Functional Dizziness as a Spatial Cognitive Dysfunction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spatial Navigation Test
Virtual Morris Water Maze (vMWM)
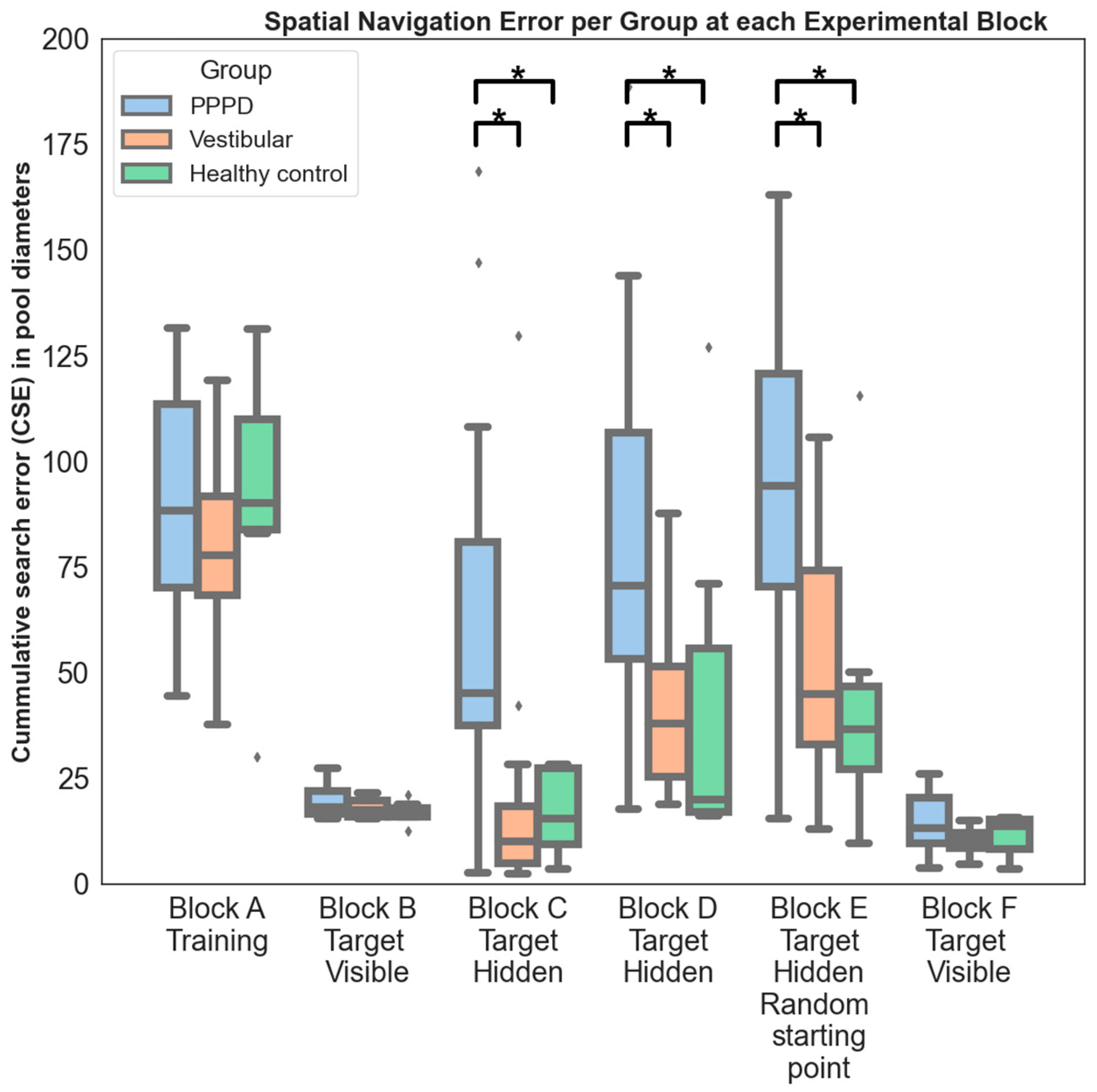
- Block A
- consisted of two training trials: (i) free navigation was allowed for familiarization with joystick movements; (ii) participants were instructed to navigate towards the visual cues on the room’s walls as a practice exercise.
- Block B
- encompassed four consecutive trials. A visible red square platform, measuring 0.17 × 0.17 virtual units was consistently located in the pool’s north-eastern quadrant. Participants initiated each trial at the southern end of the pool, facing north. Successful arrival at the platform was indicated by a rewarding sound, signaling trial completion. This block was designed to familiarize participants with the virtual environment and the test protocol. The maximal trial duration was set to 1 min for this and all subsequent blocks, irrespective of whether the target was reached or not. Blocks A and B serve as initial tests to identify any motor or group-specific issues related to manipulating the joystick and navigating within the virtual environment. We have chosen to include data from these blocks to emphasize that differences observed in subsequent blocks are indeed attributable to variations in navigational skills and not influenced by other potential confounding factors.
