Dual-Process Theory of Thought and Inhibitory Control: An ALE Meta-Analysis
Abstract
1. Introduction
1.1. The Dual-Process Theory of Thought
1.2. Neural Correlates of Fast and Slow Thinking
2. Materials and Methods
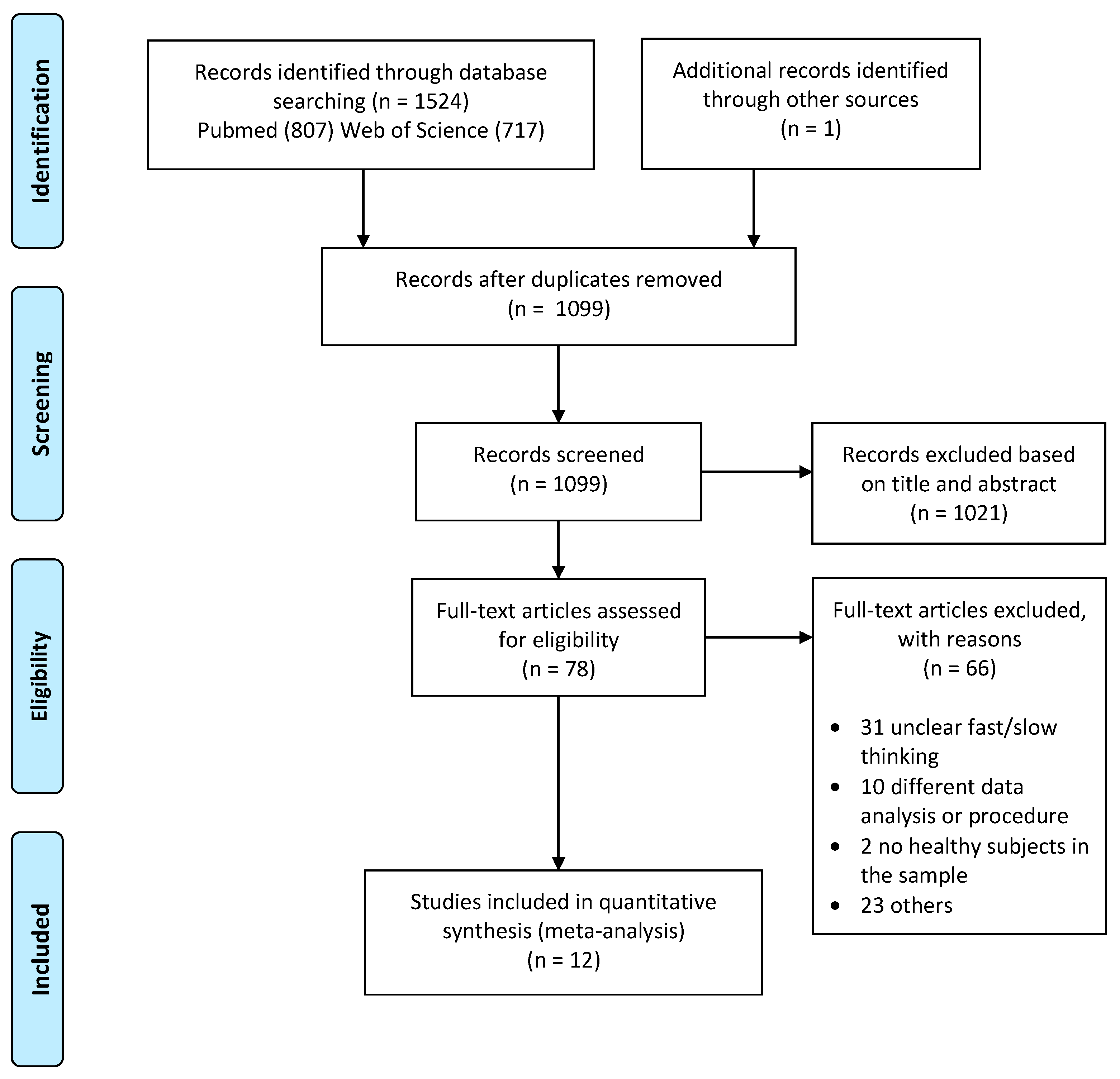
2.1. Literature Search and Selection
2.2. Activation Likelihood Estimation (ALE)
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frankish, K.; Evans, J.S.B.T. The duality of mind: An historical perspective. In Two Minds: Dual Processes and Beyond; Oxford University Press: Oxford, UK, 2009; pp. 1–29. [Google Scholar]
- Da Silva, S. System 1 vs. System 2 Thinking. PsyCh 2023, 5, 1057–1076. [Google Scholar] [CrossRef]
- Augusto, R. Two Kinds of Process or Two Kinds of Processing? Disambiguating Dual-Process Theories. Rev. Philos. Psychol. 2023, 1–22, in press. [Google Scholar] [CrossRef]
- Sherman, J.; Gawronski, B.; Trope, Y. (Eds.) Dual Process Theories of the Social Mind; Guilford Press: New York, NY, USA, 2014. [Google Scholar]
- Evans, J.S.B.T. Thinking and Reasoning: A Very Short Introduction; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- De Neys, W. (Ed.) Dual-Process Theory 2.0; Abingdon-on-Thames: Routledge, UK, 2018. [Google Scholar]
- Evans, J.S.B.T. Reflections on reflection: The nature and function of type 2 processes in dual-process theories of reasoning. Think. Reason. 2019, 25, 383–415. [Google Scholar] [CrossRef]
- Frederick, S. Cognitive reflection and decision making. J. Econ. Perspect. 2005, 19, 25–42. [Google Scholar] [CrossRef]
- Stagnaro, M.; Pennycook, G.; Rand, D.G. Performance on the cognitive reflection test is stable across time. Judgm. Decis. Mak. 2018, 13, 260–267. [Google Scholar] [CrossRef]
- Thomson, K.S.; Oppenheimer, D.M. Investigating an alternate form of the cognitive reflection test. Judgm. Decis. Mak. 2016, 11, 99–113. [Google Scholar] [CrossRef]
- Brand, C. (Ed.) Dual-Process Theories in Moral Psychology: Interdisciplinary Approaches to Theoretical, Empirical and Practical Considerations; Springer: New York, NY, USA, 2016. [Google Scholar]
- Greene, J.D. Why are VMPFC patients more utilitarian? A dual-process theory of moral judgment explains. Trends Cogn. Sci. 2007, 11, 322–323. [Google Scholar] [CrossRef]
- Chaiken, S.; Trope, Y. (Eds.) Dual-Process Theories in Social Psychology; Guilford Press: New York, NY, USA, 1999. [Google Scholar]
- Borland, R. Understanding Hard to Maintain Behaviour Change: A Dual Process Approach; Wiley: Hoboken, NJ, USA, 2013. [Google Scholar]
- Thaler, R.H.; Sunstein, C.R. Nudge: Improving Decisions about Health, Wealth, and Happiness; Yale University Press: New Haven, CT, USA, 2008. [Google Scholar]
- Graziano, M. Dual-Process Theories of Numerical Cognition; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Bronstein, M.V.; Pennycook, G.; Bear, A.; Rand, D.G.; Cannon, T.D. Belief in fake news is associated with delusionality, dogmatism, religious fundamentalism, and reduced analytic thinking. J. Appl. Res. Mem. Cogn. 2019, 8, 108–117. [Google Scholar] [CrossRef]
- Pennycook, G.; Rand, D.G. Lazy, not biased: Susceptibility to partisan fake news is better explained by lack of reasoning than by motivated reasoning. Cognition 2019, 188, 39–50. [Google Scholar] [CrossRef]
- Bahçekapili, H.G.; Yilmaz, O. The relation between different types of religiosity and analytic cognitive style. Pers. Individ. Differ. 2017, 117, 267–272. [Google Scholar] [CrossRef]
