Auditory Cortex Asymmetry Associations with Individual Differences in Language and Cognition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Behavioral Data
2.3. Imaging Data
2.4. Statistics
3. Results
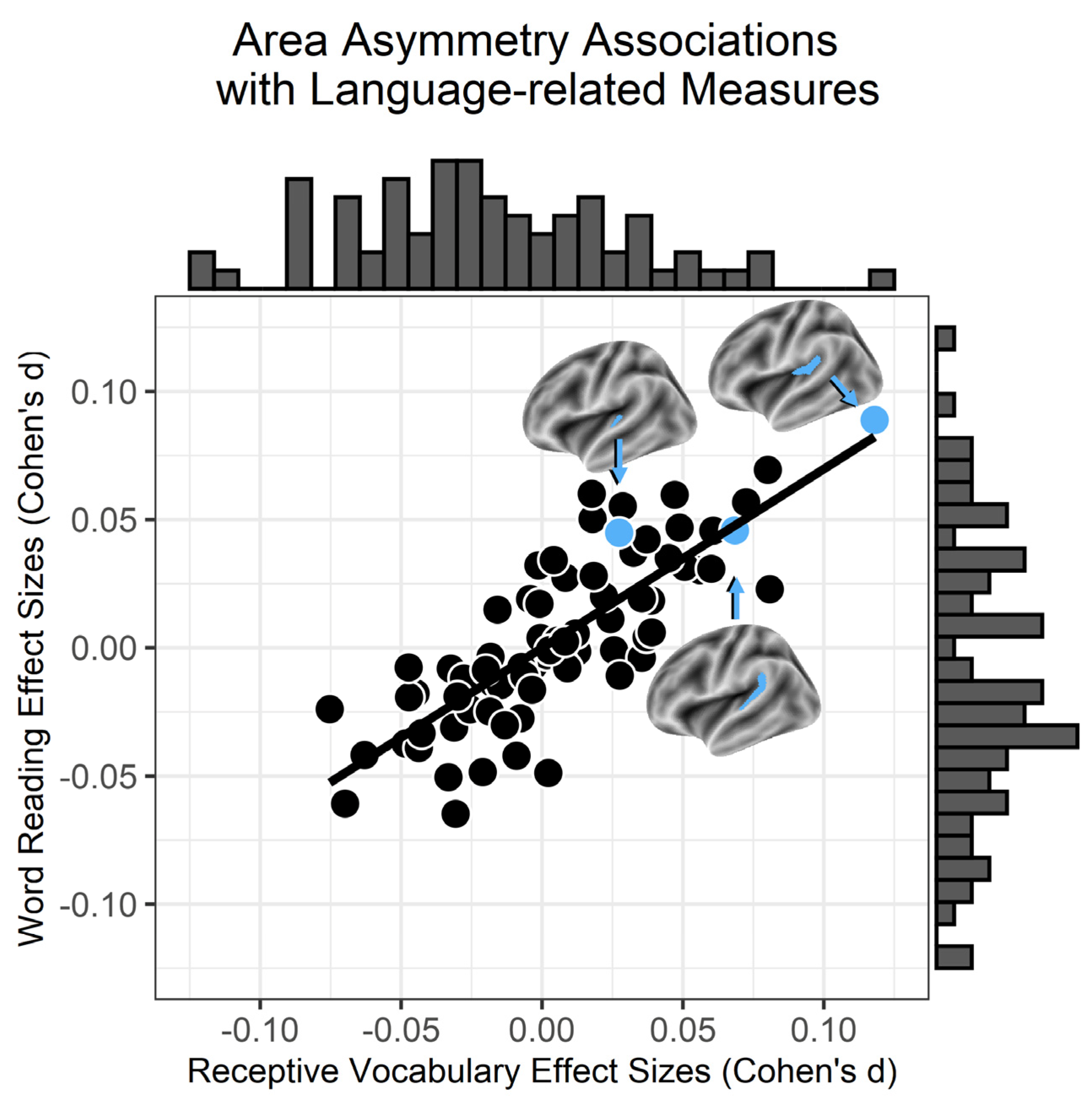
3.1. Surface Area Asymmetries and Language
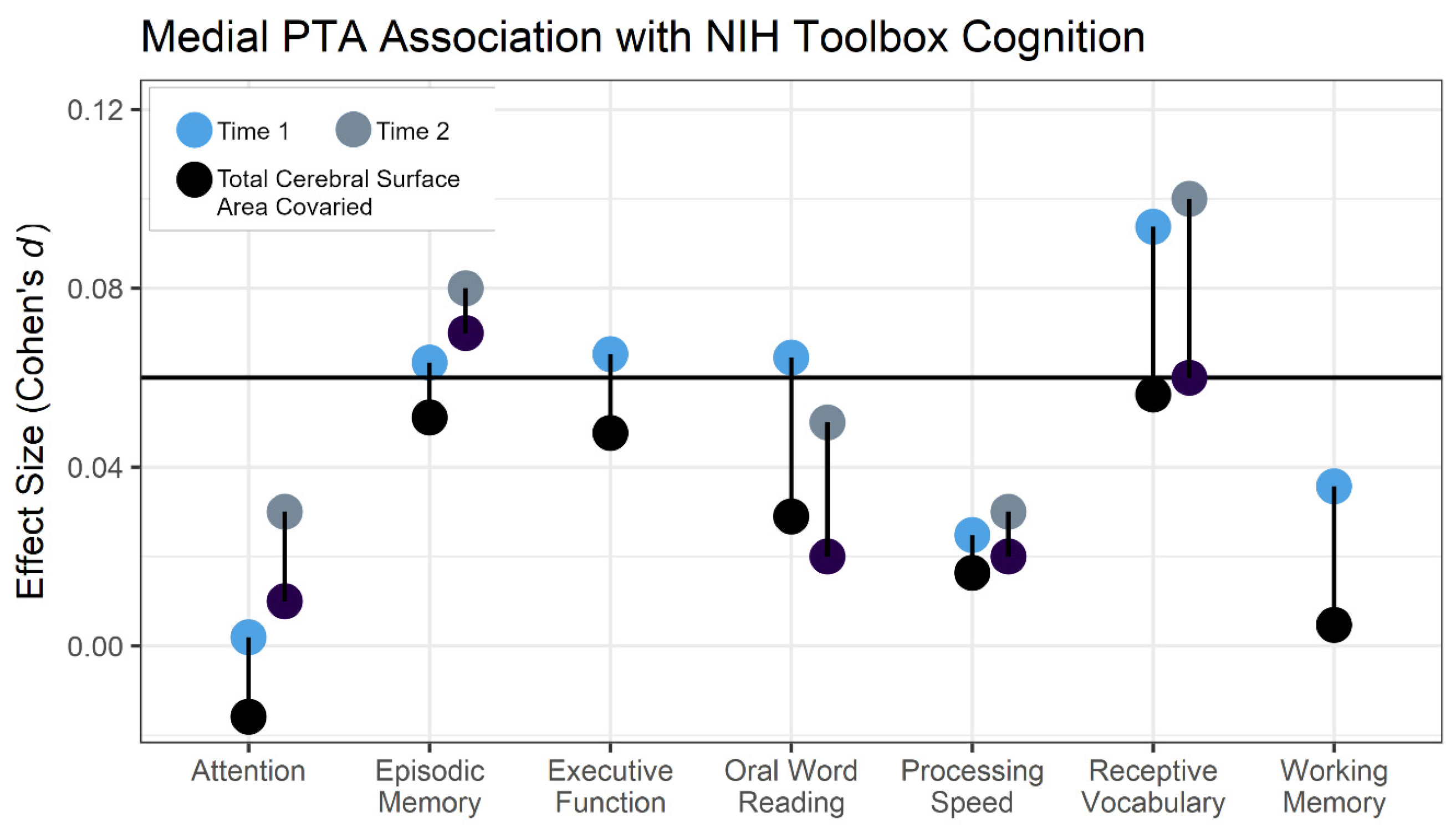
3.2. Behavioral Specificity
3.3. Sex Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galaburda, A.M.; Sherman, G.F.; Rosen, G.D.; Aboitiz, F.; Geschwind, N. Developmental dyslexia: Four consecutive patients with cortical anomalies. Ann. Neurol. 1985, 18, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Geschwind, N.; Galaburda, A.M. Cerebral lateralization. Biological mechanisms, associations, and pathology: I. A hypothesis and a program for research. Arch. Neurol. 1985, 42, 428–459. [Google Scholar] [CrossRef] [PubMed]
- Forseth, K.J.; Hickok, G.; Rollo, P.S.; Tandon, N. Language prediction mechanisms in human auditory cortex. Nat. Commun. 2020, 11, 5240. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Leonard, C.M. Structural imaging in dyslexia: The planum temporale. Ment. Retard. Dev. Disabil. Res. Rev. 2000, 6, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ramus, F.; Altarelli, I.; Jednorog, K.; Zhao, J.; Scotto di Covella, L. Neuroanatomy of developmental dyslexia: Pitfalls and promise. Neurosci. Biobehav. Rev. 2018, 84, 434–452. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Lombardino, L.J.; Leonard, C.M. Planar asymmetry tips the phonological playground and environment raises the bar. Child Dev. 2001, 72, 988–1002. [Google Scholar] [CrossRef] [PubMed]
- Gauger, L.M.; Lombardino, L.J.; Leonard, C.M. Brain morphology in children with specific language impairment. J. Speech. Lang. Hear. Res. 1997, 40, 1272–1284. [Google Scholar] [CrossRef] [PubMed]
- Tzourio-Mazoyer, N.; Mazoyer, B. Variations of planum temporale asymmetries with Heschl’s Gyri duplications and association with cognitive abilities: MRI investigation of 428 healthy volunteers. Brain Struct. Funct. 2017, 222, 2711–2726. [Google Scholar] [CrossRef]
- Altarelli, I.; Leroy, F.; Monzalvo, K.; Fluss, J.; Billard, C.; Dehaene-Lambertz, G.; Galaburda, A.M.; Ramus, F. Planum temporale asymmetry in developmental dyslexia: Revisiting an old question. Hum. Brain Mapp. 2014, 35, 5717–5735. [Google Scholar] [CrossRef]
