A 20-Year Systematic Review of the ‘Reading the Mind in the Eyes’ Test across Neurodegenerative Conditions
Abstract
:1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion Criteria
2.3. Exclusion Criteria
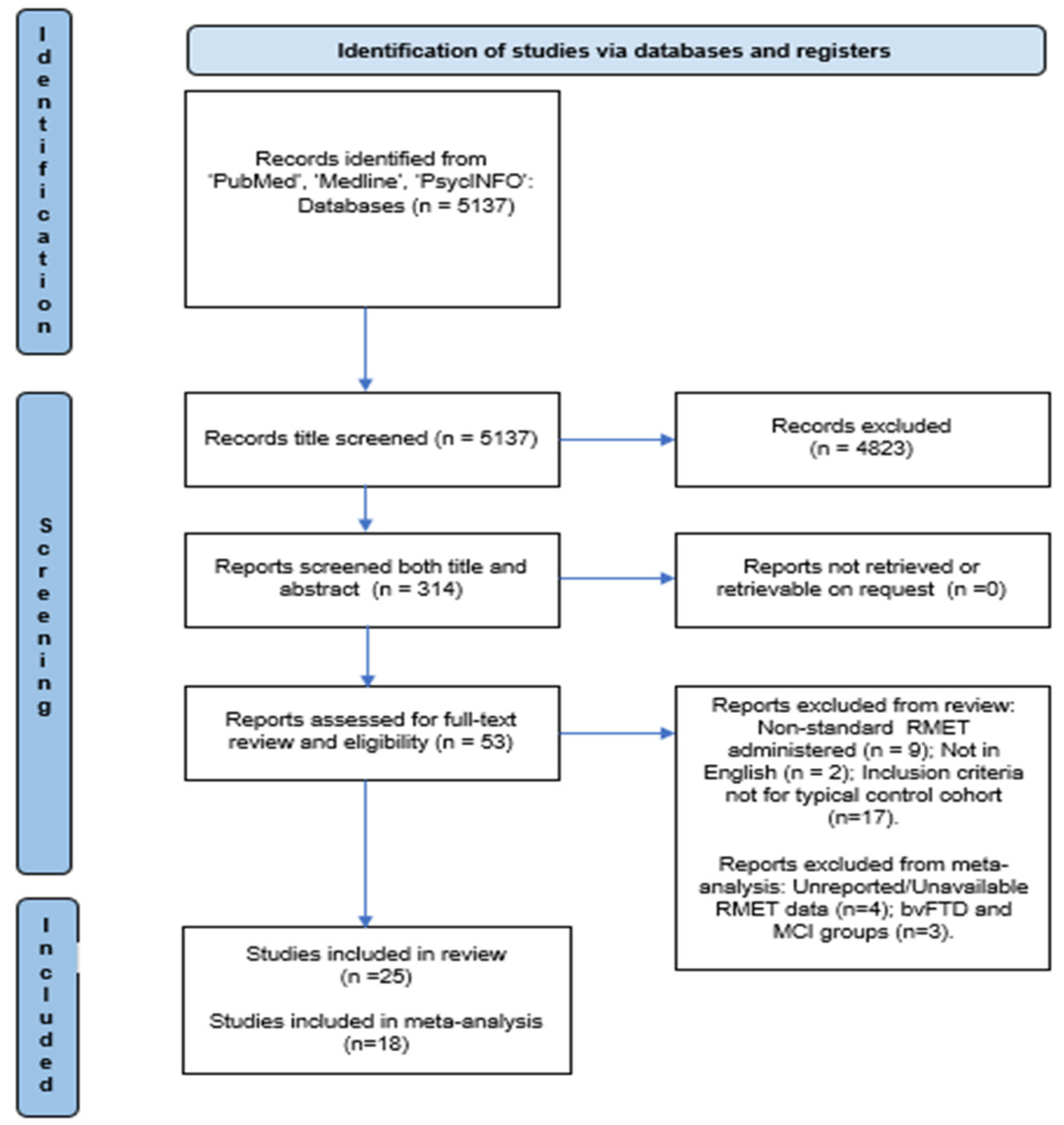
2.4. Study Selection
