive Function in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Brain Connect. 2018, 8, 333–342. [Google Scholar] [CrossRef]
- MacDonald, A.W.; Cohen, J.D.; Andrew Stenger, V.; Carter, C.S. Dissociating the Role of the Dorsolateral Prefrontal and Anterior Cingulate Cortex in Cognitive Control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef] [PubMed]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-Back Working Memory Paradigm: A Meta-Analysis of Normative Functional Neuroimaging Studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Morrell, M.J.; Vogt, B.A. Contributions of Anterior Cingulate Cortex to Behaviour. Brain 1995, 118, 279–306. [Google Scholar] [CrossRef] [PubMed]
- Stevens, F.L.; Hurley, R.A.; Taber, K.H. Anterior Cingulate Cortex: Unique Role in Cognition and Emotion. J. Neuropsychiatry Clin. Neurosci. 2011, 23, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.S.; Botvinick, M.M.; Cohen, J.D. The Contribution of the Anterior Cingulate Cortex to Executive Processes in Cognition. Rev. Neurosci. 1999, 10, 49–57. [Google Scholar] [CrossRef] [PubMed]
- King, R.L.; Brown, J.R.; Newsome, W.T.; Pauly, K.B. Effective Parameters for Ultrasound-Induced In Vivo Neurostimulation. Ultrasound Med. Biol. 2013, 39, 312–331. [Google Scholar] [CrossRef]
- Kim, H.; Chiu, A.; Lee, S.D.; Fischer, K.; Yoo, S.S. Focused Ultrasound-Mediated Non-Invasive Brain Stimulation: Examination of Sonication Parameters. Brain Stimul. 2014, 7, 748–756. [Google Scholar] [CrossRef]
- Breton-Provencher, V.; Drummond, G.T.; Sur, M. Locus Coeruleus Norepinephrine in Learned Behavior: Anatomical Modularity and Spatiotemporal Integration in Targets. Front. Neural Circuits 2021, 15, 46. [Google Scholar] [CrossRef]
- Yang, J.; Wang, Q.; Wang, F.; Wang, L.; Ming, D. Effects of Low-Intensity Focused Ultrasound Stimulation on Working Memory in Vascular Dementia Rats. In Proceedings of the International IEEE/EMBS Conference on Neural Engineering, NER, Virtual Event, 4–6 May 2021; IEEE Computer Society: Washington, DC, USA, 2021; Volume 2021, pp. 1061–1064. [Google Scholar]
- Eguchi, K.; Shindo, T.; Ito, K.; Ogata, T.; Kurosawa, R.; Kagaya, Y.; Monma, Y.; Ichijo, S.; Kasukabe, S.; Miyata, S.; et al. Whole-Brain Low-Intensity Pulsed Ultrasound Therapy Markedly Improves Cognitive Dysfunctions in Mouse Models of Dementia—Crucial Roles of Endothelial Nitric Oxide Synthase. Brain Stimul. 2018, 11, 959–973. [Google Scholar] [CrossRef]
- Huang, S.L.; Chang, C.W.; Lee, Y.H.; Yang, F.Y. Protective Effect of Low-Intensity Pulsed Ultrasound on Memory Impairment and Brain Damage in a Rat Model of Vascular Dementia. Radiology 2017, 282, 113–122. [Google Scholar] [CrossRef]
- Yoo, S.S.; Yoon, K.; Croce, P.; Cammalleri, A.; Margolin, R.W.; Lee, W. Focused Ultrasound Brain Stimulation to Anesthetized Rats Induces Long-Term Changes in Somatosensory Evoked Potentials. Int. J. Imaging Syst. Technol. 2018, 28, 106–112. [Google Scholar] [CrossRef]
- Clennell, B.; Steward, T.G.J.; Elley, M.; Shin, E.; Weston, M.; Drinkwater, B.W.; Whitcomb, D.J. Transient Ultrasound Stimulation Has Lasting Effects on Neuronal Excitability. Brain Stimul. 2021, 14, 217–225. [Google Scholar] [CrossRef]
- Dallapiazza, R.F.; Timbie, K.F.; Holmberg, S.; Gatesman, J.; Lopes, M.B.; Price, R.J.; Miller, G.W.; Elias, W.J. Noninvasive Neuromodulation and Thalamic Mapping with Low-Intensity Focused Ultrasound. J. Neurosurg. 2018, 128, 875–884. [Google Scholar] [CrossRef]
- Niu, X.; Yu, K.; He, B. Transcranial Focused Ultrasound Induces Sustained Synaptic Plasticity in Rat Hippocampus. Brain Stimul. 2022, 15, 352–359. [Google Scholar] [CrossRef]
- Yoo, S.; Mittelstein, D.R.; Hurt, R.C.; Lacroix, J.; Shapiro, M.G. Focused Ultrasound Excites Cortical Neurons via Mechanosensitive Calcium Accumulation and Ion Channel Amplification. Nat. Commun. 2022, 13, 493. [Google Scholar] [CrossRef]