- Pennycook, G.; Cheyne, J.A.; Seli, P.; Koehler, D.J.; Fugelsang, J.A. Analytic cognitive style predicts religious and paranormal belief. Cognition 2012, 123, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Pennycook, G.; Ross, R.M.; Koehler, D.J.; Fugelsang, J.A. Atheists and agnostics are more reflective than religious believers: Four empirical studies and a meta-analysis. PLoS ONE 2016, 11, e0153039. [Google Scholar] [CrossRef] [PubMed]
- Shenhav, A.; Rand, D.G.; Greene, J.D. Divine intuition: Cognitive style influences belief in God. J. Exp. Psychol. 2012, 141, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, G.; Zemla, J. Cognitive style predicts how people explain mental magic tricks. Acta Psychol. 2021, 218, 103347. [Google Scholar] [CrossRef]
- Kahneman, D. Thinking, Fast and Slow; Macmillan: London, UK, 2011. [Google Scholar]
- Gronchi, G.; Giovannelli, F. Dual process theory of thought and default mode network: A possible neural foundation of fast thinking. Front. Psychol. 2018, 9, 1237. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.S.B.; Barston, J.L.; Pollard, P. On the conflict between logic and belief in syllogistic reasoning. Mem. Cognit. 1983, 11, 295–306. [Google Scholar] [CrossRef]
- Ball, L.J.; Thompson, V.A.; Stupple, E.J.N. Conflict and dual process theory: The case of belief bias. In Dual Process Theory; De Neys, W., Ed.; Routledge/Taylor & Francis Group: Oxfordshire, UK, 2018; pp. 100–120. [Google Scholar]
- Evans, J.S.B.T. Thinking and believing. In Mental Models in Reasoning; Garcia-Madruga, J., Carriedo, N., Gonzalez-Labra, M.J., Eds.; UNED: Madrid, Spain, 2000; pp. 41–56. [Google Scholar]
- Evans, J.S.B.; Curtis-Holmes, J. Rapid responding increases belief bias: Evidence for the dual-process theory of reasoning. Think. Reason. 2005, 11, 382–389. [Google Scholar] [CrossRef]
- De Neys, W.; Glumicic, T. Conflict monitoring in dual process theories of thinking. Cognition 2008, 106, 1248–1299. [Google Scholar] [CrossRef]
- Evans, J.S.B.; Stanovich, K.E. Dual-process theories of higher cognition: Advancing the debate. Perspect. Psychol. Sci. 2013, 8, 223–241. [Google Scholar] [CrossRef]
- Denes-Raj, V.; Epstein, S. Conflict between intuitive and rational processing: When people behave against their better judgment. J. Pers. Soc. Psychol. 1994, 66, 819–829. [Google Scholar] [CrossRef]
- Sloman, S.A. The empirical case for two systems of reasoning. Psychol. Bull. 1996, 119, 3–22. [Google Scholar] [CrossRef]
- Newell, B.R.; Lagnado, D.A.; Shanks, D.R. Straight Choices: The Psychology of Decision Making; Psychology Press: New York, NY, USA, 2015. [Google Scholar]
- Thompson, V.A. Why it matters: The implications of autonomous processes for dual process theories—Commentary on Evans & Stanovich. Perspect. Psychol. Sci. 2013, 8, 253–256. [Google Scholar] [PubMed]
- Pennycook, G. A perspective on the theoretical foundation of dual process models. In Dual Process Theory; De Neys, W., Ed.; Routledge/Taylor & Francis Group: Oxfordshire, UK, 2018; pp. 5–27. [Google Scholar]
- Falkenstein, M.; Hoormann, J.; Hohnsbein, J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999, 101, 267–291. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.; Ramautar, J.R.; De Ruiter, M.B.; Band, G.P.; Ridderinkhof, K.R. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology 2004, 41, 9–20. [Google Scholar] [CrossRef]
- MacLeod, C.M. Half a century of research on the Stroop effect: An integrative review. Psychol. Bull. 1991, 109, 163–203. [Google Scholar] [CrossRef]
- Banks, A.P. Comparing dual process theories: Evidence from event-related potentials. In Dual Process Theory; De Neys, W., Ed.; Routledge/Taylor & Francis Group: Oxfordshire, UK, 2018; pp. 66–86. [Google Scholar]
