Assessment and Training of Perceptual-Motor Function: Performance of College Wrestlers Associated with History of Concussion
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cieśliński, I.; Gierczuk, D.; Sadowski, J. Identification of success factors in elite wrestlers—An exploratory study. PLoS ONE 2021, 16, e0247565. [Google Scholar] [CrossRef] [PubMed]
- Gierczuk, D.; Bujak, Z.; Cieslinski, I.; Lyakh, V.; Sadowski, J. Response time and effectiveness in elite Greco-Roman wrestlers under simulated fight conditions. J. Strength Cond. Res. 2018, 32, 3433–3440. [Google Scholar] [CrossRef] [PubMed]
- Gierczuk, D.; Lyakh, V.; Sadowski, J.; Bujak, Z. Speed of reaction and fighting effectiveness in elite Greco-Roman wrestlers. Percept. Mot. Ski. 2017, 124, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Chandran, A.; Boltz, A.J.; Morris, S.N.; Robison, H.J.; Nedimyer, A.K.; Collins, C.L.; Register-Mihalik, J.K. Epidemiology of concussions in National Collegiate Athletic Association (NCAA) sports: 2014/15–2018/19. Am. J. Sports Med. 2022, 50, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Brett, B.; Wu, Y.-C.; Mustafi, S.M.; Saykin, A.J.; Koch, K.M.; Nencka, A.S.; Giza, C.C.; Goldman, J.; Guskiewicz, K.M.; Mihalik, J.P.; et al. The association between persistent white-matter abnormalities and repeat injury after sport-related concussion. Front. Neurol. 2020, 10, 1345. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A.L.; Nagai, T.; Webster, K.E.; Hewett, T.E. Musculoskeletal injury risk after sport-related concussion: A systematic review and meta-analysis. Am. J. Sports Med. 2018, 47, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Roach, M.H.; Aderman, M.J.; Ross, J.D.; Kelly, T.F.; Malvasi, S.R.; Posner, M.A.; Svoboda, S.J.; Pasquina, P.F.; Cameron, K.L. Risk of upper extremity musculoskeletal injury within the first year after a concussion. Orthop. J. Sports Med. 2023, 11, 23259671231163570. [Google Scholar] [CrossRef] [PubMed]
- Crivelli, D.; Balconi, M. Neuroassessment in sports: An integrative approach for performance and potential evaluation in athletes. Front. Psychol. 2022, 13, 747852. [Google Scholar] [CrossRef]
- Gokeler, A.; McKeon, P.O.; Hoch, M.C. Shaping the functional task environment in sports injury rehabilitation: A framework to integrate perceptual-cognitive training in rehabilitation. Athl. Train. Sports Health Care 2020, 12, 283–292. [Google Scholar] [CrossRef]
