EEG Network Analysis of Depressive Emotion Interference Spatial Cognition Based on a Simulated Robotic Arm Docking Task
Abstract
:1. Introduction
| Articles | Scales | Neural Signals | Resting State | Cognition Task | Brain Network | Frequency Band |
|---|---|---|---|---|---|---|
| [30] | DSM-IV | DTI | √ | / | / | / |
| [31] | GDS-15 | PET | / | executive function, memory | / | / |
| [32] | DSM-IV, CES-D | / | / | interview | / | / |
| [33] | BDI-II, OASIS, K10 | EEG | / | Emotional processing | Coherence | √ |
| [24] | CES-D, HAMD | MRI | √ | / | general linear model | / |
| [23] | CES-D, HAMD | MRI | √ | / | Seed-based | / |
| [7] | DSM-IV, HAM-D, FAST | / | / | verbal memory | / | / |
| [8] | MADRS | / | / | Wisconsin Card Sorting Task | / | / |
| [9] | BDI-II, SDS | EEG | / | Emotional processing | / | / |
| ours | CES-D, BDI-II | EEG | √ | Docking task | PLV | √ |
2. Materials and Methods
2.1. Subjects
2.2. Experimental Design
2.3. EEG Signal Acquisition and Pre-Processing
2.4. Operation Time Analysis
2.5. EEG Network Analysis
3. Results
3.1. Behavior Data Results
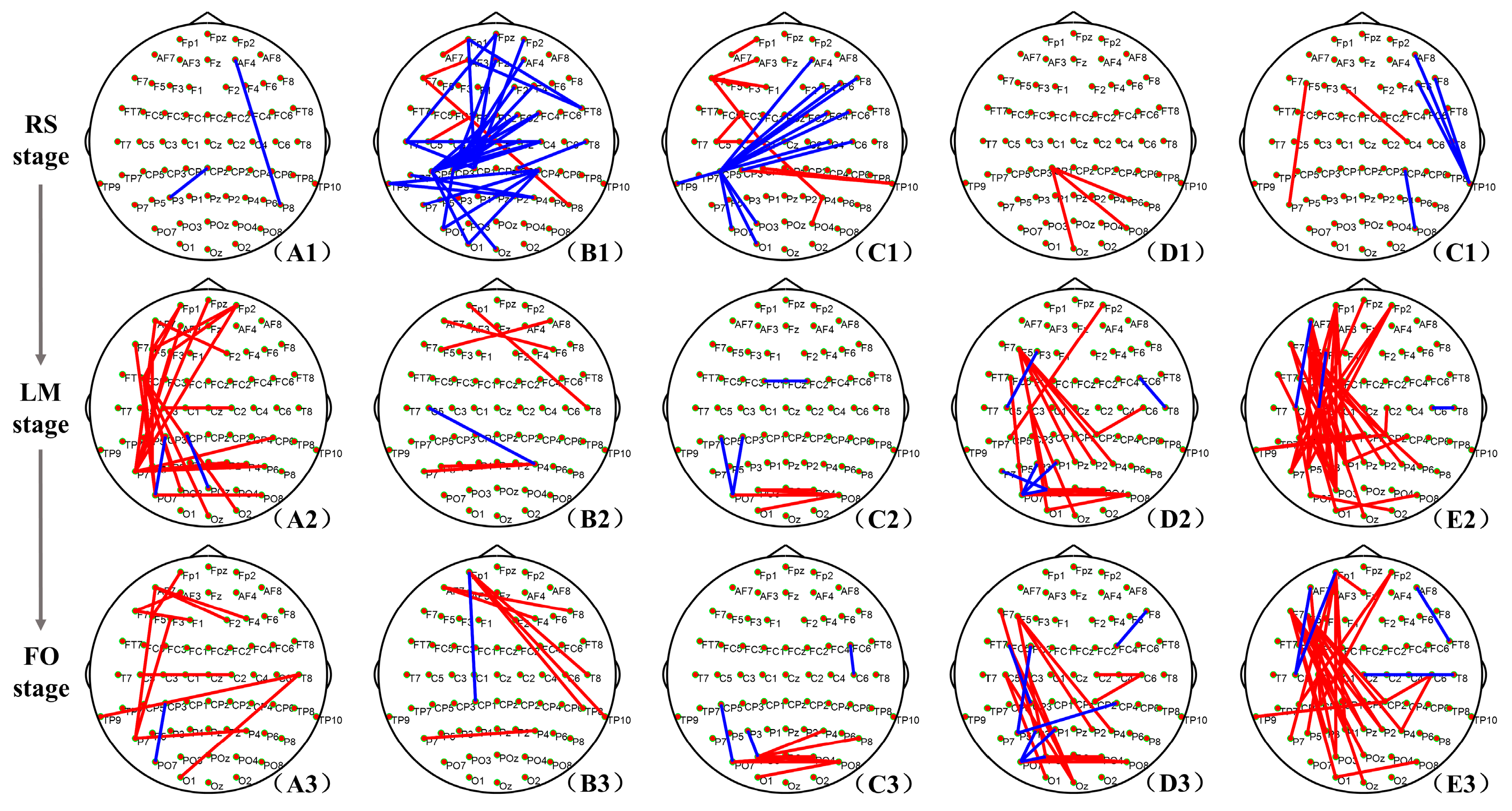
3.2. Brain Network Connections with a Significant Difference
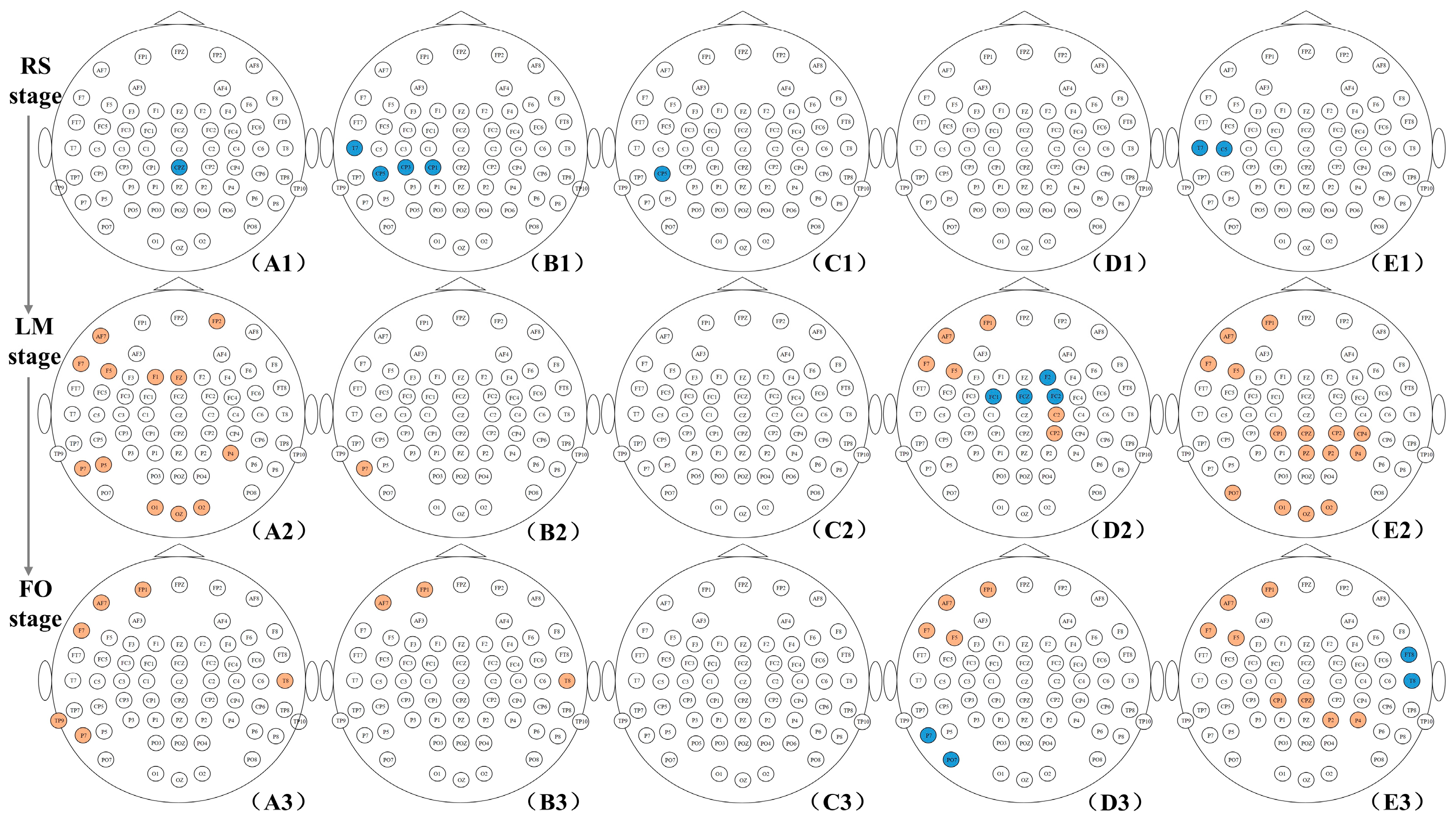
3.3. Difference in Small-Word Properties between Two Groups
3.4. Relationship between Small-Word Properties and the CES-D Scores
4. Discussion
- Depressive emotion impairs spatial cognition, resulting in longer operation time
- The weaker network connections in the RS stage of the DE group
- The enhancement in frontoparietal connections in LM and FO stages of the DE group
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mario, J. Francisco Understanding Subthreshold Depression. Shanghai Arch. Psychiatry 2012, 24, 292–293. [Google Scholar] [CrossRef]
- Volz, H.P.; Stirnwei, J.; Kasper, S.; Hans-Jürgen, M.; Seifritz, E. Subthreshold Depression–Concept, Operationalisation and Epidemiological Data A Scoping Review. Int. J. Psychiatry Clin. Pract. 2022, 27, 92–106. [Google Scholar] [CrossRef]
- Mi, W.; Dong, Q.; Zhang, L. Research Progress on Cognitive Impairment and Influencing Factors in Adolescents with Depression. J. New Med. 2023, 54, 13–16. [Google Scholar]
- Mouras, J.; King, J. Cognitive Impairment and Endorsement of Depression. Clin. Neuropsychol. 2022, 36, 756–757. [Google Scholar]