- Goel, V.; Buchel, C.; Frith, C.; Dolan, R.J. Dissociation of mechanisms underlying syllogistic reasoning. NeuroImage 2000, 12, 504–514. [Google Scholar] [CrossRef]
- Goel, V.; Dolan, R.J. Differential involvement of left prefrontal cortex in inductive and deductive reasoning. Cognition 2004, 93, B109–B121. [Google Scholar] [CrossRef]
- Stollstorff, M.; Vartanian, O.; Goel, V. Levels of conflict in reasoning modulate right lateral prefrontal cortex. Brain Res. 2012, 1428, 24–32. [Google Scholar] [CrossRef]
- De Neys, W.D.; Vartanian, O.; Goel, V. Smarter than we think: When our brains detect that we are biased. Psychol. Sci. 2008, 19, 483–489. [Google Scholar] [CrossRef]
- Aron, A.R.; Fletcher, P.C.; Bullmore, E.T.; Sahakian, B.J.; Robbins, T.W. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat. Neurosci. 2003, 6, 115–116. [Google Scholar] [CrossRef]
- Gavazzi, G.; Giovannelli, F.; Currò, T.; Mascalchi, M.; Viggiano, M.P. Contiguity of proactive and reactive inhibitory brain areas: A cognitive model based on ALE meta-analyses. Brain Imaging Behav. 2021, 15, 2199–2214. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Giovannelli, F.; Noferini, C.; Cincotta, M.; Cavaliere, C.; Salvatore, M.; Mascalchi, M.; Viggiano, M.P. Subregional prefrontal cortex recruitment as a function of inhibitory demand: An fMRI metanalysis. Neurosci. Biobehav. Rev. 2023, 152, 105285. [Google Scholar] [CrossRef] [PubMed]
- Kerns, J.G. Anterior cingulate conflict monitoring and adjustments in control. Science 2004, 303, 1023–1026. [Google Scholar] [CrossRef] [PubMed]
- Vartanian, O.; Beatty, E.L.; Smith, I.; Blackler, K.; Lam, Q.; Forbes, S.; De Neys, W. The reflective mind: Examining individual differences in susceptibility to base rate neglect with fMRI. J. Cogn. Neurosci. 2018, 30, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, D.; Menon, D.K.; Stamatakis, E.A. Default mode contributions to automated information processing. Proc. Natl. Acad. Sci. USA 2017, 114, 12821–12826. [Google Scholar] [CrossRef]
- Tops, M.; Schlinkert, C.; Tjew-A-Sin, M.; Samur, D.; Koole, S.L. Protective inhibition of self-regulation and motivation: Extending a classic Pavlovian principle to social and personality functioning. In Handbook of Biobehavioral Approaches to Self-Regulation; Gendolla, G.H.E., Tops, M., Koole, S.L., Eds.; Springer: New York, NY, USA, 2015; pp. 69–85. [Google Scholar]
- Tops, M.; Quirin, M.; Boksem, M.A.; Koole, S.L. Large-scale neural networks and the lateralization of motivation and emotion. Int. J. Psychophysiol. 2017, 119, 41–49. [Google Scholar] [CrossRef]
- Tsujii, T.; Watanabe, S. Neural correlates of dual-task effect on belief-bias syllogistic reasoning: A near-infrared spectroscopy study. Brain Res. 2009, 1287, 118–125. [Google Scholar] [CrossRef]
- Tsujii, T.; Masuda, S.; Akiyama, T.; Watanabe, S. The role of inferior frontal cortex in belief-bias reasoning: An rTMS study. Neuropsychologia 2010, 48, 2005–2008. [Google Scholar] [CrossRef]
- Tsujii, T.; Sakatani, K.; Masuda, S.; Akiyama, T.; Watanabe, S. Evaluating the roles of the inferior frontal gyrus and superior parietal lobule in deductive reasoning: An rTMS study. NeuroImage 2011, 58, 640–646. [Google Scholar] [CrossRef]
- Tops, M.; Boksem, M.A.; Luu, P.; Tucker, D.M. Brain substrates of behavioral programs associated with self-regulation. Front. Psychol. 2010, 1, 152. [Google Scholar] [CrossRef]