- Harris, K.M. Calcium from Internal Stores Modifies Dendritic Spine Shape. Proc. Natl. Acad. Sci. USA 1999, 96, 12213–12215. [Google Scholar] [CrossRef]
- Vlachos, A.; Korkotian, E.; Schonfeld, E.; Copanaki, E.; Deller, T.; Segal, M. Synaptopodin Regulates Plasticity of Dendritic Spines in Hippocampal Neurons. J. Neurosci. 2009, 29, 1017–1033. [Google Scholar] [CrossRef] [PubMed]
- Korkotian, E.; Frotscher, M.; Segal, M. Synaptopodin Regulates Spine Plasticity: Mediation by Calcium Stores. J. Neurosci. 2014, 34, 11641–11651. [Google Scholar] [CrossRef] [PubMed]
- Mueller, J.K.; Legon, W.; Opitz, A.; Sato, T.F.; Tyler, W.J. Transcranial Focused Ultrasound Modulates Intrinsic and Evoked EEG Dynamics. Brain Stimul. 2014, 7, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Sato, T.F.; Opitz, A.; Mueller, J.K.; Barbour, A.; Williams, A.; Tyler, W.J. Transcranial Focused Ultrasound Modulates the Activity of Primary Somatosensory Cortex in Humans. Nat. Neurosci. 2014, 17, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Chung, Y.A.; Jung, Y.; Song, I.U.; Yoo, S.S. Simultaneous Acoustic Stimulation of Human Primary and Secondary Somatosensory Cortices Using Transcranial Focused Ultrasound. BMC Neurosci. 2016, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Kim, H.; Jung, Y.; Song, I.U.; Chung, Y.A.; Yoo, S.S. Image-Guided Transcranial Focused Ultrasound Stimulates Human Primary Somatosensory Cortex. Sci. Rep. 2015, 5, 8743. [Google Scholar] [CrossRef] [PubMed]
- Gregoriou, G.G.; Gotts, S.J.; Zhou, H.; Desimone, R. High-Frequency, Long-Range Coupling between Prefrontal and Visual Cortex during Attention. Science 2009, 324, 1207–1210. [Google Scholar] [CrossRef]
- Von Stein, A.; Sarnthein, J. Different Frequencies for Different Scales of Cortical Integration: From Local Gamma to Long Range Alpha/Theta Synchronization. Int. J. Psychophysiol. 2000, 38, 301–313. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroglu, C.; Karakaş, S.; Schürmann, M. Brain Oscillations in Perception and Memory. Int. J. Psychophysiol. 2000, 35, 95–124. [Google Scholar] [CrossRef]
- Başar, E.; Başar-Eroglu, C.; Karakaş, S.; Schürmann, M. Gamma, Alpha, Delta, and Theta Oscillations Govern Cognitive Processes. Int. J. Psychophysiol. 2001, 39, 241–248. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.C.; Jung, Y.; Chung, Y.A.; Song, I.U.; Lee, J.H.; Yoo, S.S. Transcranial Focused Ultrasound Stimulation of Human Primary Visual Cortex. Sci. Rep. 2016, 6, 34026. [Google Scholar] [CrossRef] [PubMed]
- Bush, G.; Luu, P.; Posner, M.I. Cognitive and Emotional Influences in Anterior Cingulate Cortex. Trends Cogn. Sci. 2000, 4, 215–222. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nat. Rev. Neurosci. 2002 33 2002, 3, 201–215. [Google Scholar] [CrossRef]
- Rangelov, D.; Müller, H.J.; Taylor, P.C.J. Occipital TMS at Phosphene Detection Threshold Captures Attention Automatically. Neuroimage 2015, 109, 199–205. [Google Scholar] [CrossRef]
- Legon, W.; Bansal, P.; Tyshynsky, R.; Ai, L.; Mueller, J.K. Transcranial Focused Ultrasound Neuromodulation of the Human Primary Motor Cortex. Sci. Rep. 2018, 8, 10007. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, L.; Liu, K.; Tong, S.; Yuan, T.F.; Sun, J. Transcranial Ultrasound Stimulation of the Human Motor Cortex. iScience 2021, 24, 103429. [Google Scholar] [CrossRef]
- Lewis, D.A.; Hashimoto, T.; Volk, D.W. Cortical Inhibitory Neurons and Schizophrenia. Nat. Rev. Neurosci. 2005, 6, 312–324. [Google Scholar] [CrossRef]