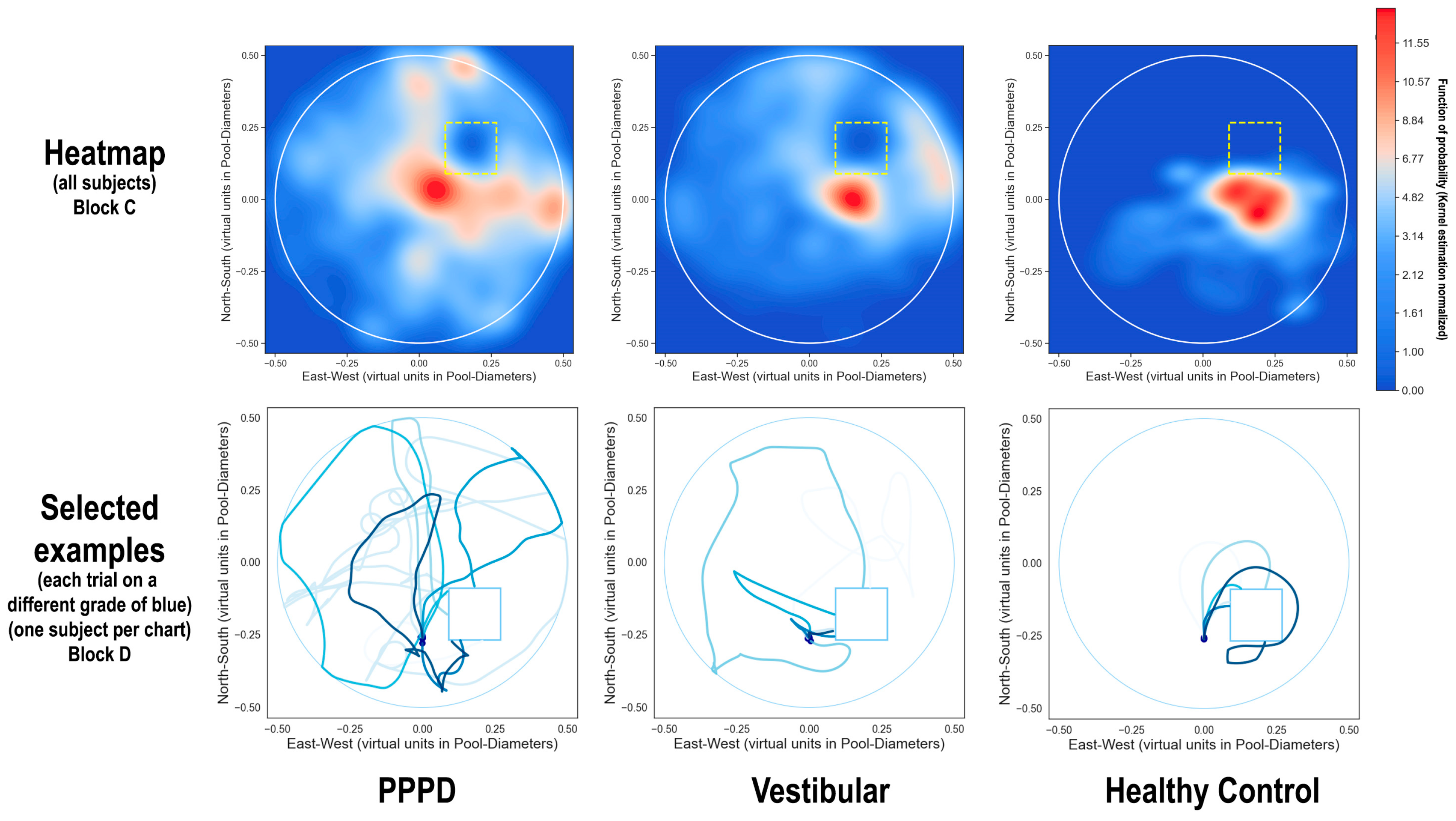
- Block C
- included seven consecutive trials. Starting from the same southern location, participants were tasked with locating a hidden platform situated in the south-western quadrant. This platform became visible only upon direct contact and emitted the same rewarding sound. Participants were instructed to remember and optimize their route to the platform across the trials. The visual cues provided were consistent: old-style airplanes (north), a sea turtle (west), “La Gioconda” by Leonardo da Vinci (south), and flowers (east). This phase represented the initial setting that necessitated the application of either egocentric or allocentric spatial navigation strategies. Participants were required to memorize the location of the hidden platform and utilize these strategies to efficiently locate it in subsequent trials.
- Block D
- was a mirrored version of Block C, comprising another set of seven trials. Here, the hidden platform was relocated to the north-western quadrant. The visual cues were changed to simple colored geometric symbols: a black cross (north), a red square (west), a pink heart (south), and a blue triangle (east). Block D closely resembles the preceding block, with the distinction of employing visual cues of markedly lower complexity, devoid of any emotional connotation. These cues consisted of simple geometric symbols as opposed to the more evocative animals or artworks used previously. The aim of this design choice was to evaluate the potential emotional influence exerted by the visual cues on the navigation process.
- Block E
- involved a sequence of seven trials similar to Blocks C and D. The hidden platform was positioned on the pool’s western side. New visual cues featured a flying condor bird (north), a sunflower field (west), Van Gogh’s “La Méridienne” (south), and an old train (east). This block introduced an increased spatial navigation challenge by incorporating random starting positions and initial facing orientations. Typically, such a “random start” setting is believed to maximize the reliance on allocentric navigational mechanisms [52].
- Block F
- consisted of four trials, similar in complexity to Block B, with a distinctly visible platform positioned in the south-east quadrant. It employed random starting points, as in Block E, to serve as a control for the assessment of joystick manipulation and movement within the virtual environment.
2.2. Global Cognition
Montreal Cognitive Assessment (MoCA)
2.3. Memory and Attention
2.3.1. Digit Span Task (DST)
2.3.2. Corsi Block-Tapping Task (CBTT)
2.4. Visuospatial and Executive Functions
2.4.1. Trail Making Test A and B (TMT-A and TMT-B)
2.4.2. Tower of London Test (ToL)
2.5. Anxiety-Depression Assessments
2.5.1. Beck Depression Inventory (BDI)
2.5.2. State-Trait Anxiety Inventory (STAI)
2.6. Vestibular- and Dizziness-Specific Assessments
2.6.1. Dizziness Handicap Inventory (DHI)
2.6.2. Analogue Visual Scale for Dizziness (AVSD)
2.6.3. Niigata Questionnaire for PPPD (NQ-PPPD)
2.7. Vestibular Function
2.7.1. Video Head Impulse Testing (vHIT)
2.7.2. Vestibular Evoked Myogenic Potentials (VEMPs)
2.8. Imaging
Magnetic Resonance Imaging
3. Results
3.1. Vestibular Function
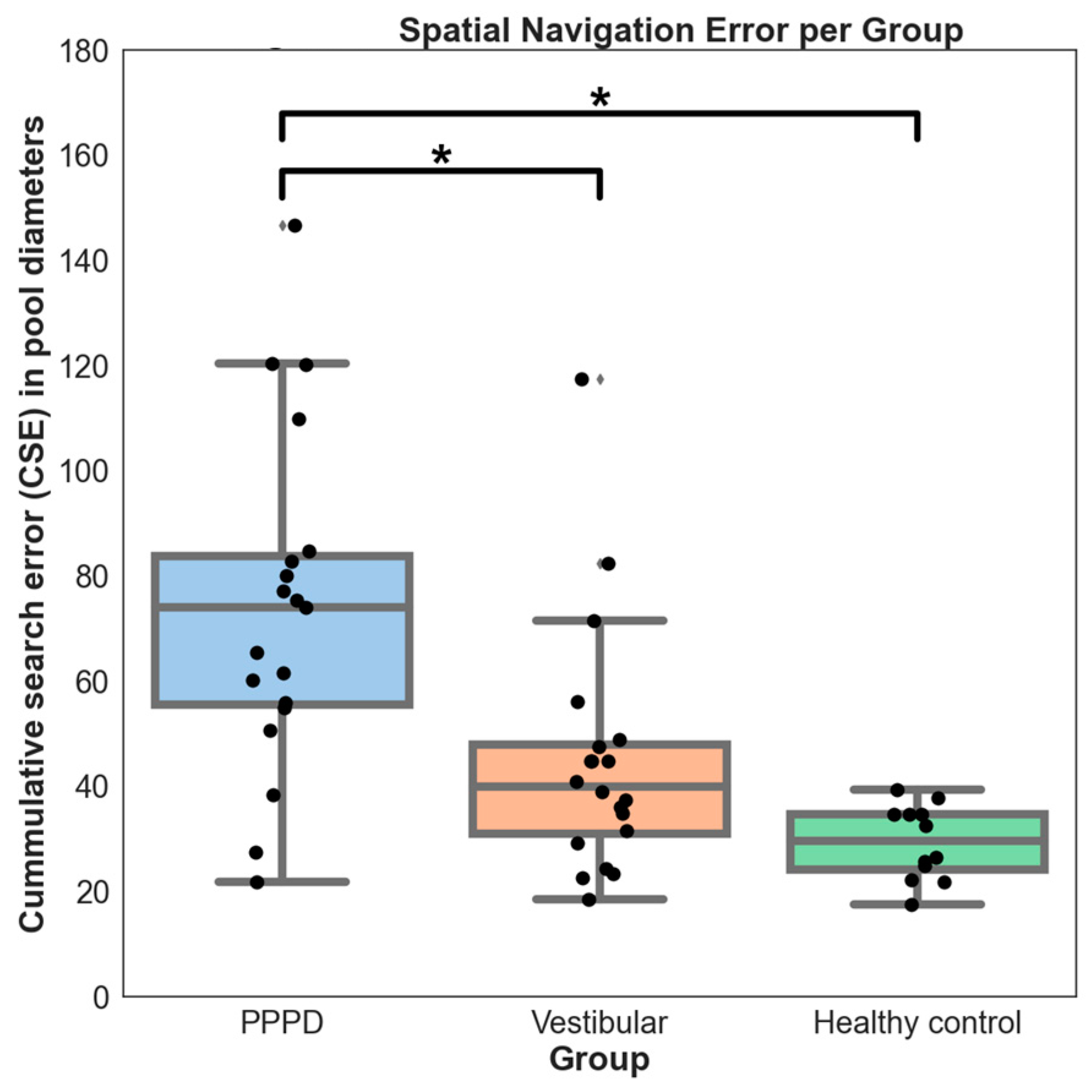
3.2. Spatial Navigation
3.3. Non-Spatial Navigation Cognitive Tests
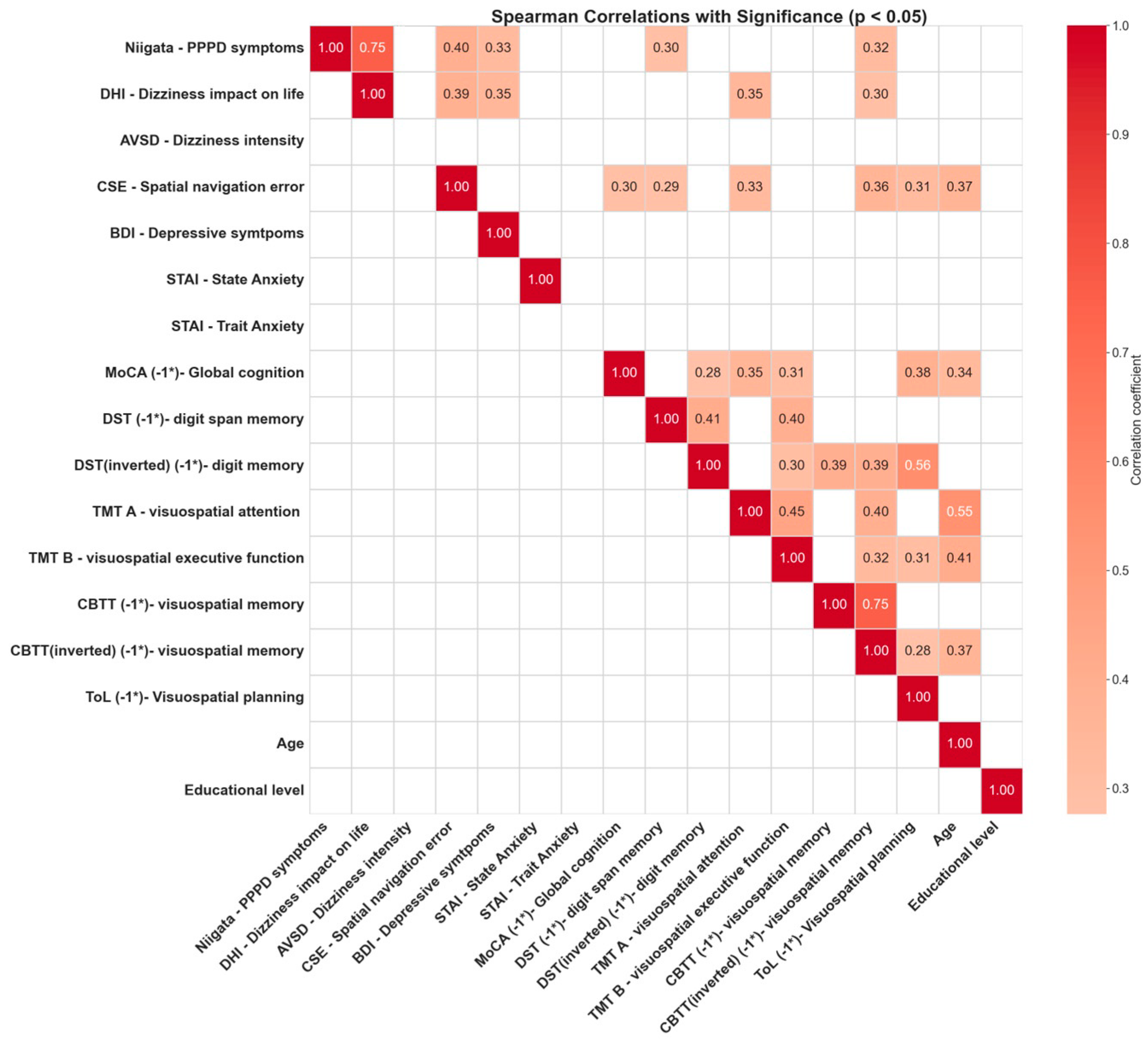
3.4. Correlations between Cognitive Tests
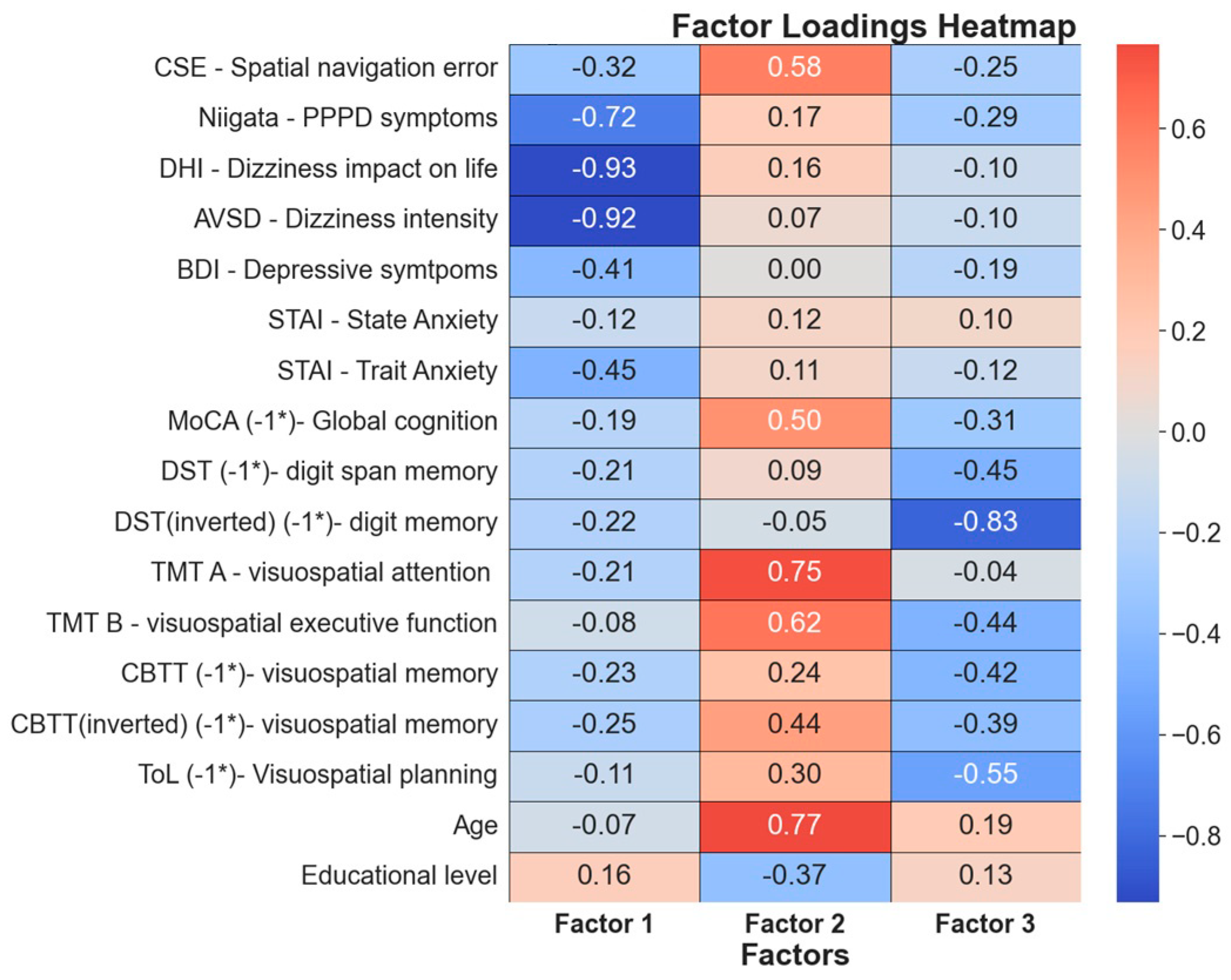
3.5. Factor Analysis
3.5.1. Factor 1: PPPD Severity and Its Impact on Daily Life
3.5.2. Factor 2: Age-Related Cognitive Changes
3.5.3. Factor 3: Advanced Cognitive Functions
4. Discussion
4.1. Impact of Non-PPPD Vestibular Disorders on Visuospatial Memory and Navigation Performance
4.2. Impact of PPPD on Higher-Demand Cognitive Tests
4.3. “Core” PPPD Phenomena
4.4. Spatial Navigation Is Distinctively Impaired in PPPD
4.5. Age and Educational Level’s Impact on Spatial Navigation and Cognitive Performance