- Tops, M.; Boksem, M.A.; Quirin, M.; IJzerman, H.; Koole, S.L. Internally directed cognition and mindfulness: An integrative perspective derived from predictive and reactive control systems theory. Front. Psychol. 2014, 5, 429. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M..; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef] [PubMed]
- Poldrack, R.A.; Fletcher, P.C.; Henson, R.N.; Worsley, K.J.; Brett, M.; Nichols, T.E. Guidelines for reporting an fMRI study. Neuroimage 2008, 40, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Pando-Naude, V.; Patyczek, A.; Bonetti, L.; Vuust, P. An ALE meta-analytic review of top-down and bottom-up processing of music in the brain. Sci. Rep. 2021, 11, 20813. [Google Scholar] [CrossRef]
- Laird, A.R.; Fox, P.M.; Price, C.J.; Glahn, D.C.; Uecker, A.M.; Lancaster, J.L.; Turkeltaub, P.E.; Kochunov, P.; Fox, P.T. ALE meta-analysis: Controlling the false discovery rate and performing statistical contrasts. Hum. Brain Mapp. 2005, 25, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Eickhoff, S.B.; Bzdok, D.; Laird, A.R.; Kurth, F.; Fox, P.T. Activation likelihood estimation meta-analysis revisited. NeuroImage 2012, 59, 2349–2361. [Google Scholar] [CrossRef] [PubMed]
- Turkeltaub, P.E.; Eden, G.F.; Jones, K.M.; Zeffiro, T.A. Meta-analysis of the functional neuroanatomy of single-word reading: Method and validation. NeuroImage 2002, 16, 765–780. [Google Scholar] [CrossRef]
- Turkeltaub, P.E.; Eickhoff, S.B.; Laird, A.R.; Fox, M.; Wiener, M.; Fox, P. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Hum. Brain Mapp. 2012, 33, 1–13. [Google Scholar] [CrossRef]
- Eickhoff, S.B.; Laird, A.R.; Grefkes, C.; Wang, L.E.; Zilles, K.; Fox, P.T. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: A random-effects approach based on empirical estimates of spatial uncertainty. Hum. Brain Mapp. 2009, 30, 2907–2926. [Google Scholar] [CrossRef]
- Goel, V.; Dolan, R.J. Explaining modulation of reasoning by belief. Cognition 2003, 87, B11–B22. [Google Scholar] [CrossRef]
- Canessa, N.; Gorini, A.; Cappa, S.F.; Piattelli-Palmarini, M.; Danna, M.; Fazio, F.; Perani, D. The effect of social content on deductive reasoning: An fMRI study. Hum. Brain. Mapp. 2005, 26, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Beierholm, U.R.; Anen, C.; Quartz, S.; Bossaerts, P. Separate encoding of model-based and model-free valuations in the human brain. NeuroImage 2011, 58, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, M.; Jou, J.; Wu, X.; Li, W.; Qiu, J. Neural bases of falsification in conditional proposition testing: Evidence from an fMRI study. Int. J. Psychophysiol. 2012, 85, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Liang, P.; Goel, V.; Jia, X.; Li, K. Different neural systems contribute to semantic bias and conflict detection in the inclusion fallacy task. Front. Hum. Neurosci. 2014, 8, 797. [Google Scholar] [CrossRef] [PubMed]
- Osherson, D.N.; Smith, E.E.; Wilkie, O.; López, A.; Shafir, E. Category-based induction. Psychol. Rev. 1990, 97, 185–200. [Google Scholar] [CrossRef]
- Liang, P.; Jia, X.; Taatgen, N.A.; Zhong, N.; Li, K. Different strategies in solving series completion inductive reasoning problems: An fMRI and computational study. Int. J. Psychophysiol. 2014, 93, 253–260. [Google Scholar] [CrossRef]
- Luo, J.; Tang, X.; Zhang, E.; Stupple, E.J. The neural correlates of belief-bias inhibition: The impact of logic training. Biol. Psychol. 2014, 103, 276–282. [Google Scholar] [CrossRef]
- von Helversen, B.; Karlsson, L.; Rasch, B.; Rieskamp, J. Neural substrates of similarity and rule-based strategies in judgment. Front. Hum. Neurosci. 2014, 8, 809. [Google Scholar] [CrossRef]