- Lai, S.; Zhong, S.; Wang, Y.; Zhang, Y.; Xue, Y.; Zhao, H.; Ran, H.; Yan, S.; Luo, Y.; He, J. The Prevalence and Characteristics of MCCB Cognitive Impairment in Unmedicated Patients with Bipolar II Depression and Major Depressive Disorder. J. Affect. Disord. 2022, 310, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Zhu, H.; Jiang, H.; Yang, J. The Neural Mechanisms Research of the Relationship between Autobiographical Memory and Subthreshold Depression. Prog. Mod. Biomed. 2014, 14, 3979–3981. [Google Scholar] [CrossRef]
- Bonnín, C.D.M.; González-Pinto, A.; Solé, B.; Reinares, M.; Vega, P. Verbal Memory as a Mediator in the Relationship between Subthreshold Depressive Symptoms and Functional Outcome in Bipolar Disorder. J. Affect. Disord. 2014, 160, 50–54. [Google Scholar] [CrossRef]
- Yepez, A.T.; Cohen, L.; Adrien, T.; Green, M.; Forester, B. Subclinical Manic Symptoms Predict Social Cognitive Impairment Mediated by Deficits in Executive Functioning in Older Adults with Mood Disorders. Am. J. Geriatr. Psychiatry 2021, 29, S107–S108. [Google Scholar] [CrossRef]
- Chen, X.; Feng, Z.; Jiang, J. Effect of Strengthened or Weakened Emotional Processing on Cognitive Control in Individuals with Subthreshold Depression: An Event-Related Potential Study. J. Third Mil. Med. Univ. 2016, 38, 1991–1996. [Google Scholar]
- Li, M.; Zhang, L.; Zhang, M.; Cao, T.; Wang, S. Cognitive and Brain Mechanisms of Subthreshold Depression. Psychol. Mon. 2023, 18, 227–229. [Google Scholar] [CrossRef]
- Geary, D.C. Spatial Ability as a Distinct Domain of Human Cognition: An Evolutionary Perspective. Intelligence 2022, 90, 101616. [Google Scholar] [CrossRef]
- Farran, E.K.; Blades, M.; Hudson, K.D.; Sockeel, P.; Courbois, Y. Spatial Exploration Strategies in Childhood: Exploration Behaviours Are Predictive of Navigation Success. Cogn. Dev. 2022, 61, 101153. [Google Scholar] [CrossRef]
- Kronovsek, A.; Hermand, E.; Berthoz, A.; Castilla, A.; Gallou-Guyot, M.; Daviet, J.C.; Perrochon, A. Age-Related Decline in Visuo-Spatial Working Memory Is Reflected by Dorsolateral Prefrontal Activation and Cognitive Capabilities. Behav. Brain Res. Int. J. 2021, 398, 112981. [Google Scholar] [CrossRef] [PubMed]
- Cak, S.; Say, B.; Misirlisoy, M. Effects of Working Memory, Attention, and Expertise on Pilots’ Situation Awareness. Cogn. Technol. Work 2019, 22, 85–94. [Google Scholar] [CrossRef]
- Kaber, D.B.; Jin, S.; Zahabi, M.; Pankok, C., Jr. The Effect of Driver Cognitive Abilities and Distractions on Situation Awareness and Performance under Hazard Conditions. Transp. Res. Part F Psychol. Behav. 2016, 42, 177–194. [Google Scholar] [CrossRef]