- Faubert, J.; Sidebottom, L. Perceptual-cognitive training of athletes. J. Clin. Sport. Psychol. 2012, 6, 85–102. [Google Scholar] [CrossRef]
- Walton, C.C.; Keegan, R.J.; Martin, M.; Hallock, H. The potential role for cognitive training in sport: More research needed. Front. Psychol. 2018, 9, 1121. [Google Scholar] [CrossRef] [PubMed]
- Wilke, J.; Groneberg, D.; Banzer, W.; Giesche, F. Perceptual–cognitive function and unplanned athletic movement task performance: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 7481. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Gabbett, T.; McNeil, D. Reducing injury risk and improving skill: How a psycho-perceptual-motor approach can benefit high-performance sport. Sports Health 2023, 15, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Serpell, B.G.; Young, W.B.; Ford, M. Are the perceptual and decision-making components of agility trainable? A preliminary investigation. J. Strength Cond. Res. 2011, 25, 1240–1248. [Google Scholar] [CrossRef]
- Cardoso, F.d.S.L.; Neves, J.A.; Roca, A.; Teoldo, I. The association between perceptual-cognitive processes and response time in decision making in young soccer players. J. Sports Sci. 2021, 39, 926–935. [Google Scholar] [CrossRef] [PubMed]
- van Maarseveen, M.J.; Oudejans, R.R.; Mann, D.L.; Savelsbergh, G.J. Perceptual-cognitive skill and the in situ performance of soccer players. Q. J. Exp. Psychol. 2018, 71, 455–470. [Google Scholar] [CrossRef] [PubMed]
- Burcal, C.J.; Needle, A.R.; Custer, L.; Rosen, A.B. The effects of cognitive loading on motor behavior in injured individuals: A systematic review. Sports Med. 2019, 49, 1233–1253. [Google Scholar] [CrossRef] [PubMed]
- Leone, C.; Feys, P.; Moumdjian, L.; D’Amico, E.; Zappia, M.; Patti, F. Cognitive-motor dual-task interference: A systematic review of neural correlates. Neurosci. Biobehav. Rev. 2017, 75, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Kucyi, A.; Esterman, M.; Capella, J.; Green, A.; Uchida, M.; Biederman, J.; Gabrieli, J.D.E.; Valera, E.M.; Whitfield-Gabrieli, S. Prediction of stimulus-independent and task-unrelated thought from functional brain networks. Nat. Commun. 2021, 12, 1793. [Google Scholar] [CrossRef]
- Dixon, M.L.; De La Vega, A.; Mills, C.; Andrews-Hanna, J.; Spreng, R.N.; Cole, M.W.; Christoff, K. Heterogeneity within the frontoparietal control network and its relationship to the default and dorsal attention networks. Proc. Nat. Acad. Sci. USA 2018, 115, E1598–E1607. [Google Scholar] [CrossRef]
