Neural Correlates of Positive Outcome Expectancy for Aggression: Evidence from Voxel-Based Morphometry and Resting-State Functional Connectivity Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Assessment of Aggressive Positive Outcome Expectancy
2.3. Imaging Data Acquisition
2.4. VBM Analysis
2.4.1. Structural MRI Data Pre-Processing
2.4.2. Structural MRI Data Statistical Analysis
2.5. RSFC Analysis
2.5.1. Resting-State MRI Data Pre-Processing
2.5.2. Resting-State Functional Connectivity Data Statistical Analysis
2.6. Prediction Analysis
3. Results
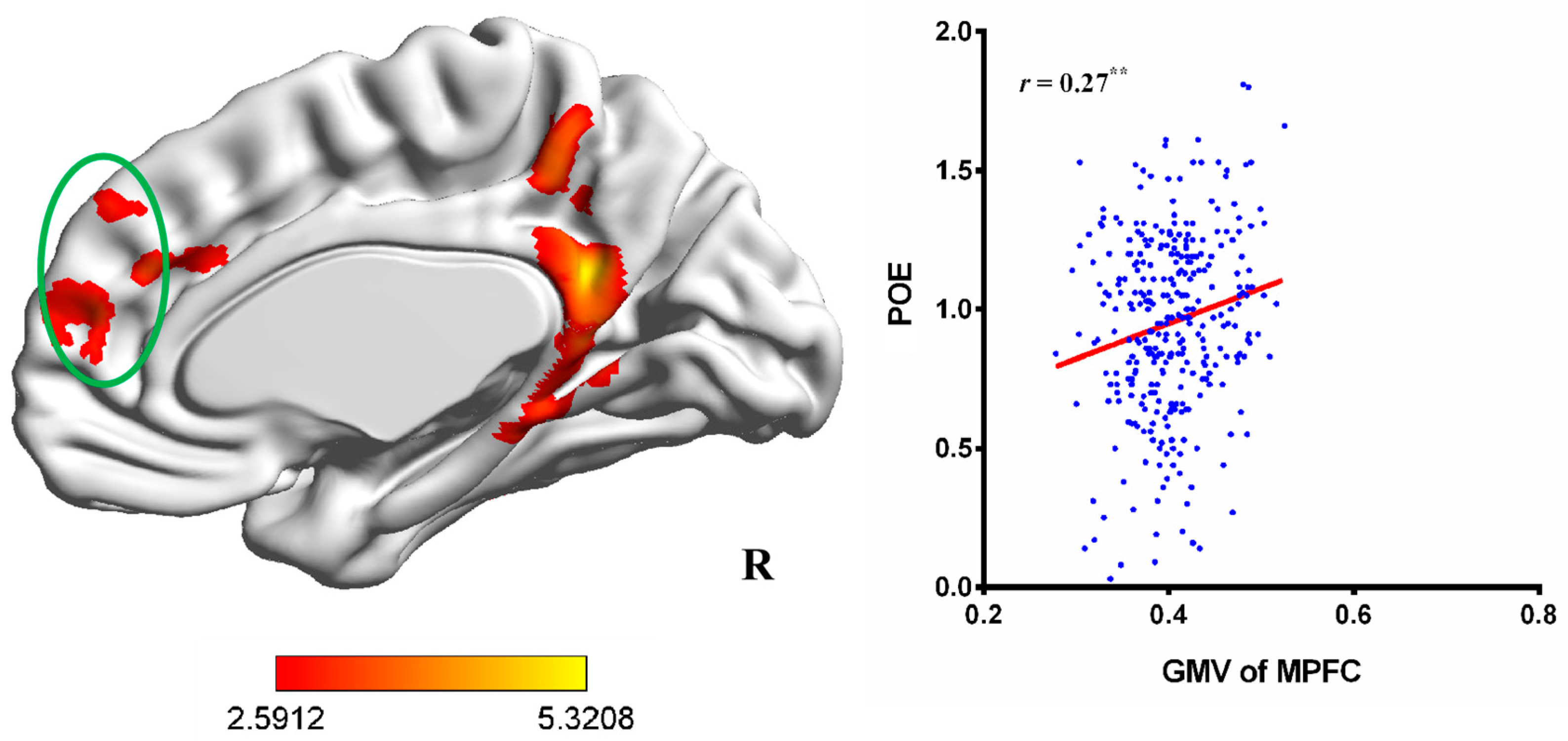
3.1. VBM Results
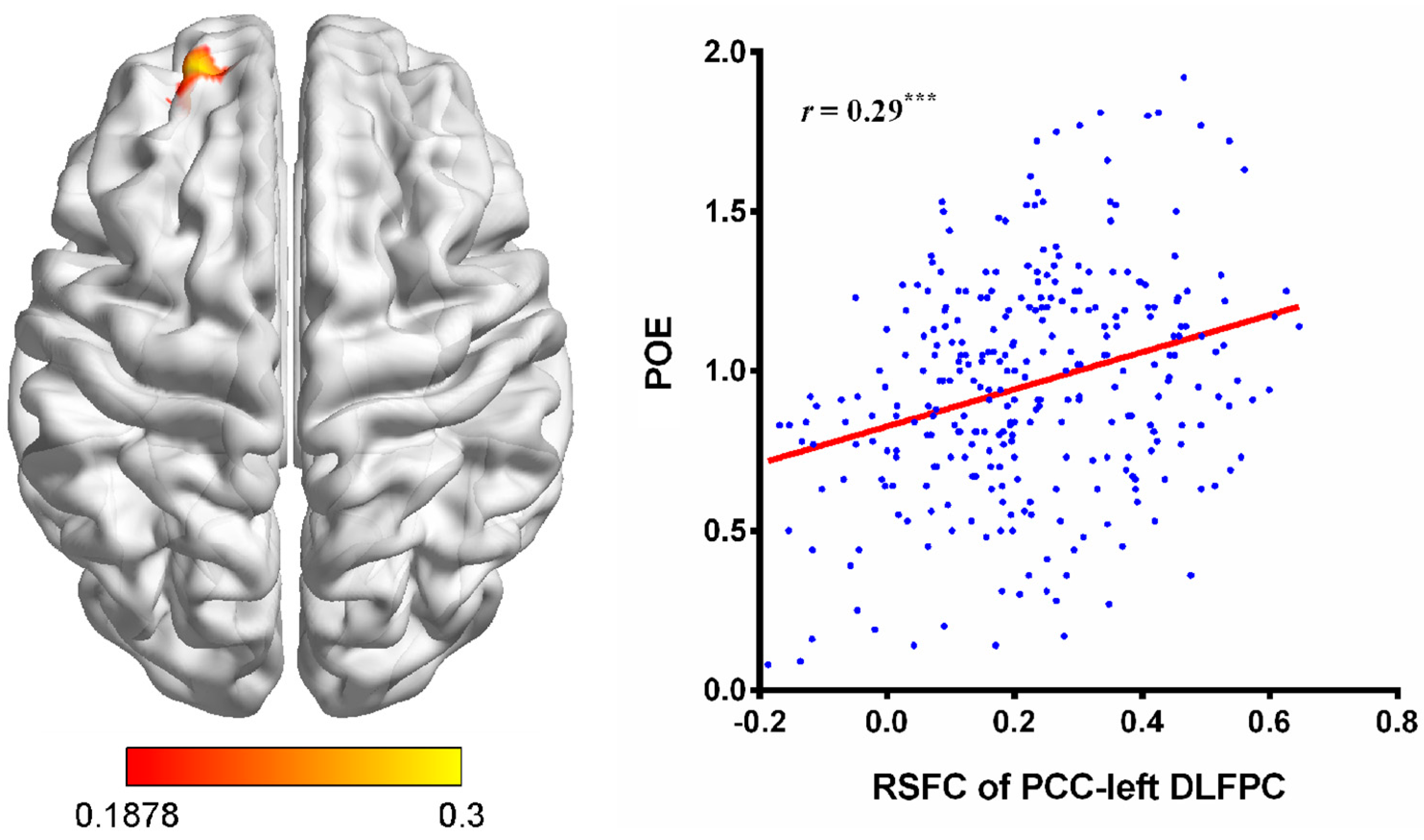
3.2. RSFC Results
4. Discussion
5. Limitations and Future Directions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Allen, J.J.; Anderson, C.A.; Bushman, B.J. The General Aggression Model. Curr. Opin. Psychol. 2018, 19, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, R.G.; Dodge, K.A. Real-time decision making and aggressive behavior in youth: A heuristic model of response evaluation and decision (RED). Aggress. Behav. 2006, 32, 604–624. [Google Scholar] [CrossRef] [PubMed]
- Crick, N.R.; Dodge, K.A. A review and reformulation of social information-processing mechanisms in children’s social adjustment. Psychol. Bull. 1994, 115, 74–101. [Google Scholar] [CrossRef]
- Allen, J.J.; Anderson, C.A. Aggression and Violence: Definitions and Distinctions. In The Wiley Handbook of Violence and Aggression; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 1–14. [Google Scholar] [CrossRef]
- Avnaim, S.; Murphy, C.M.; Miles-McLean, H.A. How Do Women in Treatment for Intimate Partner Violence Perpetration Perceive Their Abusive Behavior? Psychol. Violence 2022, 12, 324–332. [Google Scholar] [CrossRef]
- Wei, J.-M.; Xia, L.-X. Generating a Moderated Mediation Model of Positive Outcome Expectancy and Aggression. Behav. Sci. 2023, 13, 729. [Google Scholar] [CrossRef] [PubMed]
- Marks, A.D.G.; Hine, D.W.; Manton, G.C.; Thorsteinsson, E.B. Can Outcome Expectancies Help Explain Sex Differences in Direct and Indirect Aggression? J. Appl. Soc. Psychol. 2012, 42, 151–169. [Google Scholar] [CrossRef]
- Rutter, A.; Hine, D.W. Sex differences in workplace aggression: An investigation of moderation and mediation effects. Aggress. Behav. 2005, 31, 254–270. [Google Scholar] [CrossRef]
- Dodge, K.A.; Coie, J.D. Social-information-processing factors in reactive and proactive aggression in children’s peer groups. J. Pers. Soc. Psychol. 1987, 53, 1146–1158. [Google Scholar] [CrossRef]
- Wrangham, R.W. Two types of aggression in human evolution. Proc. Natl. Acad. Sci. USA 2018, 115, 245–253. [Google Scholar] [CrossRef]
- Crick, N.R.; Dodge, K.A. Social Information-Processing Mechanisms in Reactive and Proactive Aggression. Child Dev. 1996, 67, 993–1002. [Google Scholar] [CrossRef]