4.6. Advance Cognitive Functions
4.7. Interplay between PPPD Symptomatology and Cognitive Navigational Dysfunction
4.8. PPPD Shows Disruption in Higher-Order Cognitive Functions, Particularly Spatial Navigation: Possible Impact on Cognitive Maps
- Challenges in visuospatial planning and execution in demanding tasks like the TMT-B and ToL, while simpler cognitive functions like memory (assessed by DST and CBTT) remain intact.
- Issues with creating and using allocentric spatial maps, as seen in the vMWM paradigm.
- A link between spatial navigation impairment severity and PPPD, suggesting a correlation with higher cognitive functions, including spatial navigation, which could be more pronounced in a subset of patients, according to our factor analysis.
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Group | Statistical Testing between Groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Ear | Canal | PPPD | Vestibular (Non-PPPD) | Healthy Control | ANOVA (F; p-Value) | Specific Groups Presenting Significant Difference after Post Hoc Tukey (p-Value < 0.05) | Post Hoc Tukey (p-Value) | |
| Video Head Impulse Test (vHIT) Gain: mean (standard deviation) (Gain <0.7: %) Percentage of patients exhibiting gain lower than 0.7 (Sacc: %) Percentage of patients exhibiting corrective saccades | Right | Anterior | 0.88 (0.1) Gain <0.7: 6.3% Sacc: 0% | 0.9 (0.10) Gain <0.7: 8.5% Sacc: 0% | 0.91 (0.07) Gain <0.7: 0% Sacc: 0% | F = 0.16; p = 0.85 | ||
| Lateral | 0.92 (0.08) Gain <0.7: 5.2% Sacc: 21% | 0.94 (0.07) Gain <0.7: 0% Sacc: 20% | 0.96 (0.02) Gain <0.7: 0% Sacc: 0% | F = 0.19; p = 0.82 | ||||
| Posterior | 0.85 (0.07) Gain <0.7: 20% Sacc: 11% | 0.88 (0.08) Gain <0.7: 26.2% Sacc: 25% | 0.92 (0.03) Gain <0.7: 0% Sacc: 0% | F = 4.38; p = 0.038 | PPPD < Healthy Control Vestibular < Healthy Control | p = 0.042 p = 0.048 | ||
| Left | Anterior | 0.87 (0.08) Gain <0.7: 21% Sacc: 11% | 0.86 (0.07) Gain <0.7: 10% Sacc: 0% | 0.9 (0.08) Gain <0.7: 8.3% Sacc: 0% | F = 0.86; p = 0.42 | |||
| Lateral | 0.86 (0.08) Gain <0.7: 23.3% Sacc: 37% | 0.85 (0.11) Gain <0.7: 20% Sacc: 25% | 0.91(0.03) Gain <0.7: 0% Sacc: 0% | F = 3,92; p = 0.031 | PPPD < Healthy Control Vestibular < Healthy Control | p = 0.041 p = 0.044 | ||
| Posterior | 0.92 (0.09) Gain <0.7: 10.5% Sacc: 11% | 0.90 (0.13) Gain <0.7: 15% Sacc: 10% | 0.93 (0.05) Gain <0.7: 0% Sacc: 0% | F = 0.03; p = 0.96 | ||||
| Ear with higher gain | Anterior | 0.9 (0.09) Gain <0.7: 8% Sacc: 0% | 0.92 (0.07) Gain <0.7: 5% Sacc: 0% | 0.94 (0.03) Gain <0.7: 0% Sacc: 0% | F = 0.36; p = 0.69 | |||
| Lateral | 0.93 (0.08) Gain <0.7: 5% Sacc: 21% | 0.94 (0.07) Gain <0.7: 8.5% Sacc: 25% | 0.93 (0.02) Gain <0.7: 0% Sacc: 0% | F = 0.16; p = 0.84 | ||||
| Posterior | 0.94 (0.08) Gain <0.7: 5.2% Sacc: 10% | 0.97 (0.09) Gain <0.7: 0% Sacc: 0.5% | 0.94 (0.04) Gain <0.7: 0% Sacc: 0% | F = 0.62; p = 0.54 | ||||
| Ear with lower gain | Anterior | 0.84 (0.08) Gain <0.7: 31.5% Sacc: 11% | 0.85 (0.07) Gain <0.7: 25% Sacc: 0% | 0.86 (0.06) Gain <0.7: 16.6% Sacc: 0% | F = 0.18; p = 0.83 | |||
| Lateral | 0.85 (0.08) Gain <0.7: 26.3% Sacc: 36% | 0.86 (0.11) Gain <0.7: 20% Sacc: 27% | 0.9 (0.03) Gain <0.7: 0% Sacc: 0% | F = 3.97; p= 0.029 | PPPD < Healthy Control Vestibular < Healthy Control | p = 0.037 p = 0.041 | ||
| Posterior | 0.84 (0.07) Gain <0.7: 26.3% Sacc: 10% | 0.84 (0.09) Gain <0.7: 35% Sacc: 30% | 0.9 (0.03) Gain <0.7: 0% Sacc: 0% | F= 3.21; p = 0.043 | PPPD < Healthy Control Vestibular < Healthy Control | p = 0.048 p = 0.049 | ||
| Vestibular Evoked Myogenic Potentials (VEMPs) Amplitude (μV): mean (standard deviation) | oVEMP | Right | 25.4 (11.3) | 28.1 (20.1) | 26.3 (16.3) | F = 1.24; p = 0.24 | ||
| Left | 16 (10.1) | 20.8 (19.8) | 24 (16) | F = 1.16; p = 0.32 | ||||
| Best Ear | 29.3 (10.9) | 33.4 (19.5) | 27.8 (16.83) | F = 2.43; p = 0.098 | ||||
| Worse Ear | 12.1 (6.1) | 15.4 (11.5) | 26.3 (11.1) | F= 3.77; p= 0.030 | PPPD < Healthy Control Vestibular < Healthy Control | p = 0.023 p = 0.037 | ||