- Rieck, J.R.; DeSouza, B.; Baracchini, G.; Grady, C.L. Reduced modulation of BOLD variability as a function of cognitive load in healthy aging. Neurobiol. Aging. 2022, 112, 215–230. [Google Scholar] [CrossRef] [PubMed]
- Hadlow, S.M.P.D.; Mann, D.L.; Portus, M.R.; Abernethy, B. Modified perceptual training in sport: A new classification framework. J. Sci. Med. Sport 2018, 21, 950–958. [Google Scholar] [CrossRef]
- Brown, J.A.; Dalecki, M.; Hughes, C.; Macpherson, A.K.; Sergio, L.E. Cognitive-motor integration deficits in young adult athletes following concussion. BMC Sports Sci. Med. Rehabil. 2015, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Hurtubise, J.; Gorbet, D.; Hamandi, Y.; Macpherson, A.; Sergio, L. The effect of concussion history on cognitive-motor integration in elite hockey players. Concussion 2016, 1, CNC17. [Google Scholar] [CrossRef]
- Santos, F.V.; Yamaguchi, F.; Buckley, T.A.; Caccese, J.B. Virtual reality in concussion management: From lab to clinic. J. Clin. Transl. Res. 2020, 5, 148–154. [Google Scholar]
- Schuermans, J.; Van Hootegem, A.; Van den Bossche, M.; Van Gendt, M.; Witvrouw, E.; Wezenbeek, E. Extended reality in musculoskeletal rehabilitation and injury prevention—A systematic review. Phys. Ther. Sport 2022, 55, 229–240. [Google Scholar] [CrossRef]
- Wilkerson, G.B.; Colston, M.A.; Acocello, S.N.; Hogg, J.A.; Carlson, L.M. Subtle impairments of perceptual-motor function and well-being are detectable among military cadets and college athletes with self-reported history of concussion. Front. Sports Act. Living 2023, 5, 1046572. [Google Scholar] [CrossRef]
- Wilkerson, G.B.; Lansey, J.C.; Noblett, C.N.; Sarris, C.E. Test-retest reliability of immersive virtual reality measures of perceptual-motor performance. Percept. Mot. Ski. 2023, 130, 2484–2504. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Applied Logistic Regression, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2000. [Google Scholar]
- Olivier, J.; May, W.L.; Bell, M.L. Relative effect sizes for measures of risk. Commun. Stat. Theory Methods 2017, 46, 6774–6781. [Google Scholar] [CrossRef]
- Matthews, R.A. Moving towards the post p < 0.05 era via the analysis of credibility. Am. Stat. 2019, 73, 202–212. [Google Scholar]
- Cao, J.; Zhang, S. Multiple comparison procedures. JAMA 2014, 312, 543–544. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York, NY, USA, 1988. [Google Scholar]
- Chang, Y. Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front. Hum. Neurosci. 2014, 8, 35. [Google Scholar] [CrossRef]