- Chen, J.; Yang, L.; Li, Y.; Liu, G.; Wu, Y.; Wang, D.; Jia, T. Hemispheric Dominance on Visuo-Spatial Cognitive Function in Patients with First-Episode Depression. J. Shanxi Med. Univ. 2012, 12, 896–900. [Google Scholar]
- Szczepanski, S.M.; Konen, C.S.; Kastner, S. Mechanisms of Spatial Attention Control in Frontal and Parietal Cortex. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 148. [Google Scholar] [CrossRef]
- Cona, G.; Scarpazza, C. Where Is the “Where” in the Brain? A Meta-analysis of Neuroimaging Studies on Spatial Cognition. Hum. Brain Mapp. 2019, 40, 1867–1886. [Google Scholar] [CrossRef]
- Scolari, M.; Kastner, S. Mechanisms of Attentional Control in Fronto-Parietal Cortex across Spatial Positions. J. Vis. 2013, 13, 288–298. [Google Scholar] [CrossRef]
- Xu, G.; Lan, Y.; He, X. Modulatory Effects of Continuous Theta Burst Stimulation over the Right Frontoparietal Network on Visuospatial Attention. Chin. J. Rehabil. Med. 2013, 28, 714–718. [Google Scholar]
- Hasanzadeh, F.; Mohebbi, M.; Rostami, R. Graph Theory Analysis of Directed Functional Brain Networks in Major Depressive Disorder Based on EEG Signal. J. Neural Eng. 2020, 17, 26010. [Google Scholar] [CrossRef] [PubMed]
- Dell’Acqua, G.; Ghiasi, S.; Messerotti Benvenuti, S.; Greco, A.; Gentili, C.; Valenza, G. Increased Functional Connectivity within Alpha and Theta Frequency Bands in Dysphoria: A Resting-State EEG Study. J. Affect. Disord. 2021, 281, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Egorova, N.; Yang, X.Q.; Zhang, W.Y.; Chen, J.; Yang, X.Y.; Hu, L.J.; Sun, S.; Tu, Y.; Kong, J. Subthreshold Depression Is Associated with Impaired Resting-State Functional Connectivity of the Cognitive Control Network. Transl. Psychiatry 2015, 5, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.W.; Xin, S.C.; Ou, Y.M.; Zhang, W.Y.; Liang, Y.L.; Chen, J.; Yang, X.Q.; Chen, X.Y.; Guo, T.W.; Yang, X.J. Enhanced Default Mode Network Connectivity with Ventral Striatum in Subthreshold Depression Individuals. J. Psychiatr. Res. 2016, 76, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Verga, L.; Kotz, S.A. Spatial Attention Underpins Social Word Learning in the Right Fronto-Parietal Network. NeuroImage 2019, 195, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.H.; Ito, T.; Solomyak, L.I.; Chen, R.H.; Mill, R.D.; Kulkarni, K.R.; Cole, M.W. Global Connectivity of the Fronto-Parietal Cognitive Control Network Is Related to Depression Symptoms in the General Population. Netw. Neurosci. 2018, 3, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K.; Lee, T.L.; Chan, A.S. Depressive and Anxiety Symptoms Are Related to Decreased Lateral Prefrontal Cortex Functioning during Cognitive Control in Older People. Biol. Psychol. 2021, 166, 108224. [Google Scholar] [CrossRef]