- Bandura, A. Aggression: A Social Learning Analysis; Prentice–Hall: Englewood Cliffs, NJ, USA, 1973. [Google Scholar]
- Tedeschi, J.T.; Felson, R.B. Violence, Aggression, and Coercive Actions; American Psychological Association: Washington, DC, USA, 1994. [Google Scholar]
- Oostermeijer, S.; Nieuwenhuijzen, M.; van de Ven, P.M.; Popma, A.; Jansen, L.M.C. Social information processing problems related to reactive and proactive aggression of adolescents in residential treatment. Pers. Individ. Differ. 2016, 90, 54–60. [Google Scholar] [CrossRef]
- Pornari, C.D.; Wood, J. Peer and cyber aggression in secondary school students: The role of moral disengagement, hostile attribution bias, and outcome expectancies. Aggress. Behav. 2010, 36, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Miles-McLean, H.A.; LaMotte, A.D.; Murphy, C.M. Positive and Negative Outcome Expectancies of Partner Abuse Among Men in Partner Violence Treatment. Psychol. Violence 2021, 11, 329–338. [Google Scholar] [CrossRef]
- Chaibi, I.; Bouchatta, O.; Bennis, M.; Ba-M’hamed, S. The Role of the Anterior Cingulate Cortex in Aggression and Impulsivity. Behav. Neurosci. 2023, 137, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Cupaioli, F.A.; Zucca, F.A.; Caporale, C.; Lesch, K.-P.; Passamonti, L.; Zecca, L. The neurobiology of human aggressive behavior: Neuroimaging, genetic, and neurochemical aspects. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110059. [Google Scholar] [CrossRef] [PubMed]
- DeYoung, C.G.; Hirsh, J.B.; Shane, M.S.; Papademetris, X.; Rajeevan, N.; Gray, J.R. Testing Predictions From Personality Neuroscience: Brain Structure and the Big Five. Psychol. Sci. 2010, 21, 820–828. [Google Scholar] [CrossRef]
- Gong, X.; Quan, F.; Wang, L.; Zhu, W.; Lin, D.; Xia, L.-X. The relationship among regional gray matter volume in the brain, Machiavellianism and social aggression in emerging adulthood: A voxel-based morphometric study. Curr. Psychol. 2022, 42, 25160–25170. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, W.; Xiao, M.; Zhang, Q.; Zhao, Y.; Zhang, H.; Chen, X.; Zheng, Y.; Xia, L.-X. Hostile Attribution Bias Mediates the Relationship Between Structural Variations in the Left Middle Frontal Gyrus and Trait Angry Rumination. Front. Psychol. 2018, 9, 526. [Google Scholar] [CrossRef]
- Fletcher, E.; Gavett, B.; Harvey, D.; Farias, S.T.; Olichney, J.; Beckett, L.; DeCarli, C.; Mungas, D. Brain volume change and cognitive trajectories in aging. Neuropsychology 2018, 32, 436–449. [Google Scholar] [CrossRef]
- Kong, F.; Zhao, J.; You, X.; Xiang, Y. Gratitude and the brain: Trait gratitude mediates the association between structural variations in the medial prefrontal cortex and life satisfaction. Emotion 2020, 20, 917–926. [Google Scholar] [CrossRef]