- Durning, S.J.; Costanzo, M.E.; Artino, A.R.; Graner, J.; van der Vleuten, C.; Beckman, T.J.; Wittich, C.M.; Roy, M.J.; Holmboe, E.S.; Schuwirth, L. Neural basis of nonanalytical reasoning expertise during clinical evaluation. Brain Behav. 2015, 5, e00309. [Google Scholar] [CrossRef]
- Megías, A.; Navas, J.F.; Petrova, D.; Cándido, A.; Maldonado, A.; Garcia-Retamero, R.; Catena, A. Neural mechanisms underlying urgent and evaluative behaviors: An fMRI study on the interaction of automatic and controlled processes. Hum. Brain Mapp. 2015, 36, 2853–2864. [Google Scholar] [CrossRef]
- Pennycook, G.; Cheyne, J.A.; Barr, N.; Koehler, D.J.; Fugelsang, J.A. Cognitive style and religiosity: The role of conflict detection. Mem. Cognit. 2014, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, B.; de Bruin, A.B.H.; Marsman, J.C.; Lorist, M.M.; Schmidt, H.G.; Aleman, A.; Snoek, J.W. Thinking fast or slow? Functional magnetic resonance imaging reveals stronger connectivity when experienced neurologists diagnose ambiguous cases. Brain Commun. 2020, 2, fcaa023. [Google Scholar] [CrossRef] [PubMed]
- Talairach, J.; Tournoux, P. Co-Planar Stereotaxic Atlas of the Human Brain; Thieme Medical Publishers: Tokyo, Japan, 1988; Volume 270, p. 90128-5. [Google Scholar]
- Goel, V.; Gold, B.; Kapur, S.; Houle, S. The seats of reason? An imaging study of deductive and inductive reasoning. NeuroReport 1997, 8, 1305–1310. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.; Gold, B.; Kapur, S.; Houle, S. Neuroanatomical correlates of human reasoning. J. Cogn. Neurosci. 1998, 10, 293–302. [Google Scholar] [CrossRef]
- Swann, N.C.; Tandon, N.; Pieters, T.A.; Aron, A.R. Intracranial electroencephalography reveals different temporal profiles for dorsal- and Ventro-lateral prefrontal cortex in preparing to stop action. Cereb. Cortex 2013, 23, 2479–2488. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
- Botvinick, M.M.; Braver, T.S.; Barch, D.M.; Carter, C.S.; Cohen, J.D. Conflict monitoring and cognitive control. Psychol. Rev. 2001, 108, 624–652. [Google Scholar] [CrossRef]
- Eckert, M.A.; Menon, V.; Walczak, A.; Ahlstrom, J.; Denslow, S.; Horwitz, A.; Dubno, J.R. At the heart of the ventral attention system: The right anterior insula. Hum. Brain Mapp. 2009, 30, 2530–2541. [Google Scholar] [CrossRef]
- Kinomura, S.; Larsson, J.; Guly, S.B.; Roland, P.E. Activation by attention of the human reticular formation and thalamic Intralaminar nuclei. Science 1996, 271, 512–515. [Google Scholar] [CrossRef]
- Taylor, K.S.; Seminowicz, D.A.; Davis, K.D. Two systems of resting state connectivity between the insula and cingulate cortex. Hum. Brain Mapp. 2009, 30, 2731–2745. [Google Scholar] [CrossRef] [PubMed]
- Yanaka, H.T.; Saito, D.N.; Uchiyama, Y.; Sadato, N. Neural substrates of phasic alertness: A functional magnetic resonance imaging study. Neurosci. Res. 2010, 68, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Bunge, S.A.; Dudukovic, N.M.; Thomason, M.E.; Vaidya, C.J.; Gabrieli, J.D. Immature frontal lobe contributions to cognitive control in children: Evidence from fMRI. Neuron 2022, 33, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Lenge, M.; Bartolini, E.; Bianchi, A.; Agovi, H.; Mugnai, F.; Guerrini, R.; Giordano, F.; Viggiano, M.P.; Mascalchi, M. Left inferior frontal cortex can compensate the inhibitory functions of right inferior frontal cortex and pre-supplementary motor area. J. Neuropsychol. 2018, 13, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Sharot, T.; Kanai, R.; Marston, D.; Korn, C.W.; Rees, G.; Dolan, R.J. Selectively altering belief formation in the human brain. Proc. Natl. Acad. Sci. USA 2012, 109, 17058–17062. [Google Scholar] [CrossRef] [PubMed]