- Lee, T.W.; Liu, H.L.; Wai, Y.Y.; Ko, H.J.; Lee, S.H. Abnormal Neural Activity in Partially Remitted Late-Onset Depression: An fMRI Study of One-Back Working Memory Task. Psychiatry Res. Neuroimaging 2013, 213, 133–141. [Google Scholar] [CrossRef]
- Lin, S.-C.; Lee, S.H.; Huang, C.M.; Chen, G.Y.; Ho, P.S.; Liu, H.L.; Chen, Y.L.; Lee, T.M.; Wu, S.C. Increased Brain Entropy of Resting-State fMRI Mediates the Relationship between Depression Severity and Mental Health-Related Quality of Life in Late-Life Depressed Elderly. J. Affect. Disord. 2019, 250, 270–277. [Google Scholar] [CrossRef]
- Vulser, H.; Paillère Martinot, M.L.; Artiges, E.; Miranda, R.; Penttilä, J.; Grimmer, Y.; van Noort, B.M.; Stringaris, A.; Struve, M.; Fadai, T.; et al. Early Variations in White Matter Microstructure and Depression Outcome in Adolescents With Subthreshold Depression. Am. J. Psychiatry 2018, 175, 1255–1264. [Google Scholar] [CrossRef]
- Zhang, Z.; Wei, F.; Shen, X.N.; Ma, Y.H.; Chen, K.L.; Dong, Q.; Tan, L.; Yu, J.T.; Alzheimer's Disease Neuroimaging Initiative. Associations of Subsyndromal Symptomatic Depression with Cognitive Decline and Brain Atrophy in Elderly Individuals without Dementia: A Longitudinal Study. J. Affect. Disord. 2020, 274, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Wyman, M.F.; Jonaitis, E.M.; Ward, E.C.; Zuelsdorff, M.; Gleason, C.E. Depressive Role Impairment and Subthreshold Depression in Older Black and White Women: Race Differences in the Clinical Significance Criterion. Int. Psychogeriatr. 2020, 32, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jing, Z.; Hu, B.; Sun, S. An EEG-Based Study on Coherence and Brain Networks in Mild Depression Cognitive Process. In Proceedings of the 2016 IEEE International Conference on Bioinformatics and Biomedicine, Shenzhen, China, 15–18 December 2016; IEEE Xplore: New York, NY, USA, 2017; Volume 1, pp. 1275–1282. [Google Scholar]
- Zhu, S.; Tang, W.; Wang, D.; Xiao, Y. Influence of Basic Cognition and Operation Abilities of Performance and Human Error in Manipulator Fine Docking. Manned Spacefl. 2022, 28, 54–61. [Google Scholar]
- Lui, H.L.; Lee, T.F.; Ross, F.; Yeung, S. Psychometric Evaluation of the Center for Epidemiological Studies Depression Scale in Chinese Poststroke Older Adults. J. Nurs. Scholarsh. 2006, 38, 366–369. [Google Scholar]