- Basser, P.J.; Jones, D.K. Diffusion-tensor MRI: Theory, experimental design and data analysis—A technical review. NMR Biomed. 2002, 15, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Zhu, W.; Wei, J.-M.; Lei, X.; Xia, L.-X. The relationship among resting-state brain activity and connectivity, agreeableness and displaced aggression: Two possible mediation models. J. Affect. Disord. 2019, 256, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Chen, J.; Tian, X.; Wu, X.; Matkurban, K.; Qiu, J.; Xia, L.-X. The brain correlates of hostile attribution bias and their relation to the displaced aggression. J. Affect. Disord. 2022, 317, 204–211. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R. The brain’s default network and its adaptive role in internal mentation. Neuroscientist 2012, 18, 251–270. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Saxe, R.; Yarkoni, T. Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage 2014, 91, 324–335. [Google Scholar] [CrossRef]
- Bo O’Connor, B.; Fowler, Z. How Imagination and Memory Shape the Moral Mind. Pers. Soc. Psychol. Rev. 2023, 27, 226–249. [Google Scholar] [CrossRef]
- Halvorson, M.A.; Lengua, L.J.; Smith, G.T.; King, K.M. Pathways of personality and learning risk for addictive behaviors: A systematic review of mediational research on the acquired preparedness model. J. Pers. 2023, 91, 613–637. [Google Scholar] [CrossRef]
- Schunk, D.H.; DiBenedetto, M.K. Motivation and Social Cognitive Theory. Contemp. Educ. Psychol. 2020, 60, 101832. [Google Scholar] [CrossRef]
- Fontaine, R.G.; Yang, C.; Dodge, K.A.; Bates, J.E.; Pettit, G.S. Testing an individual systems model of response evaluation and decision (RED) and antisocial behavior across adolescence. Child Dev. 2008, 79, 462–475. [Google Scholar] [CrossRef] [PubMed]
- Guerra, N.G.; Slaby, R.G. Evaluative factors in social problem solving by aggressive boys. J. Abnorm. Child Psychol. 1989, 17, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Schacter, D.L.; Benoit, R.G.; Szpunar, K.K. Episodic Future Thinking: Mechanisms and Functions. Curr. Opin. Behav. Sci. 2017, 17, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Szpunar, K.K.; Spreng, R.N.; Schacter, D.L. A taxonomy of prospection: Introducing an organizational framework for future-oriented cognition. Proc. Natl. Acad. Sci. USA 2014, 111, 18414–18421. [Google Scholar] [CrossRef] [PubMed]
- Addis, D.R. Mental Time Travel? A Neurocognitive Model of Event Simulation. Rev. Philos. Psychol. 2020, 11, 233–259. [Google Scholar] [CrossRef]
- Coccaro, E.F.; Fanning, J.; Lee, R. Development of a social emotional information processing assessment for adults (SEIP-Q). Aggress. Behav. 2017, 43, 47–59. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Grilli, M.D. Mapping the imaginative mind: Charting new paths forward. Curr. Dir. Psychol. Sci. 2021, 30, 82–89. [Google Scholar] [CrossRef]