- Carver, C.S.; Johnson, S.L.; Joormann, J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychol. Bull. 2008, 134, 912–943. [Google Scholar] [CrossRef]
- Pan, N.; Wang, S.; Zhao, Y.; Lai, H.; Qin, K.; Li, J.; Biswal, B.B.; Sweeney, J.A.; Gong, Q. Brain gray matter structures associated with trait impulsivity: A systematic review and voxel-based meta-analysis. Hum. Brain Mapp. 2021, 42, 2214–2235. [Google Scholar] [CrossRef]
- Baron, J.; Scott, S.; Fincher, K.; Metz, S.E. Why does the Cognitive Reflection Test (sometimes) predict utilitarian moral judgment (and other things)? J. Appl. Res. Mem. Cogn. 2015, 4, 265–284. [Google Scholar] [CrossRef]
- Littrell, S.; Fugelsang, J.; Risko, E.F. Not so fast: Individual differences in impulsiveness are only a modest predictor of cognitive reflection. Pers. Individ. Differ. 2020, 154, 109678. [Google Scholar] [CrossRef]
- Oldrati, V.; Patricelli, J.; Colombo, B.; Antonietti, A. The role of dorsolateral prefrontal cortex in inhibition mechanism: A study on cognitive reflection test and similar tasks through neuromodulation. Neuropsychology 2016, 91, 499–508. [Google Scholar] [CrossRef]
- Aron, A.R. From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biol. Psychiatry 2011, 69, e55–e68. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, D.J.; Pekar, J.J.; Mostofsky, S.H. Meta-analysis of go/no-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychology 2008, 46, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Jonides, J.; Irwin, D.E. Capturing attention. Cognition 1981, 10, 145–150. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kahneman, D.; Frederick, S. Representativeness revisited: Attribute substitution in intuitive judgment. In Heuristics and Biases: The Psychology of Intuitive Judgment; Gilovich, T., Griffin, D., Kahneman, D., Eds.; Cambridge University Press: Cambridge, UK, 2002; pp. 49–81. [Google Scholar]
- Schneider, W.; Schiffrin, R.M. Automatic vs. controlled processing. Psychol. Rev. 1977, 84, 1–64. [Google Scholar] [CrossRef]
- Dorigoni, A.; Rajsic, J.; Bonini, N. Does cognitive reflection predict attentional control in visual tasks? Acta Psychol. 2022, 226, 103562. [Google Scholar] [CrossRef]
- Attali, Y.; Bar-Hillel, M. The false allure of fast lures. Judgm. Decis. Mak. 2020, 15, 93–111. [Google Scholar] [CrossRef]
- Erceg, N.; Galic, Z.; Ružojcic, M. A reflection on cognitive reflection-testing convergent/divergent validity of two measures of cognitive reflection. Judgm. Decis. Mak. 2020, 15, 741–755. [Google Scholar] [CrossRef]

| Study | Year | Sample | Task | fMRI | ||||
|---|---|---|---|---|---|---|---|---|
| Number | Age (Mean, SD or Range) | Gender (f/m) | Contrast | Number of Foci | ||||
| 1 | Goel et al. [66] | 2003 | 14 | 30.8 ± 4.3 | 7/7 | Deductive reasoning task. A total of 120 syllogisms (15 different forms) and 40 baseline items organized in a nested 2 × 2 design (Belief × Task). The belief factor consisted of two levels: belief-laden (80 syllogisms and 40 baseline items) and belief-neutral (40 syllogisms and 20 baseline items) items. In the task factor, the first level (a reasoning condition) involved stimuli that constituted arguments (120 trials, as in the examples above and below). Half of these were valid, while the other half were not valid. The second-level (baseline condition) trials were generated by taking these arguments and switching around the third sentence, such that the three sentences did not constitute arguments. | Slow vs. Fast Fast vs. Slow | 1/1 |