- Jackson-Koku, G. Beck Depression Inventory. Occup. Med. 2016, 18, 174–175. [Google Scholar] [CrossRef]
- Ibrahimi, D.; Mendiola-Santibanez, J.D.; Martinez, E.C.; Rodriguez-Resendiz, J.; Pacheco, I.T. Cortical Activity at Baseline and During Light Stimulation in Patients With Strabismus and Amblyopia. IEEE Access 2021, 9, 22430–22446. [Google Scholar] [CrossRef]
- Schuett, E.; Mackenzie, I.G.; Kaup, B.; Dudschig, C. Replacing Vertical Actions by Mouse Movements: A Web-Suited Paradigm for Investigating Vertical Spatial Associations. Psychol. Res. 2023, 87, 194–209. [Google Scholar] [CrossRef]
- Barker, R.M.; Bialystok, E. Processing Differences between Monolingual and Bilingual Young Adults on an Emotion N-Back Task. Brain Cogn. 2019, 134, 29–43. [Google Scholar] [CrossRef]
- Krause, F.C.; Linardatos, E.; Fresco, D.M.; Moore, M.T. Facial Emotion Recognition in Major Depressive Disorder: A Meta-Analytic Review. J. Affect. Disord. 2021, 293, 320–328. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, C.; Wang, C.; Zhang, Y.; Gao, S. Design and Implementation of Mental WorkLoad Prediction Software for Teleoperation of Robotic Arm. J. Beijing Univ. Civ. Eng. Archit. 2020, 36, 91–98. [Google Scholar]
- Freese, M.; Singh, S.P.N.; Ozaki, F.; Matsuhira, N. Virtual Robot Experimentation Platform V-REP: A Versatile 3D Robot Simulator. In Proceedings of the Simulation, Modeling, and Programming for Autonomous Robots–Second International Conference, SIMPAR 2010, Darmstadt, Germany, 15–18 November 2010; Springer: Berlin/Heidelberg, Germany, 2010; Volume 6472, pp. 51–62. [Google Scholar]
- Mishra, D.K.; Thomas, A.; Kuruvilla, J.; Kalyanasundaram, P.; Prasad, K.R.; Haldorai, A. Design of Mobile Robot Navigation Controller Using Neuro-Fuzzy Logic System. Comput. Electr. Eng. 2022, 101, 108044. [Google Scholar] [CrossRef]
- HyvRinen, A. The Fixed-Point Algorithm and Maximum Likelihood Estimation for Independent Component Analysis. Neural Process. Lett. 1999, 10, 1–5. [Google Scholar] [CrossRef]
- Tang, W.; Chen, S.; Xiao, Y.; Jiang, G.; Tian, Z. Study on Mental Workload in Manipulator Teleoperation Mission. Manned Spacefl. 2017, 23, 688–696. [Google Scholar]