| cVEMP | Right | 159.5 (154.2) | 123.9 (122.3) | 233.1 (133.2) | F = 2.36; p = 0.10 | |||
| Left | 133.5 (115.7) | 124 (97.6) | 233.9 (154.2) | F = 3.60; p = 0.034 | Vestibular < Healthy Control | p = 0.039 | ||
| Best Ear | 168.6 (150.5) | 159.6 (122.1) | 250.7 (117.5) | F = 1.73; p = 0.18 | ||||
| Worse Ear | 124.4 (117.7) | 88.3 (72.9) | 216.3 (97.3) | F = 5.36; p = 0.007 | Vestibular < Healthy Control | p = 0.0058 | ||
References
- Ishizuka, K.; Shikino, K.; Yamauchi, Y.; Yanagita, Y.; Yokokawa, D.; Ikegami, A.; Tsukamoto, T.; Noda, K.; Uehara, T.; Ikusaka, M. The Clinical Key Features of Persistent Postural Perceptual Dizziness in the General Medicine Outpatient Setting: A Case Series Study of 33 Patients. Intern. Med. 2020, 59, 2857. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Lee, J.O.; Choi, J.Y.; Kim, J.S. Etiologic Distribution of Dizziness and Vertigo in a Referral-Based Dizziness Clinic in South Korea. J. Neurol. 2020, 267, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Staibano, P.; Lelli, D.; Tse, D. A Retrospective Analysis of Two Tertiary Care Dizziness Clinics: A Multidisciplinary Chronic Dizziness Clinic and an Acute Dizziness Clinic. J. Otolaryngol. Head Neck Surg. 2019, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.P.; Eckhardt-Henn, A.; Horii, A.; Jacob, R.; Strupp, M.; Brandt, T.; Bronstein, A. Diagnostic Criteria for Persistent Postural-Perceptual Dizziness (PPPD): Consensus Document of the Committee for the Classification of Vestibular Disorders of the Bárány Society. J. Vestib. Res. 2017, 27, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.P. Persistent Postural-Perceptual Dizziness. Neurol. Clin. 2023, 41, 647–664. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, M.; Staab, J.P. Functional Dizziness. Curr. Opin. Neurol. 2017, 30, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.P. Persistent Postural-Perceptual Dizziness; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Dieterich, M.; Staab, J.P.; Brandt, T. Functional (Psychogenic) Dizziness. Handb. Clin. Neurol. 2016, 139, 447–468. [Google Scholar] [CrossRef] [PubMed]
- Cousins, S.; Kaski, D.; Cutfield, N.; Arshad, Q.; Ahmad, H.; Gresty, M.A.; Seemungal, B.M.; Golding, J.; Bronstein, A.M. Predictors of Clinical Recovery from Vestibular Neuritis: A Prospective Study. Ann. Clin. Transl. Neurol. 2017, 4, 340–346. [Google Scholar] [CrossRef]
- Trinidade, A.; Goebel, J.A. Persistent Postural-Perceptual Dizziness—A Systematic Review of the Literature for the Balance Specialist. Otol. Neurotol. 2018, 39, 1291–1303. [Google Scholar] [CrossRef]
- Trinidade, A.; Harman, P.; Stone, J.; Staab, J.P.; Goebel, J.A. Assessment of Potential Risk Factors for the Development of Persistent Postural-Perceptual Dizziness: A Case-Control Pilot Study. Front. Neurol. 2021, 11, 601883. [Google Scholar] [CrossRef]
- Breinbauer, H.A.; Contreras, M.D.; Lira, J.P.; Guevara, C.; Castillo, L.; Ruëdlinger, K.; Muñoz, D.; Delano, P.H. Spatial Navigation Is Distinctively Impaired in Persistent Postural Perceptual Dizziness. Front. Neurol. 2020, 10, 1361. [Google Scholar] [CrossRef] [PubMed]
- Yagi, C.; Morita, Y.; Kitazawa, M.; Yamagishi, T.; Ohshima, S.; Izumi, S.; Takahashi, K.; Horii, A. Subtypes of Persistent Postural-Perceptual Dizziness. Front. Neurol. 2021, 12, 652366. [Google Scholar] [CrossRef] [PubMed]
- Im, J.J.; Na, S.; Jeong, H.; Chung, Y.A. A Review of Neuroimaging Studies in Persistent Postural-Perceptual Dizziness (PPPD). Nucl. Med. Mol. Imaging 2021, 55, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, X.; Ju, Y.; Zhao, X. Neuroimaging Studies in Persistent Postural–Perceptual Dizziness and Related Disease: A Systematic Review. J. Neurol. 2022, 269, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Indovina, I.; Passamonti, L.; Mucci, V.; Chiarella, G.; Lacquaniti, F.; Staab, J.P. Brain Correlates of Persistent Postural-Perceptual Dizziness: A Review of Neuroimaging Studies. J. Clin. Med. 2021, 10, 4274. [Google Scholar] [CrossRef] [PubMed]