| 2 | Canessa et al. [67] | 2005 | 12 | 23.5 (21–26) | 7/5 | Deductive reasoning task. Two versions of the Wason selection task: the first version described an arbitrary relation between two actions (descriptive (DES): “If someone does …, then he does …”), whereas the other described an exchange of goods between two persons (social exchange (SE): “If you give me …, then I give you …”). | Fast vs. Slow | 9 |
| 3 | Beierholm et al. [68] | 2011 | 23 | n.r. | n.r. | Novel economic task, where three doors were visually presented. Participants were instructed to choose the order of the doors. After 6–8 s, the location of the money was revealed behind one of the doors, and subjects were rewarded according to the following: 0.50 USD if the money was behind their first choice, 0 USD if it was behind their second choice, and −0.50 USD if the money was behind the third choice. They were explicitly instructed to ignore anything they learned about the distribution of money and that the sequence of locations for the money was random. Behavioral data were employed to fit two models aimed at quantifying subjective valuation and updating signals corresponding to fast and slow thinking. | Fast vs. Slow | 40 |
| 4 | Liu et al. [69] | 2012 | 14 | 21.8 (17–25) | 6/8 | Deductive reasoning task. Twenty-eight conditional reasoning statements (based on the Wason selection task). | Slow vs. Fast | 9 |
| 5 | Liang et al. [70] | 2014 | 15 | 23.6 ± 3.1 | 7/8 | Inductive reasoning task. One hundred twenty trials of a categorical induction task (modeled on stimuli from Osherson et al., 1990 [71]). Each trial was composed of pairs of arguments, and participants were instructed to indicate which one of the two arguments was stronger. Stimuli were divided into two conditions (explicit quantification vs. implicit quantification). Subjects’ responses to each trial were used to further divide the stimuli into fallacy or non-fallacy response trials. | Fast vs. Slow | 6 |
| 6 | Liang et al. [72] | 2014 | 23 | 24.1 ± 3.7 | 11/12 | Inductive reasoning task. Thirty number-series induction tasks, thirty letter-series induction tasks, twenty-four number judgment baseline tasks and twenty-four letter judgment baseline tasks were organized into a 2 × 2 factorial design (Content × Task). Content factor: number-related vs. letter-related content. Task factor: series completion task vs. baseline condition. | Fast vs. Slow | 17 |
| 7 | Luo et al. [73] | 2014 | 16 | 23 (20–28) | 8/8 | Deductive reasoning task. One hundred twenty items (encompassing four different conditional reasoning forms) for the condition. Participants were required to draw a conclusion based on the premises. This study was organized into a 2 × 2 design (Type of Problem × Logical Training). Type-of-problem factor: conflict problems (in which the logical conclusion is inconsistent with one’s beliefs) and non-conflict problems (in which the logical conclusion is consistent with one’s beliefs). Logical training factor: naive participants vs. post logic training. | Slow vs. Fast | 5 |
| 8 | von Helversen et al. [74] | 2014 | 23 | 20.13 ± 2.67 | 17/6 | Categorial induction task. Participants were required to make fictitious quantitative judgements on 9 items (3 for each scenario) using a scale with 100 possible values. Each item was described by six binary cues and a criterion value. The task was based on learning to estimate the correct criterion value of items given the item’s cue values. Participants were instructed to use either a similarity-based exemplar strategy or a rule-based strategy. The actual use of the two strategies was determined by means of a computational model. | Slow vs. Fast Fast vs. Slow | 7/9 |