- Hallett, M. Neurophysiology of Dystonia: The Role of Inhibition. Neurobiol. Dis. 2011, 42, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Legon, W.; Punzell, S.; Dowlati, E.; Adams, S.E.; Stiles, A.B.; Moran, R.J. Altered Prefrontal Excitation/Inhibition Balance and Prefrontal Output: Markers of Aging in Human Memory Networks. Cereb. Cortex 2016, 26, 4315–4326. [Google Scholar] [CrossRef] [PubMed]
- Hameroff, S.; Trakas, M.; Duffield, C.; Annabi, E.; Gerace, M.B.; Boyle, P.; Lucas, A.; Amos, Q.; Buadu, A.; Badal, J.J. Transcranial Ultrasound (TUS) Effects on Mental States: A Pilot Study. Brain Stimul. 2013, 6, 409–415. [Google Scholar] [CrossRef]
- Sanguinetti, J.L.; Hameroff, S.; Smith, E.E.; Sato, T.F.; Daft, C.M.W.; Tyler, W.J.; Allen, J.J.B. Transcranial Focused Ultrasound to the Right Prefrontal Cortex Improves Mood and Alters Functional Connectivity in Humans. Front. Hum. Neurosci. 2020, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Aron, A.R.; Robbins, T.W.; Poldrack, R.A. Inhibition and the Right Inferior Frontal Cortex. Trends Cogn. Sci. 2004, 8, 170–177. [Google Scholar] [CrossRef]
- Chiu, P.H.; Holmes, A.J.; Pizzagalli, D.A. Dissociable Recruitment of Rostral Anterior Cingulate and Inferior Frontal Cortex in Emotional Response Inhibition. Neuroimage 2008, 42, 988–997. [Google Scholar] [CrossRef]
- Golkar, A.; Lonsdorf, T.B.; Olsson, A.; Lindstrom, K.M.; Berrebi, J.; Fransson, P.; Schalling, M.; Ingvar, M.; Öhman, A. Distinct Contributions of the Dorsolateral Prefrontal and Orbitofrontal Cortex during Emotion Regulation. PLoS ONE 2012, 7, e48107. [Google Scholar] [CrossRef]
- Legon, W.; Ai, L.; Bansal, P.; Mueller, J.K. Neuromodulation with Single-Element Transcranial Focused Ultrasound in Human Thalamus. Hum. Brain Mapp. 2018, 39, 1995–2006. [Google Scholar] [CrossRef]
- Fries, P. Neuronal Gamma-Band Synchronization as a Fundamental Process in Cortical Computation. Annu. Rev. Neurosci. 2009, 32, 209–224. [Google Scholar] [CrossRef] [PubMed]
- Guillery, R.W.; Feig, S.L.; Lozsádi, D.A. Paying Attention to the Thalamic Reticular Nucleus. Trends Neurosci. 1998, 21, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D.; Shah, S.A.; Hudson, A.E.; Nauvel, T.; Kalik, S.F.; Purpura, K.P. Gating of Attentional Effort through the Central Thalamus. J. Neurophysiol. 2013, 109, 1152–1163. [Google Scholar] [CrossRef] [PubMed]
- Staines, W.R.; Black, S.E.; Graham, S.J.; McIlroy, W.E. Somatosensory Gating and Recovery from Stroke Involving the Thalamus. Stroke 2002, 33, 2642–2651. [Google Scholar] [CrossRef] [PubMed]
- Saalmann, Y.B.; Kastner, S. The Cognitive Thalamus. Front. Syst. Neurosci. 2015, 9, 39. [Google Scholar] [CrossRef]
- Ab Aziz, C.B.; Ahmad, A.H. The Role of the Thalamus in Modulating Pain. Malays. J. Med. Sci. 2006, 13, 11–18. [Google Scholar]
- Wallace, B.A.; Ashkan, K.; Benabid, A.L. Deep Brain Stimulation for the Treatment of Chronic, Intractable Pain. Neurosurg. Clin. N. Am. 2004, 15, 343–357. [Google Scholar] [CrossRef]
- Rasche, D.; Rinaldi, P.C.; Young, R.F.; Tronnier, V.M. Deep Brain Stimulation for the Treatment of Various Chronic Pain Syndromes. Neurosurg. Focus 2006, 21, E8. [Google Scholar] [CrossRef]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The Effect of Pain on Cognitive Function: A Review of Clinical and Preclinical Research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef]
- Badran, B.W.; Caulfield, K.A.; Stomberg-Firestein, S.; Summers, P.M.; Dowdle, L.T.; Savoca, M.; Li, X.; Austelle, C.W.; Short, E.B.; Borckardt, J.J.; et al. Sonication of the Anterior Thalamus with MRI-Guided Transcranial Focused Ultrasound (TFUS) Alters Pain Thresholds in Healthy Adults: A Double-Blind, Sham-Controlled Study. Brain Stimul. 2020, 13, 1805–1812. [Google Scholar] [CrossRef]