- Blakemore, S.J.; Robbins, T.W. Decision-making in the adolescent brain. Nat. Neurosci. 2012, 15, 1184–1191. [Google Scholar] [CrossRef]
- Gilboa, A.; Marlatte, H. Neurobiology of Schemas and Schema-Mediated Memory. Trends Cogn. Sci. 2017, 21, 618–631. [Google Scholar] [CrossRef]
- Haruno, M.; Kawato, M. Heterarchical reinforcement-learning model for integration of multiple cortico-striatal loops: fMRI examination in stimulus-action-reward association learning. Neural Netw. 2006, 19, 1242–1254. [Google Scholar] [CrossRef]
- Kustubayeva, A.M.; Nelson, E.B.; Smith, M.L.; Allendorfer, J.B.; Eliassen, J.C. Functional MRI study of feedback-based reinforcement learning in depression. Front. Neurosci. 2022, 16, 1028121. [Google Scholar] [CrossRef] [PubMed]
- Blair, R.J. The neurobiology of psychopathic traits in youths. Nat. Rev. Neurosci. 2013, 14, 786–799. [Google Scholar] [CrossRef] [PubMed]
- Becker, N.; Kalpouzos, G.; Salami, A.; Laukka, E.J.; Brehmer, Y. Structure-function associations of successful associative encoding. Neuroimage 2019, 201, 116020. [Google Scholar] [CrossRef] [PubMed]
- Hanseeuw, B.; Dricot, L.; Kavec, M.; Grandin, C.; Seron, X.; Ivanoiu, A. Associative encoding deficits in amnestic mild cognitive impairment: A volumetric and functional MRI study. Neuroimage 2011, 56, 1743–1748. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, V.E.; Gilboa, A. What is a memory schema? A historical perspective on current neuroscience literature. Neuropsychologia 2014, 53, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.X.; Grady, C.; Moscovitch, M. Effects of Prior-Knowledge on Brain Activation and Connectivity During Associative Memory Encoding. Cereb. Cortex 2017, 27, 1991–2009. [Google Scholar] [CrossRef]
- Liu, X.; Hairston, J.; Schrier, M.; Fan, J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neurosci. Biobehav. Rev. 2011, 35, 1219–1236. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Huang, J.; Wang, Y.; Niu, Y.; Lui, S.S.Y.; Hui, L.; Chan, R.C.K. Revisiting reward impairments in schizophrenia spectrum disorders: A systematic review and meta-analysis for neuroimaging findings. Psychol. Med. 2023, 53, 7189–7202. [Google Scholar] [CrossRef]
- Gerlach, K.D.; Spreng, R.N.; Madore, K.P.; Schacter, D.L. Future planning: Default network activity couples with frontoparietal control network and reward-processing regions during process and outcome simulations. Soc. Cogn. Affect. Neurosci. 2014, 9, 1942–1951. [Google Scholar] [CrossRef]
- Harmon-Jones, E.; Gable, P.A. On the role of asymmetric frontal cortical activity in approach and withdrawal motivation: An updated review of the evidence. Psychophysiology 2018, 55, e12879. [Google Scholar] [CrossRef]