- Jernigan, T.L.; Brown, S.A.; Coordinators, A.C. Introduction. Dev. Cogn. Neurosci. 2018, 32, 1–3. [Google Scholar] [CrossRef]
- Gooch, D.; Thompson, P.; Nash, H.M.; Snowling, M.J.; Hulme, C. The development of executive function and language skills in the early school years. Ann. Dyslexia 2016, 57, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Cutting, L.E.; Materek, A.; Cole, C.A.; Levine, T.M.; Mahone, E.M. Effects of fluency, oral language, and executive function on reading comprehension performance. Ann. Dyslexia 2009, 59, 34–54. [Google Scholar] [CrossRef] [PubMed]
- Galaburda, A.M.; Corsiglia, J.; Rosen, G.D.; Sherman, G.F. Planum temporale asymmetry, reappraisal since Geschwind and Levitsky. Neuropsychologia 1987, 25, 853–868. [Google Scholar] [CrossRef]
- Rosen, G.D.; Herman, A.E.; Galaburda, A.M. Sex differences in the effects of early neocortical injury on neuronal size distribution of the medial geniculate nucleus in the rat are mediated by perinatal gonadal steroids. Cereb. Cortex 1999, 9, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pfannkuche, K.A.; Bouma, A.; Groothuis, T.G. Does testosterone affect lateralization of brain and behaviour? A meta-analysis in humans and other animal species. Philos. Trans. R Soc. Lond. B Biol. Sci. 2009, 364, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.M.; Towler, S.; Welcome, S.; Halderman, L.K.; Otto, R.; Eckert, M.A.; Chiarello, C. Size matters: Cerebral volume influences sex differences in neuroanatomy. Cereb. Cortex 2008, 18, 2920–2931. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Pasqualetti, P.; Volterra, V.; Caselli, M.C. Gender differences in early stages of language development. Some evidence and possible explanations. J. Neurosci. Res. 2023, 101, 643–653. [Google Scholar] [CrossRef] [PubMed]
- Rademacher, J.; Morosan, P.; Schleicher, A.; Freund, H.-J.; Zilles, K. Human primary auditory cortex in women and men. Neuroreport 2001, 12, 1561–1565. [Google Scholar] [CrossRef]
- Brun, C.C.; Lepore, N.; Luders, E.; Chou, Y.-Y.; Madsen, S.K.; Toga, A.W.; Thompson, P.M. Sex differences in brain structure in auditory and cingulate regions. Neuroreport 2009, 20, 930. [Google Scholar] [CrossRef]