- Miljevic, A.; Bailey, N.; Murphy, O.W.; Perera, M.P.; Fitzgerald, P. Alterations in EEG Functional Connectivity in Individuals with Depression: A Systematic Review. J. Affect. Disord. 2023, 328, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Echeverri, C.J.; Salazar-Colores, S.; Rodríguez-Reséndiz, J.; Gómez-Loenzo, R.A. A New Approach for Motor Imagery Classification Based on Sorted Blind Source Separation, Continuous Wavelet Transform, and Convolutional Neural Network. Sensors 2019, 19, 4541–4555. [Google Scholar] [CrossRef] [PubMed]
- Luz-María, S.-R.; Juvenal, R.-R.; Gloria Nélida, A.-R.; María-Luisa, G.-G. Impact of EEG Parameters Detecting Dementia Diseases: A Systematic Review. IEEE Access 2021, 9, 78060–78074. [Google Scholar]
- Wu, Q.; Dey, N.; Shi, F.; Crespo, R.G.; Sherratt, R.S. Emotion Classification on Eye-Tracking and Electroencephalograph Fused Signals Employing Deep Gradient Neural Networks. Appl. Soft Comput. 2021, 110, 107752. [Google Scholar] [CrossRef]
- Fahimi, F.; Zhang, Z.; Goh, W.B.; Lee, T.S.; Ang, K.K.; Guan, C. Inter-Subject Transfer Learning with End-to-End Deep Convolutional Neural Network for EEG-Based BCI. J. Neural Eng. 2018, 16, 26007. [Google Scholar] [CrossRef]
- Yang, K.; Tong, L.; Zeng, Y.; Lu, R.; Zhang, R.; Gao, Y.; Yan, B. Exploration of Effective Electroencephalography Features for the Recognition of Different Valence Emotions. Front. Neurosci. 2022, 16, 1010951. [Google Scholar] [CrossRef]
- Nandi, S.; Sarkar, S.K.; Chen, X. Adapting to One- and Two-Way Classified Structures of Hypotheses While Controlling the False Discovery Rate. J. Stat. Plan. Inference 2021, 215, 95–108. [Google Scholar] [CrossRef]
- Tran, T.; Milanovic, M.; Holshausen, K.; Bowie, C.R. What Is Normal Cognition in Depression? Prevalence and Functional Correlates of Normative versus Idiographic Cognitive Impairment. Neuropsychology 2021, 35, 33–41. [Google Scholar] [CrossRef] [PubMed]
- McVoy, M.; Aebi, M.E.; Loparo, K.; Lytle, S.; Morris, A.; Woods, N.; Deyling, E.; Tatsuoka, C.; Kaffashi, F.; Lhatoo, S.; et al. Resting-State Quantitative Electroencephalography Demonstrates Differential Connectivity in Adolescents with Major Depressive Disorder. J. Child Adolesc. Psychopharmacol. 2019, 29, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Velichkovsky, B.; Sultanova, F.; Tatarinov, D.V. Explicit and Implicit Processing of Facial Expressions in Depression. Exp. Psychol. 2021, 14, 24–36. [Google Scholar]
- Teng, C.; Wang, M.; Luo, Y.; Wang, Y.; Zhang, Y.; Xu, J. The Study of Frontal Functional Network in Patients With Major Depression Disorder Under Resting State. Int. J. Psychophysiol. 2021, 168, S103–S104. [Google Scholar] [CrossRef]