| 9 | Durning et al. [75] | 2015 | 10 * | 29.6 ± 2 | 3/7 | Medical diagnosis task. Participants were presented with medical scenarios, and they were required to answer “what is the most likely diagnosis?” by choosing among 5 options. Participants were then given seven seconds to choose an answer option using finger response items, which would be expected to require both analytical and non-analytical reasoning. The final phase (“reflection” phase) was then entered; in this phase, participants were instructed to reflect on how they had arrived at the diagnosis, which primarily required (or accentuated) analytical reasoning. | Fast vs. Slow | 17 |
| 10 | Megìas et al. [76] | 2015 | 56 | 22.24 ± 2.7 | 39/17 | Novel risky driving evaluation task. Participants performed an urgent task (to brake or not in a given traffic situation) and an evaluative task (to evaluate whether the traffic situation entailed risk or not) during the experiment. Each task comprised 140 trials (70 risky situations and 70 non-risky situations). | Fast vs. Slow | 21 |
| 11 | Vartanian et al. [49] | 2018 | 44 | 35.5 ± 11.3 | 13/31 | Probabilistic reasoning task. Forty-eight base rate problems (24 conflict, 24 non-conflict) selected from Cheyne, et al.’s (2014) [77] item pool. | Slow vs. Fast | 7 |
| 12 | van den Berg et al. [78] | 2020 | 16 | 51 (46–57) | 4/12 | Novel medical diagnosis task. Participants were required to diagnose 26 neurological cases. Each case had both fast-thinking (prototypical information) and slow-thinking (ambiguous information) versions to elicit the different types of reasoning. | Slow vs. Fast Fast vs. Slow | 8/9 |
| Cluster | x | y | z | p | Z | Label (Nearest Gray Matter within 5 mm) |
|---|---|---|---|---|---|---|
| 1 | −2 | 26 | 44 | 5.20 × 10−7 | 4.884085 | Left cerebrum. Frontal lobe. Medial frontal gyrus. Gray matter. Brodmann area 8 |
| 12 | 30 | 52 | 4.88 × 10−4 | 3.2970774 | Right cerebrum. Frontal lobe. Superior frontal gyrus. Gray matter. Brodmann area 6 | |
| 8 | 24 | 34 | 0.0013236 | 3.0059886 | Right cerebrum. Frontal lobe. Cingulate gyrus. Gray matter. Brodmann area 32 | |
| 2 | −30 | 24 | 4 | 1.68 × 10−6 | 4.6475234 | Left cerebrum. Sub-lobar. Insula. Gray matter. Brodmann area 13 |
| −34 | 30 | −2 | 9.45 × 10−4 | 3.1068544 | Left cerebrum. Frontal lobe. Inferior frontal gyrus. Gray matter. Brodmann area 45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gronchi, G.; Gavazzi, G.; Viggiano, M.P.; Giovannelli, F. Dual-Process Theory of Thought and Inhibitory Control: An ALE Meta-Analysis. Brain Sci. 2024, 14, 101. https://doi.org/10.3390/brainsci14010101
Gronchi G, Gavazzi G, Viggiano MP, Giovannelli F. Dual-Process Theory of Thought and Inhibitory Control: An ALE Meta-Analysis. Brain Sciences. 2024; 14(1):101. https://doi.org/10.3390/brainsci14010101
Chicago/Turabian StyleGronchi, Giorgio, Gioele Gavazzi, Maria Pia Viggiano, and Fabio Giovannelli. 2024. "Dual-Process Theory of Thought and Inhibitory Control: An ALE Meta-Analysis" Brain Sciences 14, no. 1: 101. https://doi.org/10.3390/brainsci14010101
APA StyleGronchi, G., Gavazzi, G., Viggiano, M. P., & Giovannelli, F. (2024). Dual-Process Theory of Thought and Inhibitory Control: An ALE Meta-Analysis. Brain Sciences, 14(1), 101. https://doi.org/10.3390/brainsci14010101