- Cain, J.A.; Visagan, S.; Johnson, M.A.; Crone, J.; Blades, R.; Spivak, N.M.; Shattuck, D.W.; Monti, M.M. Real Time and Delayed Effects of Subcortical Low Intensity Focused Ultrasound. Sci. Rep. 2021, 11, 6100. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef] [PubMed]
- Schiff, N.D. Recovery of Consciousness after Brain Injury: A Mesocircuit Hypothesis. Trends Neurosci. 2010, 33, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.; Im, J.J.; Park, J.S.; Na, S.H.; Lee, W.; Yoo, S.S.; Song, I.U.; Chung, Y.A. A Pilot Clinical Study of Low-Intensity Transcranial Focused Ultrasound in Alzheimer’s Disease. Ultrasonography 2021, 40, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Matt, E.; Dörl, G.; Beisteiner, R. Transcranial Pulse Stimulation (TPS) Improves Depression in AD Patients on State-of-the-Art Treatment. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12245. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, F.; He, M.J.; Chen, S.J. The Effects and Mechanisms of Transcranial Ultrasound Stimulation Combined with Cognitive Rehabilitation on Post-Stroke Cognitive Impairment. Neurol. Sci. 2022, 43, 4315–4321. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Kim, S.E.; Lee, J.; Hwang, S.; Yoo, S.S.; Lee, H.W. Neuromodulation Using Transcranial Focused Ultrasound on the Bilateral Medial Prefrontal Cortex. J. Clin. Med. 2022, 11, 3809. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.; Dolan, R. The Role of the Prefrontal Cortex in Higher Cognitive Functions. Cogn. Brain Res. 1996, 5, 175–181. [Google Scholar] [CrossRef]
- Kesner, R.P.; Churchwell, J.C. An Analysis of Rat Prefrontal Cortex in Mediating Executive Function. Neurobiol. Learn. Mem. 2011, 96, 417–431. [Google Scholar] [CrossRef]
- Curtis, C.E.; D’Esposito, M. Persistent Activity in the Prefrontal Cortex during Working Memory. Trends Cogn. Sci. 2003, 7, 415–423. [Google Scholar] [CrossRef]
- Bonelli, R.M.; Cummings, J.L. Frontal-Subcortical Circuitry and Behavior. Dialogues Clin. Neurosci. 2022, 9, 141–151. [Google Scholar] [CrossRef]
- Kroczka, S.; Stepien, K.; Skoczen, S. Screening of Cognitive Impairment in Childhood Acute Lymphoblastic Leukemia Survivors with P300 Event-Related Potentials. Pediatr. Blood Cancer 2020, 67, S170–S171. [Google Scholar]
- Lähteenmäki, P.M.; Holopainen, I.; Krause, C.M.; Helenius, H.; Salmi, T.T.; Heikki, L.A. Cognitive Functions of Adolescent Childhood Cancer Survivors Assessed by Event-Related Potentials. Med. Pediatr. Oncol. 2001, 36, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Brace, K.M.; Lee, W.W.; Cole, P.D.; Sussman, E.S. Childhood Leukemia Survivors Exhibit Deficiencies in Sensory and Cognitive Processes, as Reflected by Event-Related Brain Potentials after Completion of Curative Chemotherapy: A Preliminary Investigation. J. Clin. Exp. Neuropsychol. 2019, 41, 814–831. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
VanGilder, P.; Tanner, J.; Krull, K.R.; Sitaram, R. Clinical Potential of Transcranial Focused Ultrasound for Neurorehabilitation in Pediatric Cancer Survivors. Brain Sci. 2024, 14, 218. https://doi.org/10.3390/brainsci14030218
VanGilder P, Tanner J, Krull KR, Sitaram R. Clinical Potential of Transcranial Focused Ultrasound for Neurorehabilitation in Pediatric Cancer Survivors. Brain Sciences. 2024; 14(3):218. https://doi.org/10.3390/brainsci14030218
Chicago/Turabian StyleVanGilder, Paul, Justin Tanner, Kevin R. Krull, and Ranganatha Sitaram. 2024. "Clinical Potential of Transcranial Focused Ultrasound for Neurorehabilitation in Pediatric Cancer Survivors" Brain Sciences 14, no. 3: 218. https://doi.org/10.3390/brainsci14030218
APA StyleVanGilder, P., Tanner, J., Krull, K. R., & Sitaram, R. (2024). Clinical Potential of Transcranial Focused Ultrasound for Neurorehabilitation in Pediatric Cancer Survivors. Brain Sciences, 14(3), 218. https://doi.org/10.3390/brainsci14030218