- Hellyer, P.J.; Scott, G.; Shanahan, M.; Sharp, D.J.; Leech, R. Cognitive flexibility through metastable neural dynamics is disrupted by damage to the structural connectome. J. Neurosci. 2015, 35, 9050–9063. [Google Scholar] [CrossRef]
- Baracchini, G.; Mišić, B.; Setton, R.; Mwilambwe-Tshilobo, L.; Girn, M.; Nomi, J.S.; Uddin, L.Q.; Turner, G.R.; Spreng, R.N. Inter-regional BOLD signal variability is an organizational feature of functional brain networks. Neuroimage 2021, 237, 118149. [Google Scholar] [CrossRef]
- Wang, C.-H.; Liang, W.-K.; Moreau, D. Differential modulation of brain signal variability during cognitive control in athletes with different domains of expertise. Neuroscience 2020, 425, 267–279. [Google Scholar] [CrossRef]
- Kelly, A.C.; Uddin, L.Q.; Biswal, B.B.; Castellanos, F.X.; Milham, M.P. Competition between functional brain networks mediates behavioral variability. Neuroimage 2008, 39, 527–537. [Google Scholar] [CrossRef]
- Roberts, S.D.; Wilson, A.; Rahimi, A.; Gorbet, D.; Sergio, L.; Stevens, W.D.; Wojtowicz, M. Investigation of baseline attention, executive control, and performance variability in female varsity athletes. Brain Imag. Behav. 2022, 16, 1636–1645. [Google Scholar] [CrossRef]
- Uddin, L.Q. Cognitive and behavioural flexibility: Neural mechanisms and clinical considerations. Nat. Rev. Neurosci. 2021, 22, 167–179. [Google Scholar] [CrossRef]
- Garrett, D.D.; Epp, S.M.; Kleemeyer, M.; Lindenberger, U.; Polk, T.A. Higher performers upregulate brain signal variability in response to more feature-rich visual input. Neuroimage 2020, 217, 116836. [Google Scholar] [CrossRef]
- Grady, C.L.; Garrett, D.D. Brain signal variability is modulated as a function of internal and external demand in younger and older adults. Neuroimage 2018, 169, 510–523. [Google Scholar] [CrossRef]
- Mennes, M.; Zuo, X.-N.; Kelly, C.; Di Martino, A.; Zang, Y.-F.; Biswal, B.; Castellanos, F.X.; Milham, M.P. Linking inter-individual differences in neural activation and behavior to intrinsic brain dynamics. Neuroimage 2011, 54, 2950–2959. [Google Scholar] [CrossRef]
- Gazzellini, S.; Napolitano, A.; Bauleo, G.; Bisozzi, E.; Lispi, M.L.; Ardu, E.; Castelli, E.; Benso, F. Time–frequency analyses of reaction times and theta/beta EEG ratio in pediatric patients with traumatic brain injury: A preliminary study. Dev. Neurorehabil. 