- Kong, X.-Z.; Mathias, S.R.; Guadalupe, T.; Group, E.L.W.; Glahn, D.C.; Franke, B.; Crivello, F.; Tzourio-Mazoyer, N.; Fisher, S.E.; Thompson, P.M. Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. Proc. Natl. Acad. Sci. USA 2018, 115, E5154–E5163. [Google Scholar] [CrossRef]
- Garavan, H.; Bartsch, H.; Conway, K.; Decastro, A.; Goldstein, R.; Heeringa, S.; Jernigan, T.; Potter, A.; Thompson, W.; Zahs, D. Recruiting the ABCD sample: Design considerations and procedures. Dev. Cogn. Neurosci. 2018, 32, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.B.; Fisher, C.B.; Bookheimer, S.; Brown, S.A.; Evans, J.H.; Hopfer, C.; Hudziak, J.; Montoya, I.; Murray, M.; Pfefferbaum, A. Biomedical ethics and clinical oversight in multisite observational neuroimaging studies with children and adolescents: The ABCD experience. Dev. Cogn. Neurosci. 2018, 32, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Auchter, A.M.; Mejia, M.H.; Heyser, C.J.; Shilling, P.D.; Jernigan, T.L.; Brown, S.A.; Tapert, S.F.; Dowling, G.J. A description of the ABCD organizational structure and communication framework. Dev. Cogn. Neurosci. 2018, 32, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, S.; Dikmen, S.S.; Heaton, R.K.; Tulsky, D.S.; Zelazo, P.D.; Bauer, P.J.; Carlozzi, N.E.; Slotkin, J.; Blitz, D.; Wallner-Allen, K.; et al. Cognition assessment using the NIH Toolbox. Neurology 2013, 80, S54–S64. [Google Scholar] [CrossRef] [PubMed]
- Gershon, R.C.; Cook, K.F.; Mungas, D.; Manly, J.J.; Slotkin, J.; Beaumont, J.L.; Weintraub, S. Language measures of the NIH toolbox cognition battery. J. Int. Neuropsychol. Soc. 2014, 20, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.J.; Cannonier, T.; Conley, M.I.; Cohen, A.O.; Barch, D.M.; Heitzeg, M.M.; Soules, M.E.; Teslovich, T.; Dellarco, D.V.; Garavan, H.; et al. The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. Dev. Cogn. Neurosci. 2018, 32, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Winkler, A.M.; Greve, D.N.; Bjuland, K.J.; Nichols, T.E.; Sabuncu, M.R.; Haberg, A.K.; Skranes, J.; Rimol, L.M. Joint analysis of cortical area and thickness as a replacement for the analysis of the volume of the cerebral cortex. Cereb. Cortex 2018, 28, 738–749. [Google Scholar] [CrossRef]