- Ven, P.V.D.; Hollingworth, W.; Nakamura, C.A.; Scazufca, M.; Araya, R.; Moreno-Agostino, D.; Peters, T.J.; Fajersztajn, L. Depressive and Subthreshold Depressive Symptomatology among Older Adults in a Socioeconomically Deprived Area in Brazil. Int. J. Geriatr. Psychiatry 2022, 37, 1–8. [Google Scholar]
- Save, E.; Poucet, B. Hippocampal-parietal Cortical Interactions in Spatial Cognition. Hippocampus 2015, 10, 491–499. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, F.; Zhang, Q.; Naya, Y. Distinct Networks Coupled with Parietal Cortex for Spatial Representations inside and Outside the Visual Field. NeuroImage 2022, 252, 119041. [Google Scholar] [CrossRef]
- Smith, E.E.; Cavanagh, J.F.; Allen, J.J.B. Intracranial Source Activity (eLORETA) Related to Scalp-Level Asymmetry Scores and Depression Status. Psychophysiology 2017, 55, 1–53. [Google Scholar] [CrossRef]
- Ho, P.S.; Lin, C.; Chen, G.Y.; Liu, H.L.; Huang, C.M.; Lee, M.C.; Lee, S.H.; Wu, S.C. Complexity Analysis of Resting State fMRI Signals in Depressive Patients. In Proceedings of the International Conference of the IEEE Engineering in Medicine & Biology Society, Jeju, Republic of Korea, 11–15 July 2017; IEEE: New York, NY, USA, 2017; Volume 1, pp. 3190–3193. [Google Scholar]
- Sklar, A.L.; Coffman, B.A.; Salisbury, D.F. Fronto-Parietal Network Function during Cued Visual Search in the First-Episode Schizophrenia Spectrum. J. Psychiatr. Res. 2021, 141, 339–345. [Google Scholar] [CrossRef]
- Berman, M.G.; Peltier, S.; Nee, D.E.; Kross, E.; Deldin, P.J.; Jonides, J. Depression, Rumination and the Default Network. Soc. Cogn. Affect. Neurosci. 2011, 6, 548–555. [Google Scholar] [CrossRef]
- Keizer, A.W.; Verment, R.S.; Hommel, B. Enhancing Cognitive Control through Neurofeedback: A Role of Gamma-Band Activity in Managing Episodic Retrieval. NeuroImage 2010, 49, 3404–3413. [Google Scholar] [CrossRef] [PubMed]
- Harmony, T. The Functional Significance of Delta Oscillations in Cognitive Processing. Front. Integr. Neurosci. 2013, 7, 83. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, G.G. EEG Delta Oscillations as a Correlate of Basic Homeostatic and Motivational Processes. Neurosci. Biobehav. Rev. 2012, 36, 677–695. [Google Scholar] [CrossRef] [PubMed]

| Groups | O Stage | LM Stage | FO Stage | D Stage | Total Time |
|---|---|---|---|---|---|
| HC | 10.91 (2.6) | 20.52 (3.2) | 19.31 (1.9) | 24.22 (3.8) | 74.96 (5.6) |
| DE | 11.53 (3.0) | 25.68 (2.9) | 26.47 (2.2) | 25.75 (4.3) | 89.43 (6.4) |
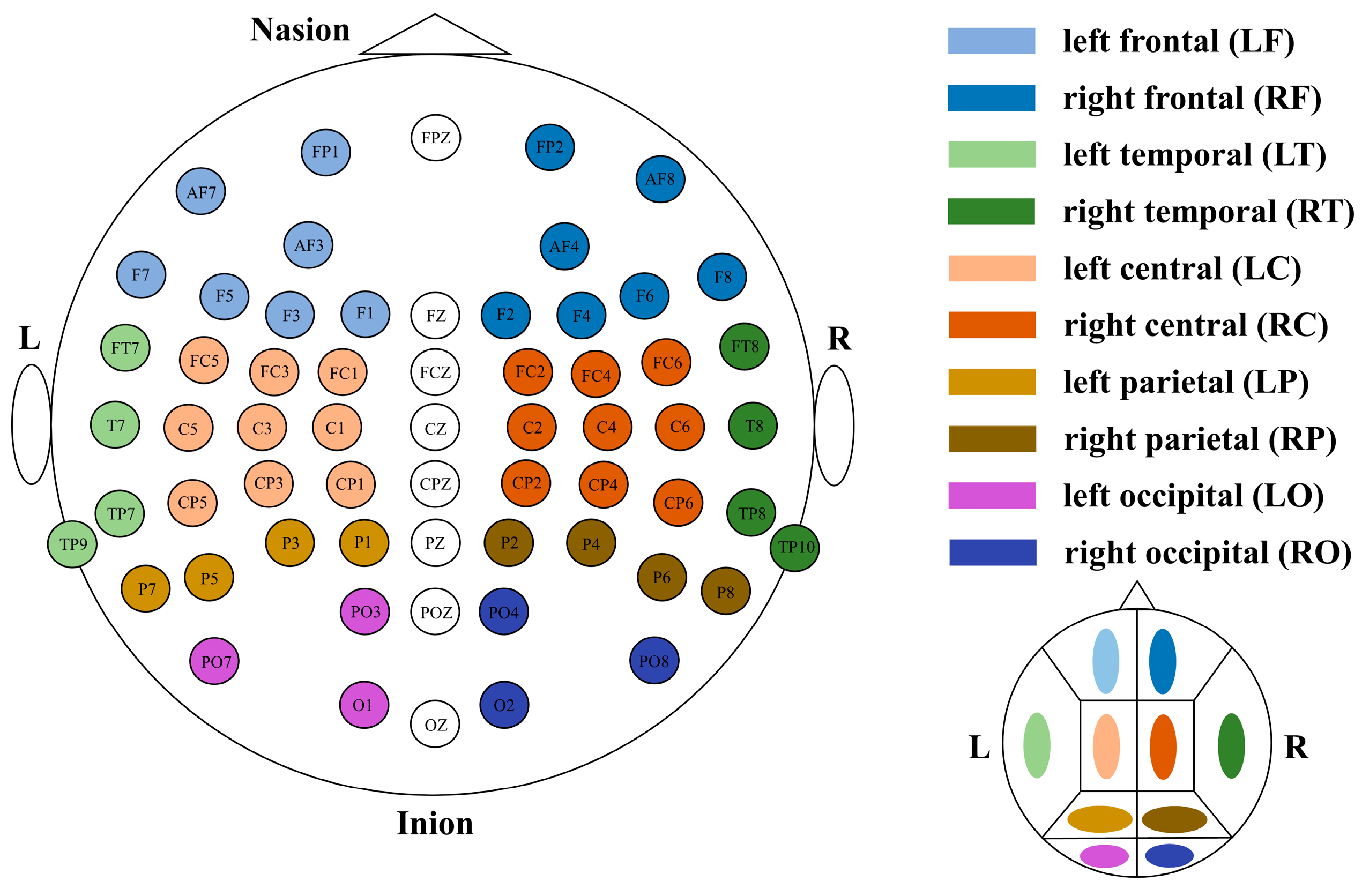