2017, 20, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Halliday, D.W.; Gawryluk, J.R.; Garcia-Barrera, M.A.; MacDonald, S.W. White matter integrity is associated with intraindividual variability in neuropsychological test performance in healthy older adults. Front. Hum. Neurosci. 2019, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Hallock, H.; Mantwill, M.; Vajkoczy, P.; Wolfarth, B.; Reinsberger, C.; Lampit, A.; Finke, C. Sport-related concussion: A cognitive perspective. Neurol. Clin. Pract. 2023, 13, e200123. [Google Scholar] [CrossRef] [PubMed]


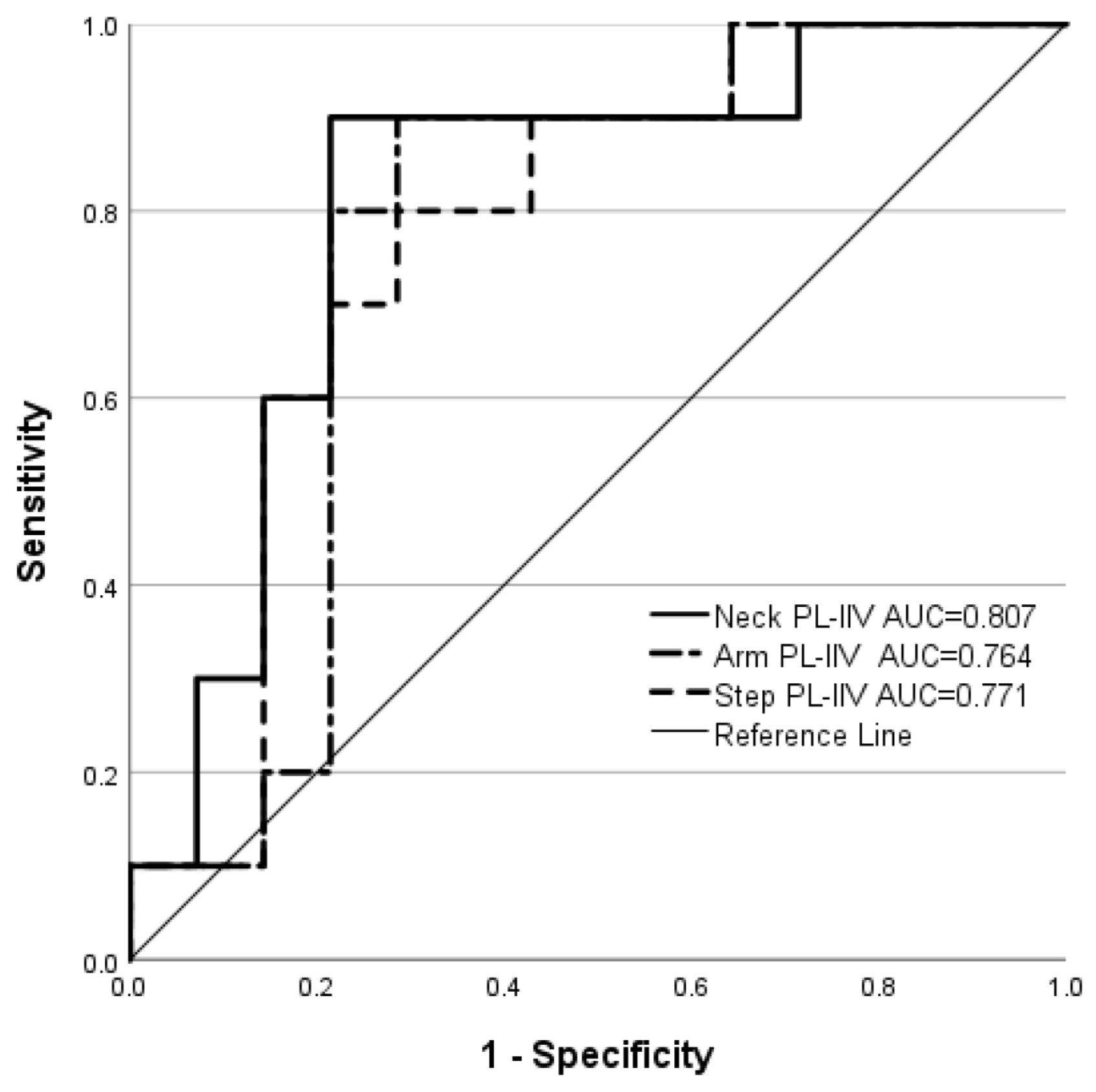

| Metric | AUC | Cut-Off Point | p * | Sensitivity | Specificity | OR | 95% CI | SL |
|---|---|---|---|---|---|---|---|---|
| Neck PL-IIV | 0.807 | ≥0.215 | 0.001 | 0.90 | 0.79 | 33.00 | 2.91, 374.31 | 10.43 |
| Arm PL-IIV | 0.764 | ≥0.217 | 0.004 | 0.90 | 0.71 | 22.50 | 2.11, 240.48 | 15.98 |
| Step PL-IIV | 0.771 | ≥0.215 | 0.018 | 0.80 | 0.71 | 10.00 | 1.44, 69.26 | 20.43 |
| Neck RT-IIV | 0.793 | ≥0.241 | 0.001 | 0.90 | 0.79 | 33.00 | 2.91, 374.36 | 10.43 |
| Arm RT-IIV | 0.771 | ≥0.247 | 0.007 | 0.80 | 0.79 | 14.67 | 1.97, 109.20 | 9.58 |
| Step RT-IIV | 0.775 | ≥0.242 | 0.007 | 0.80 | 0.79 | 14.67 | 1.97, 109.20 | 9.58 |
| Metric | Group | Geometric Mean (Loge Mean) | Group × Session Interaction | Group Difference | Session Difference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | p | ηp2 | p | ηp2 | p | ηp2 | ||
| Neck PL-IIV * | NoC | 0.193 (−1.646) | 0.133 (−2.015) | 0.231 | 0.065 | 0.005 | 0.303 | 0.060 | 0.152 |
| HxC | 0.301 (−1.200) | 0.276 (−1.286) | |||||||
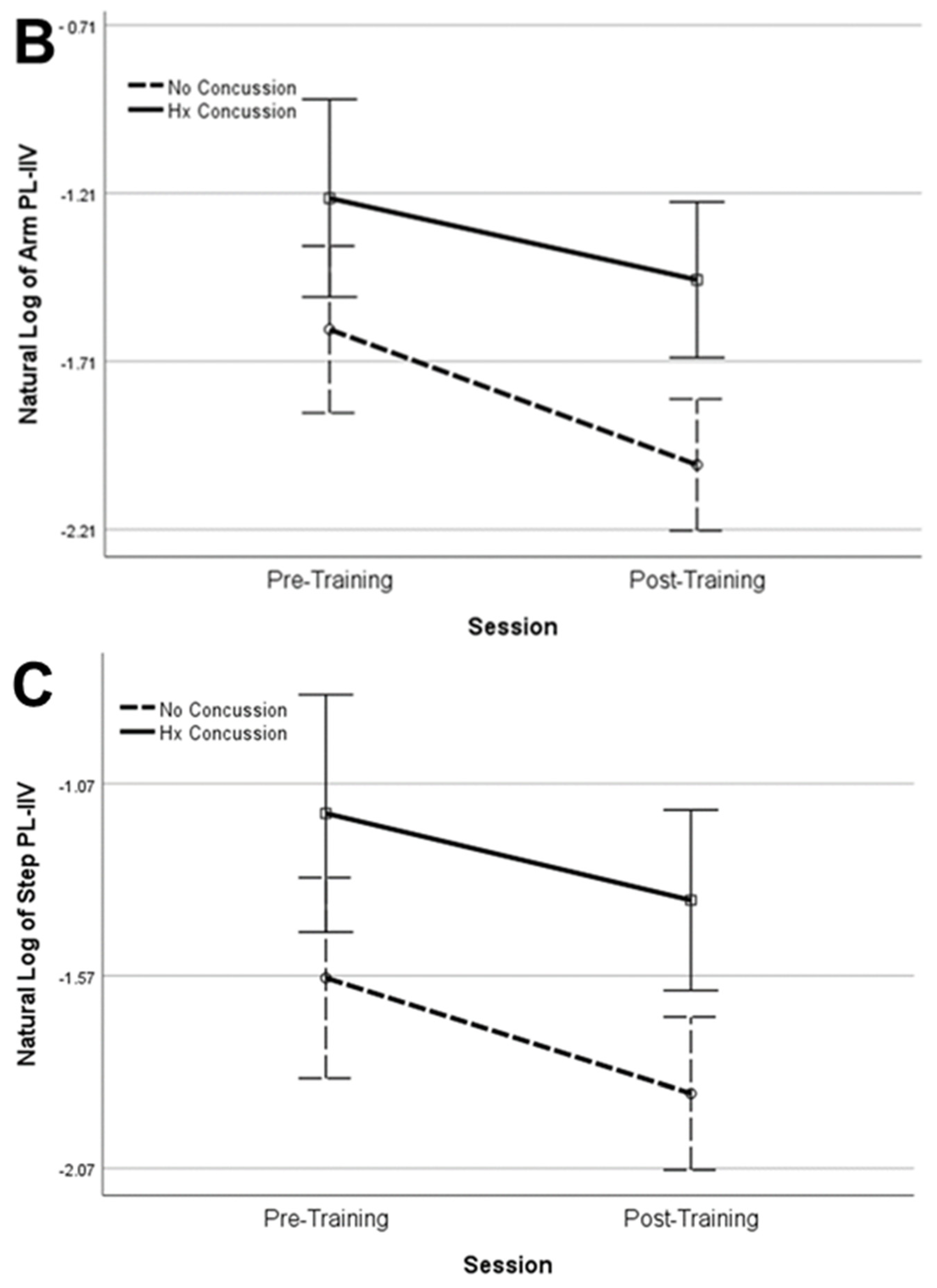
| Arm PL-IIV * | NoC | 0.199 (−1.615) | 0.133 (−2.018) | 0.339 | 0.042 | 0.005 | 0.301 | <0.001 | 0.414 |
| HxC | 0.294 (−1.225) | 0.231 (−1.468) | |||||||
| Step PL-IIV * | NoC | 0.207 (−1.576) | 0.153 (−1.876) | 0.668 | 0.009 | 0.008 | 0.282 | 0.005 | 0.303 |
| HxC | 0.317 (−1.148) | 0.253 (−1.374) | |||||||
| Neck RT-IIV * | NoC | 0.222 (−1.505) | 0.154 (−1.872) | 0.221 | 0.067 | 0.007 | 0.285 | 0.022 | 0.216 |
| HxC | 0.296 (−1.216) | 0.263 (−1.335) | |||||||
| Arm RT-IIV * | NoC | 0.214 (−1.543) | 0.136 (−1.995) | 0.073 | 0.139 | 0.003 | 0.336 | <0.001 | 0.410 |
| HxC | 0.285 (−1.256) | 0.243 (−1.415) | |||||||
| Step RT-IIV * | NoC | 0.220 (−1.513) | 0.155 (−1.863) | 0.272 | 0.055 | 0.009 | 0.269 | <0.001 | 0.448 |
| HxC | 0.302 (−1.199) | 0.246 (−1.401) | |||||||
| Neck PL-Avg * | NoC | 0.609 (−0.495) | 0.567 (−0.567) | 0.203 | 0.073 | 0.078 | 0.134 | 0.714 | 0.006 |
| HxC | 0.632 (−0.459) | 0.657 (−0.419) | |||||||
| Arm PL-Avg * | NoC | 0.718 (−0.331) | 0.603 (−0.505) | 0.925 | <0.001 | 0.301 | 0.049 | <0.001 | 0.701 |