- Castro, P.; Bancroft, M.J.; Arshad, Q.; Kaski, D. Persistent Postural-Perceptual Dizziness (PPPD) from Brain Imaging to Behaviour and Perception. Brain Sci. 2022, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Yagi, C.; Morita, Y.; Yamagishi, T.; Ohshima, S.; Izumi, S.; Takahashi, K.; Itoh, K.; Suzuki, Y.; Igarashi, H.; Horii, A. Gaze Instability after Exposure to Moving Visual Stimuli in Patients with Persistent Postural-Perceptual Dizziness. Front. Hum. Neurosci. 2022, 16, 1056556. [Google Scholar] [CrossRef]
- Li, K.; Si, L.; Cui, B.; Ling, X.; Shen, B.; Yang, X. Altered Intra- and Inter-Network Functional Connectivity in Patients with Persistent Postural-Perceptual Dizziness. Neuroimage Clin. 2020, 26, 102216. [Google Scholar] [CrossRef]
- Riccelli, R.; Passamonti, L.; Toschi, N.; Nigro, S.; Chiarella, G.; Petrolo, C.; Lacquaniti, F.; Staab, J.P.; Indovina, I. Altered Insular and Occipital Responses to Simulated Vertical Self-Motion in Patients with Persistent Postural-Perceptual Dizziness. Front. Neurol. 2017, 8, 529. [Google Scholar] [CrossRef]
- Chen, T.-A.J.; Dion Parenteau, M.-L.; Marchand, K.; Zhang, H.Z.; Dannenbaum, E.; Lamontagne, A.; Fung, J. A Pilot Study to Assess Visual Vertigo in People with Persistent Postural–Perceptual Dizziness with a New Computer-Based Tool. J. Clin. Med. 2023, 12, 1766. [Google Scholar] [CrossRef]
- Nigro, S.; Indovina, I.; Riccelli, R.; Chiarella, G.; Petrolo, C.; Lacquaniti, F.; Staab, J.P.; Passamonti, L. Reduced Cortical Folding in Multi-Modal Vestibular Regions in Persistent Postural Perceptual Dizziness. Brain Imaging Behav. 2018, 13, 798–809. [Google Scholar] [CrossRef] [PubMed]
- Powell, G.; Penacchio, O.; Derry-Sumner, H.; Rushton, S.K.; Rajenderkumar, D.; Sumner, P. Visual Stress Responses to Static Images Are Associated with Symptoms of Persistent Postural Perceptual Dizziness (PPPD). J. Vestib. Res. 2022, 32, 69–78. [Google Scholar] [CrossRef] [PubMed]
- McCaslin, D.L.; Shepard, N.T.; Hollman, J.H.; Staab, J.P. Characterization of Postural Sway in Patients With Persistent Postural-Perceptual Dizziness (PPPD) Using Wearable Motion Sensors. Otol. Neurotol. 2022, 43, E243–E251. [Google Scholar] [CrossRef] [PubMed]
- Yagi, C.; Morita, Y.; Kitazawa, M.; Nonomura, Y.; Yamagishi, T.; Ohshima, S.; Izumi, S.; Takahashi, K.; Wada, Y.; Kitahara, T.; et al. Head Roll-Tilt Subjective Visual Vertical Test in the Diagnosis of Persistent Postural-Perceptual Dizziness. Otol. Neurotol. 2021, 42, E1618–E1624. [Google Scholar] [CrossRef] [PubMed]
- Wuehr, M.; Pradhan, C.; Novozhilov, S.; Krafczyk, S.; Brandt, T.; Jahn, K.; Schniepp, R. Inadequate Interaction between Open- And Closed-Loop Postural Control in Phobic Postural Vertigo. J. Neurol. 2013, 260, 1314–1323. [Google Scholar] [CrossRef] [PubMed]
- Teggi, R.; Gatti, O.; Cangiano, J.; Fornasari, F.; Bussi, M. Functional Head Impulse Test with and Without Optokinetic Stimulation in Subjects with Persistent Postural Perceptual Dizziness (PPPD): Preliminary Report. Otol. Neurotol. 2020, 41, E70–E75. [Google Scholar] [CrossRef] [PubMed]
- Arshad, Q.; Saman, Y.; Sharif, M.; Kaski, D.; Staab, J.P. Magnitude Estimates Orchestrate Hierarchal Construction of Context-Dependent Representational Maps for Vestibular Space and Time: Theoretical Implications for Functional Dizziness. Front. Integr. Neurosci. 2022, 15, 806940. [Google Scholar] [CrossRef] [PubMed]
- Staab, J.P.; Balaban, C.D.; Furman, J.M. Threat Assessment and Locomotion: Clinical Applications of an Integrated Model of Anxiety and Postural Control. Semin. Neurol. 2013, 33, 297–306. [Google Scholar] [CrossRef]
- Holmberg, J.; Tjernström, F.; Karlberg, M.; Fransson, P.A.; Magnusson, M. Reduced Postural Differences between Phobic Postural Vertigo Patients and Healthy Subjects during a Postural Threat. J. Neurol. 2009, 256, 1258–1262. [Google Scholar] [CrossRef]
- Naranjo, E.; Cleworth, T.; Allum, J.; Inglis, J.; Lea, J.; Westerberg, B.; Carpenter, M.; Cleworth, W. Threat Effects on Human Oculo-Motor Function. Neurosci. Carpent. Neurosci. 2017, 359, 289–298. [Google Scholar] [CrossRef]