- Ohmann, H.A.; Kuper, N.; Wacker, J. Left frontal anodal tDCS increases approach motivation depending on reward attributes. Neuropsychologia 2018, 119, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, X.; Xia, L.-X. Brain structures and functional connectivity associated with individual differences in trait proactive aggression. Sci. Rep. 2019, 9, 7731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; He, L.; Xia, L.-X. The brain correlates of state proactive aggression. Neuropsychology 2022, 36, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Chester, D.S. The Role of Positive Affect in Aggression. Curr. Dir. Psychol. Sci. 2017, 26, 366–370. [Google Scholar] [CrossRef]
- Di Domenico, S.I.; Ryan, R.M. The Emerging Neuroscience of Intrinsic Motivation: A New Frontier in Self-Determination Research. Front. Hum. Neurosci. 2017, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Gu, R.; Huang, W.; Camilleri, J.; Xu, P.; Wei, P.; Eickhoff, S.B.; Feng, C. Love is analogous to money in human brain: Coordinate-based and functional connectivity meta-analyses of social and monetary reward anticipation. Neurosci. Biobehav. Rev. 2019, 100, 108–128. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.P.; Colizzi, M.; Bossong, M.G.; Allen, P.; Kempton, M.; Bhattacharyya, S. The Neural Substrate of Reward Anticipation in Health: A Meta-Analysis of fMRI Findings in the Monetary Incentive Delay Task. Neuropsychol. Rev. 2018, 28, 496–506. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef]
- Huo, H.; Seger, C.A.; Zhou, D.; Chen, Z.; Xu, T.; Zhang, R.; Feng, T.; Chen, Q. The assessment dimension of regulatory mode mediates the relation between frontoparietal connectivity and risk-taking: Evidence from voxel-base morphometry and functional connectivity analysis. Brain Cogn. 2020, 140, 105533. [Google Scholar] [CrossRef]
- Ashburner, J. A fast diffeomorphic image registration algorithm. Neuroimage 2007, 38, 95–113. [Google Scholar] [CrossRef]
- Yan, C.-G.; Zang, Y.-F. DPARSF: A MATLAB Toolbox for “Pipeline” Data Analysis of Resting-State fMRI. Front. Syst. Neurosci. 2010, 4, 13. [Google Scholar] [CrossRef] [PubMed]
- Power, J.D.; Barnes, K.A.; Snyder, A.Z.; Schlaggar, B.L.; Petersen, S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 2012, 59, 2142–2154. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, B.; Yan, C.G. Reproducibility of R-fMRI metrics on the impact of different strategies for multiple comparison correction and sample sizes. Hum. Brain Mapp. 2018, 39, 300–318. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, M.D.; Straccia, M.A.; Meyer, M.L.; Du, M.; Tan, K.M. Social, self, (situational), and affective processes in medial prefrontal cortex (MPFC): Causal, multivariate, and reverse inference evidence. Neurosci. Biobehav. Rev. 2019, 99, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M.; Tholen, M.G.; Perner, J.; Mars, R.B.; Sallet, J. Specifying the brain anatomy underlying temporo-parietal junction activations for theory of mind: A review using probabilistic atlases from different imaging modalities. Hum. Brain Mapp. 2017, 38, 4788–4805. [Google Scholar] [CrossRef] [PubMed]