| HxC | 0.760 (−0.275) | 0.641 (−0.444) | |||||||
| Step PL-Avg * | NoC | 0.709 (−0.344) | 0.613 (−0.489) | 0.513 | 0.020 | 0.182 | 0.080 | <0.001 | 0.545 |
| HxC | 0.750 (−0.288) | 0.671 (−0.400) | |||||||
| Metric * | Group | Mean ± SD | Group × Session Interaction | Group Difference | Session Difference | ||||
|---|---|---|---|---|---|---|---|---|---|
| Pre-Training | Post-Training | p | ηp2 | p | ηp2 | p | ηp2 | ||
| Neck RT-Avg | NoC | 0.931 ± 0.135 | 0.882 ± 0.116 | 0.075 | 0.137 | 0.354 | 0.039 | 0.725 | 0.006 |
| HxC | 0.934 ± 0.110 | 0.968 ± 0.134 | |||||||
| Arm RT-Avg | NoC | 1.129 ± 0.151 | 1.018 ± 0.154 | 0.606 | 0.012 | 0.240 | 0.062 | <0.001 | 0.529 |
| HxC | 1.187 ± 0.144 | 1.097 ± 0.126 | |||||||
| Step RT-Avg | NoC | 1.192 ± 0.159 | 1.096 ± 0.132 | 0.131 | 0.101 | 0.219 | 0.068 | 0.043 | 0.173 |
| HxC | 1.213 ± 0.112 | 1.198 ± 0.110 | |||||||
| ARM PL-RCS | NoC | 1.18 ± 0.44 | 1.56 ± 0.33 | 0.351 | 0.040 | 0.412 | 0.031 | <0.001 | 0.601 |
| HxC | 1.11 ± 0.37 | 1.38 ± 0.42 | |||||||
| Arm RT-RCS | NoC | 0.77 ± 0.23 | 0.94 ± 0.17 | 0.190 | 0.077 | 0.305 | 0.048 | <0.001 | 0.424 |
| HxC | 0.73 ± 0.19 | 0.81 ± 0.22 | |||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkerson, G.B.; Fleming, L.R.; Adams, V.P.; Petty, R.J.; Carlson, L.M.; Hogg, J.A.; Acocello, S.N. Assessment and Training of Perceptual-Motor Function: Performance of College Wrestlers Associated with History of Concussion. Brain Sci. 2024, 14, 68. https://doi.org/10.3390/brainsci14010068
Wilkerson GB, Fleming LR, Adams VP, Petty RJ, Carlson LM, Hogg JA, Acocello SN. Assessment and Training of Perceptual-Motor Function: Performance of College Wrestlers Associated with History of Concussion. Brain Sciences. 2024; 14(1):68. https://doi.org/10.3390/brainsci14010068
Chicago/Turabian StyleWilkerson, Gary B., Lexi R. Fleming, Victoria P. Adams, Richard J. Petty, Lynette M. Carlson, Jennifer A. Hogg, and Shellie N. Acocello. 2024. "Assessment and Training of Perceptual-Motor Function: Performance of College Wrestlers Associated with History of Concussion" Brain Sciences 14, no. 1: 68. https://doi.org/10.3390/brainsci14010068
APA StyleWilkerson, G. B., Fleming, L. R., Adams, V. P., Petty, R. J., Carlson, L. M., Hogg, J. A., & Acocello, S. N. (2024). Assessment and Training of Perceptual-Motor Function: Performance of College Wrestlers Associated with History of Concussion. Brain Sciences, 14(1), 68. https://doi.org/10.3390/brainsci14010068