- Arshad, Q. Dynamic Interhemispheric Competition and Vestibulo-Cortical Control in Humans; A Theoretical Proposition. Neuroscience 2017, 353, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Kaski, D.; Quadir, S.; Nigmatullina, Y.; Malhotra, P.A.; Bronstein, A.M.; Seemungal, B.M. Temporoparietal Encoding of Space and Time during Vestibular-Guided Orientation. Brain 2016, 139, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Bednarczuk, N.F.; Casanovas Ortega, M.; Fluri, A.-S.; Arshad, Q. Vestibulo-Cortical Hemispheric Dominance: The Link. between Anxiety and the Vestibular System? Eur. J. Neurosci. 2018, 47, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- Epstein, R.A.; Patai, E.Z.; Julian, J.B.; Spiers, H.J. The Cognitive Map in Humans: Spatial Navigation and Beyond. Nat. Neurosci. 2017, 20, 1504–1513. [Google Scholar] [CrossRef] [PubMed]
- Angelaki, D.E.; Cullen, K.E. Vestibular System: The Many Facets of a Multimodal Sense. Annu. Rev. Neurosci. 2008, 31, 125–150. [Google Scholar] [CrossRef] [PubMed]
- Ghazanfar, A.A.; Schroeder, C.E. Is Neocortex Essentially Multisensory? Trends Cogn. Sci. 2006, 10, 278–285. [Google Scholar] [CrossRef]
- Frank, S.M.; Mark, X.; Greenlee, W. The Parieto-Insular Vestibular Cortex in Humans: More than a Single Area? Rev. Sens. Process. J. Neurophysiol. 2018, 120, 1438–1450. [Google Scholar] [CrossRef]
- Cullen, K.E. Physiology of Central Pathways, 1st ed.Elsevier: Amsterdam, The Netherlands, 2016; Volume 137, ISBN 9780444634375. [Google Scholar]
- Cullen, K.E. Vestibular Processing during Natural Self-Motion: Implications for Perception and Action. Nat. Rev. Neurosci. 2019, 20, 346–363. [Google Scholar] [CrossRef]
- Cullen, K.E.; Wang, L. Predictive Coding in Early Vestibular Pathways: Implications for Vestibular Cognition. Cogn. Neuropsychol. 2020, 37, 423–426. [Google Scholar] [CrossRef]
- Carriot, J.; Jamali, M.; Cullen, K.E. Rapid Adaptation of Multisensory Integration in Vestibular Pathways. Front. Syst. Neurosci. 2015, 9, 59. [Google Scholar] [CrossRef]
- Hitier, M.; Besnard, S.; Smith, P.F. Vestibular Pathways Involved in Cognition. Front. Integr. Neurosci. 2014, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, Y. Vestibular Cognition: Building a Framework. Cogn. Neuropsychol. 2020, 37, 421–422. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, E.R.; Haggard, P. Vestibular Cognition: State-of-the-Art and Future Directions. Cogn. Neuropsychol. 2020, 37, 413–420. [Google Scholar] [CrossRef]
- Zachou, A.; Bronstein, A.M. Vestibulo-Spatial Navigation: Pathways and Sense of Direction. J. Neurophysiol. 2023, 129, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Lempert, T.; Olesen, J.; Furman, J.; Waterston, J.; Seemungal, B.; Carey, J.; Bisdorff, A.; Versino, M.; Evers, S.; Newman-Toker, D. Vestibular Migraine: Diagnostic Criteria. J. Vestib. Res. 2012, 22, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Escamez, J.A.; Carey, J.; Chung, W.-H.; Goebel, J.A.; Magnusson, M.; Mandala, M.; Newman-Toker, D.E.; Strupp, M.; Suzuki, M.; Trabalzini, F.; et al. Diagnostic Criteria for Meniere’s Disease. J. Vestib. Res. 2015, 25, 1–7. [Google Scholar] [CrossRef] [PubMed]
- von Brevern, M.; Bertholon, P.; Brandt, T.; Fife, T.; Imai, T.; Nuti, D.; Newman-Toker, D. Benign Paroxysmal Positional Vertigo: Diagnostic Criteria. J. Vestib. Res. 2015, 25, 105–117. [Google Scholar] [CrossRef]
- Strupp, M.; Kim, J.-S.; Murofushi, T.; Straumann, D.; Jen, J.C.; Rosengren, S.M.; Della Santina, C.C.; Kingma, H. Bilateral Vestibulopathy: Diagnostic Criteria Consensus Document of the Classification Committee of the Barany Society. J. Vestib. Res. 2017, 27, 177–189. [Google Scholar] [CrossRef]