- Hagler, D.J., Jr.; Hatton, S.; Cornejo, M.D.; Makowski, C.; Fair, D.A.; Dick, A.S.; Sutherland, M.T.; Casey, B.J.; Barch, D.M.; Harms, M.P.; et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage 2019, 202, 116091. [Google Scholar] [CrossRef]
- Destrieux, C.; Fischl, B.; Dale, A.; Halgren, E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 2010, 53, 1–15. [Google Scholar] [CrossRef]
- Iscan, Z.; Jin, T.B.; Kendrick, A.; Szeglin, B.; Lu, H.; Trivedi, M.; Fava, M.; McGrath, P.J.; Weissman, M.; Kurian, B.T.; et al. Test-retest reliability of freesurfer measurements within and between sites: Effects of visual approval process. Hum. Brain Mapp. 2015, 36, 3472–3485. [Google Scholar] [CrossRef]
- Veale, J.F. Edinburgh Handedness Inventory—Short Form: A revised version based on confirmatory factor analysis. Laterality 2014, 19, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Leonard, C.M.; Puranik, C.; Kuldau, J.M.; Lombardino, L.J. Normal variation in the frequency and location of human auditory cortex landmarks. Heschl’s gyrus: Where is it? Cereb. Cortex 1998, 8, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Eckert, M.A.; Vaden, K.I.; Dyslexia Data, C. A deformation-based approach for characterizing brain asymmetries at different spatial scales of resolution. J. Neurosci. Meth. 2019, 322, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ricketts, J.; Nation, K.; Bishop, D.V. Vocabulary is important for some, but not all reading skills. Sci. Stud. Read. 2007, 11, 235–257. [Google Scholar] [CrossRef]
- Mitchell, A.M.; Brady, S.A. The effect of vocabulary knowledge on novel word identification. Ann. Dyslexia 2013, 63, 201–216. [Google Scholar] [CrossRef] [PubMed]
- Baron, J. Orthographic and word-specific mechanisms in children’s reading of words. Child Dev. 1979, 50, 60–72. [Google Scholar] [CrossRef]
- Thompson, G.B. Three studies of predicted gender differences in processes of word reading. J. Educ. Res. 1987, 80, 212–219. [Google Scholar] [CrossRef]
- Wise, J.C.; Sevcik, R.A.; Morris, R.D.; Lovett, M.W.; Wolf, M. The relationship among receptive and expressive vocabulary, listening comprehension, pre-reading skills, word identification skills, and reading comprehension by children with reading disabilities. J. Speech. Lang. Hear. Res. 2007, 50, 1093–1109. [Google Scholar] [CrossRef]