- Gross, J.; Vostroknutov, A. Why do people follow social norms? Curr. Opin. Psychol. 2022, 44, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kelley, N.J.; Hortensius, R.; Schutter, D.J.L.G.; Harmon-Jones, E. The relationship of approach/avoidance motivation and asymmetric frontal cortical activity: A review of studies manipulating frontal asymmetry. Int. J. Psychophysiol. 2017, 119, 19–30. [Google Scholar] [CrossRef]
- Lotze, M.; Veit, R.; Anders, S.; Birbaumer, N. Evidence for a different role of the ventral and dorsal medial prefrontal cortex for social reactive aggression: An interactive fMRI study. Neuroimage 2007, 34, 470–478. [Google Scholar] [CrossRef]
- Nikolic, M.; Pezzoli, P.; Jaworska, N.; Seto, M.C. Brain responses in aggression-prone individuals: A systematic review and meta-analysis of functional magnetic resonance imaging (fMRI) studies of anger- and aggression-eliciting tasks. Prog. Neuropsychopharmacol. Biol. Psychiatry 2022, 119, 110596. [Google Scholar] [CrossRef]
- Simonetti, A.; Kurian, S.; Saxena, J.; Verrico, C.D.; Restaino, A.; Di Nicola, M.; Soares, J.C.; Sani, G.; Saxena, K. Cortical Correlates of Impulsive Aggressive Behavior in Pediatric Bipolar Disorder. Front. Psychiatry 2021, 12, 674707. [Google Scholar] [CrossRef] [PubMed]

| Regions | Brodmann Areas | Peak MNI Coordinates | Peak t-Value | Cluster Size | ||
|---|---|---|---|---|---|---|
| x | y | z | ||||
| Only+ | ||||||
| PCC | BA 23, 31 | 9 | −48 | 23 | 5.10 | 1889 |
| Right TPJ | BA 40 | 63 | −47 | 21 | 5.32 | 2223 |
| MPFC a | BA 9, 10 b | 0 | 41 | 39 | 3.821 | 2134 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.-M.; Xia, L.-X. Neural Correlates of Positive Outcome Expectancy for Aggression: Evidence from Voxel-Based Morphometry and Resting-State Functional Connectivity Analysis. Brain Sci. 2024, 14, 43. https://doi.org/10.3390/brainsci14010043
Wei J-M, Xia L-X. Neural Correlates of Positive Outcome Expectancy for Aggression: Evidence from Voxel-Based Morphometry and Resting-State Functional Connectivity Analysis. Brain Sciences. 2024; 14(1):43. https://doi.org/10.3390/brainsci14010043
Chicago/Turabian StyleWei, Jia-Ming, and Ling-Xiang Xia. 2024. "Neural Correlates of Positive Outcome Expectancy for Aggression: Evidence from Voxel-Based Morphometry and Resting-State Functional Connectivity Analysis" Brain Sciences 14, no. 1: 43. https://doi.org/10.3390/brainsci14010043
APA StyleWei, J.-M., & Xia, L.-X. (2024). Neural Correlates of Positive Outcome Expectancy for Aggression: Evidence from Voxel-Based Morphometry and Resting-State Functional Connectivity Analysis. Brain Sciences, 14(1), 43. https://doi.org/10.3390/brainsci14010043