- Morris, R. Developments of a Water-Maze Procedure for Studying Spatial Learning in the Rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Schoenfeld, R.; Schiffelholz, T.; Beyer, C.; Leplow, B.; Foreman, N. Variants of the Morris Water Maze Task to Comparatively Assess Human and Rodent Place Navigation. Neurobiol. Learn. Mem. 2017, 139, 117–127. [Google Scholar] [CrossRef]
- Wood, H. A Virtual Morris Maze to Assess Cognitive Impairment in Alzheimer Disease. Nat. Rev. Neurol. 2016, 12, 126. [Google Scholar] [CrossRef] [PubMed]
- Wei, E.X.; Oh, E.S.; Harun, A.; Ehrenburg, M.; Agrawal, Y. Vestibular Loss Predicts Poorer Spatial Cognition in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Dobbels, B.; Peetermans, O.; Boon, B.; Mertens, G.; Van de Heyning, P.; Van Rompaey, V. Impact of Bilateral Vestibulopathy on Spatial and Nonspatial Cognition: A Systematic Review. Ear Hear. 2019, 40, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Kremmyda, O.; Hüfner, K.; Flanagin, V.L.; Hamilton, D.A.; Linn, J.; Strupp, M.; Jahn, K.; Brandt, T. Beyond Dizziness: Virtual Navigation, Spatial Anxiety and Hippocampal Volumein Bilateral Vestibulopathy. Front. Hum. Neurosci. 2016, 10, 139. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Gignac, G.E.; Reynolds, M.R.; Kovacs, K. Digit Span Subscale Scores May Be Insufficiently Reliable for Clinical Interpretation: Distinguishing Between Stratified Coefficient Alpha and Omega Hierarchical. Assessment 2017, 26, 1554–1563. [Google Scholar] [CrossRef] [PubMed]
- Rosas, R.; Tenorio, M.; Pizarro, M.; Cumsille, P.; Bosch, A.; Arancibia, S.; Carmona-Halty, M.; Perez-Salas, C.P.; Pino, E.; Vizcarra, B.; et al. Estandarización de La Escala Wechsler de Inteligencia Para Adultos-Cuarta Edición En Chile. Psykhe 2014, 23, 1–18. [Google Scholar] [CrossRef]
- Farrell Pagulayan, K.; Busch, R.; Medina, K.; Bartok, J.; Krikorian, R. Developmental Normative Data for the Corsi Block-Tapping Task. J. Clin. Exp. Neuropsychol. 2006, 28, 1043–1052. [Google Scholar] [CrossRef]
- Kessels, R.P.C.; Van Zandvoort, M.J.E.; Postma, A.; Kappelle, L.J.; De Haan, E.H.F. The Corsi Block-Tapping Task: Standardization and Normative Data. Appl. Neuropsychol. 2000, 7, 252–258. [Google Scholar] [CrossRef]
- Bigelow, R.T.; Agrawal, Y. Vestibular Involvement in Cognition: Visuospatial Ability, Attention, Executive Function, and Memory. J. Vestib. Res. 2015, 25, 73–89. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Administration and Interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Trakoshis, S.; Ioannou, M.; Fanti, K. The Factorial Structure of the Tower Test From the Delis-Kaplan Executive Function System: A Confirmatory Factor Analysis Study. Assessment 2022, 29, 317–331. [Google Scholar] [CrossRef]
- García-Alba, J.; Esteba-Castillo, S.; López, M.Á.C.; Hidalgo, E.R.; Vidal, N.R.; Díaz, F.M.; Novell-Alsina, R. Validation and Normalization of the Tower of London-Drexel University Test 2nd Edition in an Adult Population with Intellectual Disability. Span. J. Psychol. 2017, 20, E32. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, R.; Bartok, J.; Gay, N. Tower of London Procedure: A Standard Method and Developmental Data. J. Clin. Exp. Neuropsychol. 1994, 16, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Melipillán Araneda, R.; Cova Solar, F.; Rincón González, P.; Valdivia Peralta, M. Propiedades Psicométricas Del Inventario de Depresión de Beck-II En Adolescentes Chilenos. Ter. Psicológica 2008, 26, 59–69. [Google Scholar] [CrossRef]
- Sydeman, S. State-Trait Anxiety Inventory. Encycl. Personal. Individ. Differ. 2018, 1–3. [Google Scholar] [CrossRef]
- Vera-Villarroel, P.; Celis-Atenas, K.; Córdova-Rubio, N.; Buela-Casal, G.; Spielberger, C.D. Preliminary Analysis and Normative Data of the State-Trait Anxiety Inventory (STAI) in Adolescent and Adults of Santiago, Chile. Ter. Psicológica 2007, 25, 155–162. [Google Scholar] [CrossRef]