- Girbau-Massana, D.; Garcia-Marti, G.; Marti-Bonmati, L.; Schwartz, R.G. Gray–white matter and cerebrospinal fluid volume differences in children with specific language impairment and/or reading disability. Neuropsychologia 2014, 56, 90–100. [Google Scholar] [CrossRef]
- Leonard, C.M.; Lombardino, L.J.; Walsh, K.; Eckert, M.A.; Mockler, J.L.; Rowe, L.A.; Williams, S.; DeBose, C.B. Anatomical risk factors that distinguish dyslexia from SLI predict reading skill in normal children. J. Commun. Disord. 2002, 35, 501–531. [Google Scholar] [CrossRef]
- Eckert, M.A.; Berninger, V.W.; Vaden, K.I.; Gebregziabher, M.; Tsu, L. Gray matter reatures of reading disability: A combined meta-analytic and direct analysis approach(1,2,3,4). eNeuro 2016, 3, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Pahs, G.; Rankin, P.; Helen Cross, J.; Croft, L.; Northam, G.B.; Liegeois, F.; Greenway, S.; Harrison, S.; Vargha-Khadem, F.; Baldeweg, T. Asymmetry of planum temporale constrains interhemispheric language plasticity in children with focal epilepsy. Brain 2013, 136, 3163–3175. [Google Scholar] [CrossRef] [PubMed]
- Liebenthal, E.; Desai, R.; Ellingson, M.M.; Ramachandran, B.; Desai, A.; Binder, J.R. Specialization along the left superior temporal sulcus for auditory categorization. Cereb. Cortex 2010, 20, 2958–2970. [Google Scholar] [CrossRef] [PubMed]
- Richardson, F.M.; Ramsden, S.; Ellis, C.; Burnett, S.; Megnin, O.; Catmur, C.; Schofield, T.M.; Leff, A.P.; Price, C.J. Auditory short-term memory capacity correlates with grey matter density in the left posterior superior temporal sulcus in cognitively normal and dyslexic adults. J. Cogn. Neurosci. 2011, 23, 3746. [Google Scholar] [CrossRef]
- Richlan, F. Developmental dyslexia: Dysfunction of a left hemisphere reading network. Front. Hum. Neurosci. 2012, 6, 120. [Google Scholar] [CrossRef]
- Friederici, A.D.; Chomsky, N.; Berwick, R.C.; Moro, A.; Bolhuis, J.J. Language, mind and brain. Nat. Hum. Behav. 2017, 1, 713–722. [Google Scholar] [CrossRef]
| Boys | Girls | Group Difference | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p Value | |