| LF | RF | LC | RC | LT | RT | LP | RP | LO | RO | |
|---|---|---|---|---|---|---|---|---|---|---|
| RS delta | 0.13 | 0.06 | 0.12 | 0.10 | 0.02 | −0.24 | 0.07 | −0.19 | 0.04 | 0.17 |
| RS theta | 0.03 | −0.07 | −0.10 | −0.20 | −0.31 | −0.15 | −0.20 | 0.22 | 0.05 | 0.21 |
| RS alpha | 0.21 | 0.20 | −0.32 | 0.14 | 0.06 | 0.16 | −0.13 | 0.20 | 0.10 | 0.12 |
| RS beta | 0.02 | −0.06 | −0.03 | −0.03 | −0.11 | −0.19 | −0.06 | 0.07 | 0.01 | 0.14 |
| RS gamma | 0.08 | 0.01 | 0.00 | −0.04 | −0.33 | −0.12 | 0.17 | −0.13 | 0.07 | −0.13 |
| LM delta | 0.41 | 0.23 | 0.22 | 0.22 | 0.16 | 0.27 | 0.40 | 0.19 | 0.41 | 0.38 |
| LM theta | 0.22 | 0.20 | 0.12 | 0.15 | 0.08 | 0.06 | 0.21 | 0.19 | 0.20 | 0.22 |
| LM alpha | 0.07 | 0.07 | 0.03 | 0.06 | 0.07 | 0.01 | 0.06 | 0.07 | 0.01 | 0.03 |
| LM beta | 0.38 | −0.11 | −0.06 | 0.05 | −0.10 | 0.04 | −0.12 | −0.23 | −0.20 | −0.11 |
| LM gamma | 0.35 | 0.11 | 0.15 | 0.14 | 0.17 | 0.27 | 0.22 | 0.33 | 0.36 | 0.33 |
| FO delta | 0.42 | 0.24 | 0.27 | 0.15 | 0.32 | 0.17 | 0.41 | 0.21 | 0.35 | 0.19 |
| FO theta | 0.23 | 0.13 | 0.04 | 0.09 | 0.07 | 0.16 | 0.19 | 0.11 | 0.14 | 0.14 |
| FO alpha | 0.14 | 0.10 | 0.04 | 0.10 | 0.11 | 0.06 | 0.18 | 0.14 | 0.19 | 0.13 |
| FO beta | 0.35 | −0.08 | −0.12 | 0.02 | −0.12 | −0.05 | −0.10 | −0.03 | −0.21 | −0.11 |
| FO gamma | 0.34 | −0.05 | 0.11 | 0.18 | 0.00 | 0.17 | 0.13 | 0.27 | 0.10 | 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, K.; Hu, Y.; Zeng, Y.; Tong, L.; Gao, Y.; Pei, C.; Li, Z.; Yan, B. EEG Network Analysis of Depressive Emotion Interference Spatial Cognition Based on a Simulated Robotic Arm Docking Task. Brain Sci. 2024, 14, 44. https://doi.org/10.3390/brainsci14010044
Yang K, Hu Y, Zeng Y, Tong L, Gao Y, Pei C, Li Z, Yan B. EEG Network Analysis of Depressive Emotion Interference Spatial Cognition Based on a Simulated Robotic Arm Docking Task. Brain Sciences. 2024; 14(1):44. https://doi.org/10.3390/brainsci14010044
Chicago/Turabian StyleYang, Kai, Yidong Hu, Ying Zeng, Li Tong, Yuanlong Gao, Changfu Pei, Zhongrui Li, and Bin Yan. 2024. "EEG Network Analysis of Depressive Emotion Interference Spatial Cognition Based on a Simulated Robotic Arm Docking Task" Brain Sciences 14, no. 1: 44. https://doi.org/10.3390/brainsci14010044
APA StyleYang, K., Hu, Y., Zeng, Y., Tong, L., Gao, Y., Pei, C., Li, Z., & Yan, B. (2024). EEG Network Analysis of Depressive Emotion Interference Spatial Cognition Based on a Simulated Robotic Arm Docking Task. Brain Sciences, 14(1), 44. https://doi.org/10.3390/brainsci14010044