- Caldara, B.; Asenzo, A.I.; Brusotti Paglia, G.; Ferreri, E.; Gomez, R.S.; Laiz, M.M.; Luques, M.L.; Mangoni, A.P.; Marazzi, C.; Matesa, M.A.; et al. Adaptación Cultural y Validación Del Dizziness Handicap Inventory, Versión Argentina. Acta Otolaringol. Esp. 2012, 63, 106–114. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Newman, C.W. The Development of the Dizziness Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Yagi, C.; Morita, Y.; Kitazawa, M.; Nonomura, Y.; Yamagishi, T.; Ohshima, S.; Izumi, S.; Takahashi, K.; Horii, A. A Validated Questionnaire to Assess the Severity of Persistent Postural-Perceptual Dizziness (PPPD): The Niigata PPPD Questionnaire (NPQ). Otol. Neurotol. 2019, 40, e747–e752. [Google Scholar] [CrossRef]
- Popp, P.; Wulff, M.; Finke, K.; Rühl, M.; Brandt, T.; Dieterich, M. Cognitive Deficits in Patients with a Chronic Vestibular Failure. J. Neurol. 2017, 264, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Harris, L.R. Does the Vestibular System Exert Specific or General Influences on Cognitive Processes? Cogn. Neuropsychol. 2020, 37, 430–432. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.G.; Sharon, J.D.; Lee, J.A.; Thomas, C.; Nguyen, S.A.; Meyer, T.A. Cross-Sectional Analysis of Cognitive Dysfunction in Patients with Vestibular Disorders. Ear Hear. 2020, 41, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Biju, K.; Wei, E.X.; Rebello, E.; Matthews, J.; He, Q.; McNamara, T.P.; Agrawal, Y. Performance in Real World-and Virtual Reality-Based Spatial Navigation Tasks in Patients with Vestibular Dysfunction. Otol. Neurotol. 2021, 42, E1524–E1531. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.; Biju, K.; Klatt, B.N.; Simonsick, E.; Agrawal, Y. Self-Reported Sense of Direction and Vestibular Function in the Baltimore Longitudinal Study of Aging (BLSA). J. Assoc. Res. Otolaryngol. 2021, 22, 207–214. [Google Scholar] [CrossRef]
- Schöberl, F.; Zwergal, A.; Brandt, T. Testing Navigation in Real Space: Contributions to Understanding the Physiology and Pathology of Human Navigation Control. Front. Neural Circuits 2020, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Maccallum, R.C.; Widaman, K.F.; Zhang, S.; Hong, S. Sample Size in Factor Analysis. Psychol. Methods 1999, 4, 84. [Google Scholar] [CrossRef]


| Group | ||||
|---|---|---|---|---|
| PPPD | Vestibular (Non-PPPD) | Healthy Control | ||
| Number | 19 | 20 | 12 | |
| Age * | Mean | 46.8 | 44 | 43.6 |
| Standard deviation | 14.9 | 13.9 | 15.3 | |
| Range | 21–65 | 20–63 | 25–64 | |
| Gender | Female/Male | 79%/21% | 85%/15% | 75%/25% |
| Educational level *,† | Mean score | 3.78 | 3.9 | 3.75 |
| Standard deviation | 0.53 | 0.31 | 0.46 | |
| Diagnosis (percentage of each group) | Vestibular migraine | 21.6% | 33.3% | - |
| Vestibular neuritis | 24.3% | 23.3% | - | |
| Benign positional paroxysmal vertigo | 10.8% | 23.3% | - | |
| Bilateral vestibulopathy | 2.7% | 10% | - | |
| Otoesclerosis | 2.7% | 3.3% | - | |
| Meniere’s disease | 2.7% | 3.3% | - | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Breinbauer, H.A.; Arévalo-Romero, C.; Villarroel, K.; Lavin, C.; Faúndez, F.; Garrido, R.; Alarcón, K.; Stecher, X.; Zamorano, F.; Billeke, P.; et al. Functional Dizziness as a Spatial Cognitive Dysfunction. Brain Sci. 2024, 14, 16. https://doi.org/10.3390/brainsci14010016
Breinbauer HA, Arévalo-Romero C, Villarroel K, Lavin C, Faúndez F, Garrido R, Alarcón K, Stecher X, Zamorano F, Billeke P, et al. Functional Dizziness as a Spatial Cognitive Dysfunction. Brain Sciences. 2024; 14(1):16. https://doi.org/10.3390/brainsci14010016
Chicago/Turabian StyleBreinbauer, Hayo A., Camilo Arévalo-Romero, Karen Villarroel, Claudio Lavin, Felipe Faúndez, Rosario Garrido, Kevin Alarcón, Ximena Stecher, Francisco Zamorano, Pablo Billeke, and et al. 2024. "Functional Dizziness as a Spatial Cognitive Dysfunction" Brain Sciences 14, no. 1: 16. https://doi.org/10.3390/brainsci14010016
APA StyleBreinbauer, H. A., Arévalo-Romero, C., Villarroel, K., Lavin, C., Faúndez, F., Garrido, R., Alarcón, K., Stecher, X., Zamorano, F., Billeke, P., & Delano, P. H. (2024). Functional Dizziness as a Spatial Cognitive Dysfunction. Brain Sciences, 14(1), 16. https://doi.org/10.3390/brainsci14010016