| Oral Word Reading ^ | 90.879 | 7.101 | 90.837 | 6.701 | |
| Receptive Vocabulary | 84.705 | 8.063 | 84.191 | 8.170 | *** |
| Attention | 94.165 | 9.348 | 93.817 | 0.0395 | |
| Executive Function ^ | 92.002 | 9.957 | 93.094 | 8.965 | *** |
| Episodic Memory | 102.088 | 12.032 | 103.610 | 12.070 | *** |
| Processing Speed ^ | 87.168 | 14.866 | 89.032 | 14.202 | *** |
| Working Memory | 97.036 | 12.240 | 96.234 | 11.892 | *** |
| Medial PTA † ^ | −0.174 | 0.124 | −0.207 | 0.110 | *** |
| Anterior PTA † | 0.291 | 0.263 | 0.299 | 0.253 | |
| Posterolateral PTA ^ | 0.182 | 0.214 | 0.144 | 0.204 | *** |
| Total Cortical Surface Area ^ | 197,494 | 16,850 | 180,889 | 15,512 | *** |
| Regression Model | NIH Toolbox Cognition Variable | Estimate | Standard Error | t-Score | |
|---|---|---|---|---|---|
| Level 1 | Receptive Vocabulary | 7.80−4 | 1.55−4 | 5.041 | *** |
| Model 1: | Receptive Vocabulary | 6.69−4 | 1.71−4 | 3.905 | *** |
| Level 2 | Oral Word Reading | 2.78−4 | 1.92−4 | 1.446 | |
| Model 2: | Receptive Vocabulary | 6.99−4 | 1.58−4 | 4.429 | *** |
| Level 2 | Executive Function | 3.15−4 | 1.24−4 | 2.541 | * |
| Model 3: | Receptive Vocabulary | 7.21−4 | 1.57−4 | 4.599 | *** |
| Level 2 | Episodic Memory | 2.41−4 | 9.48−5 | 2.546 | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eckert, M.A.; Vaden, K.I., Jr.; Paracchini, S. Auditory Cortex Asymmetry Associations with Individual Differences in Language and Cognition. Brain Sci. 2024, 14, 14. https://doi.org/10.3390/brainsci14010014
Eckert MA, Vaden KI Jr., Paracchini S. Auditory Cortex Asymmetry Associations with Individual Differences in Language and Cognition. Brain Sciences. 2024; 14(1):14. https://doi.org/10.3390/brainsci14010014
Chicago/Turabian StyleEckert, Mark A., Kenneth I. Vaden, Jr., and Silvia Paracchini. 2024. "Auditory Cortex Asymmetry Associations with Individual Differences in Language and Cognition" Brain Sciences 14, no. 1: 14. https://doi.org/10.3390/brainsci14010014
APA StyleEckert, M. A., Vaden, K. I., Jr., & Paracchini, S. (2024). Auditory Cortex Asymmetry Associations with Individual Differences in Language and Cognition. Brain Sciences, 14(1), 14. https://doi.org/10.3390/brainsci14010014