2.5. Risk-of-Bias Assessment and Quality of Evidence
2.6. Data Synthesis
2.7. Meta-Analysis
3. Results
3.1. Studies Selected
3.2. Description of Studies Included
3.3. Risk of Bias and Quality Evidence
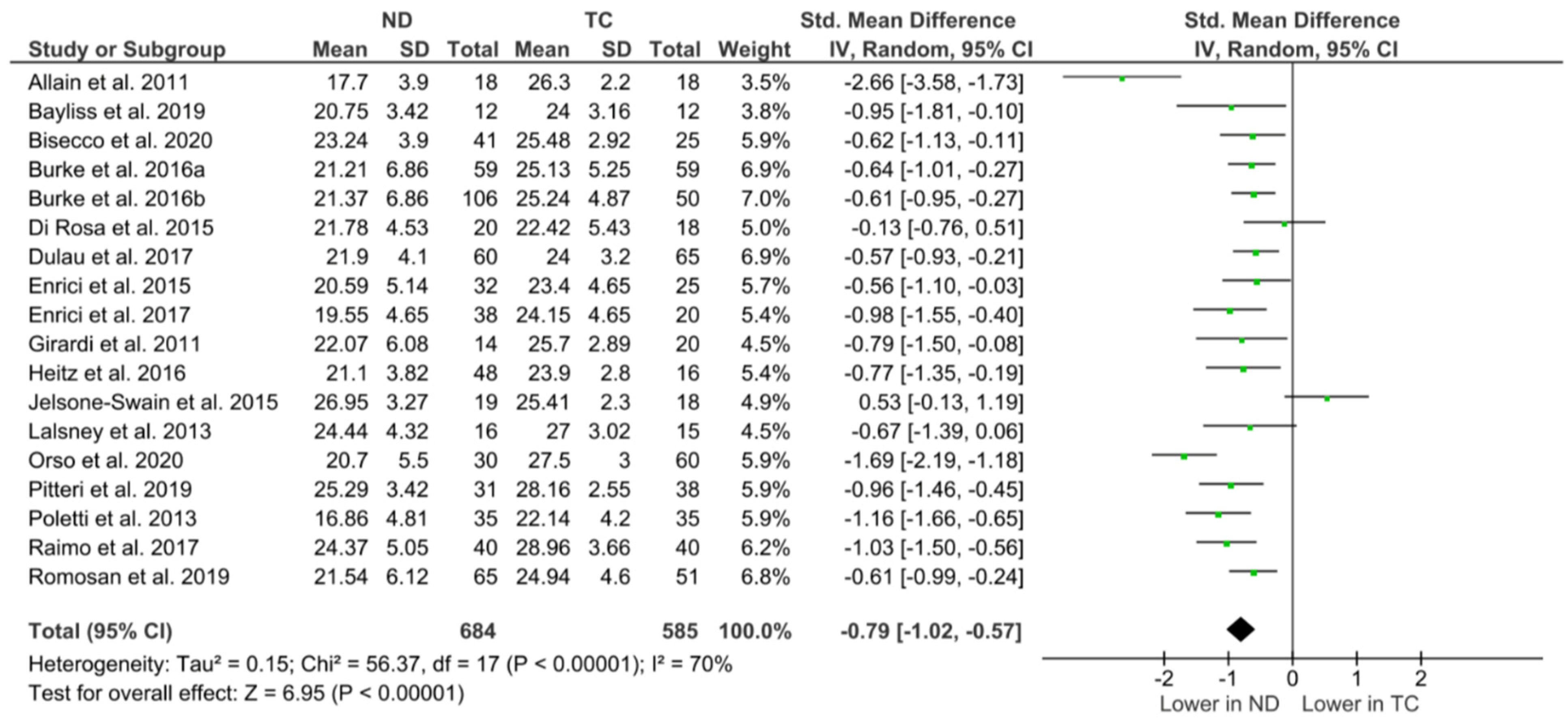
3.4. RMET Performance
Meta-Analysis and Typical Control Outcomes
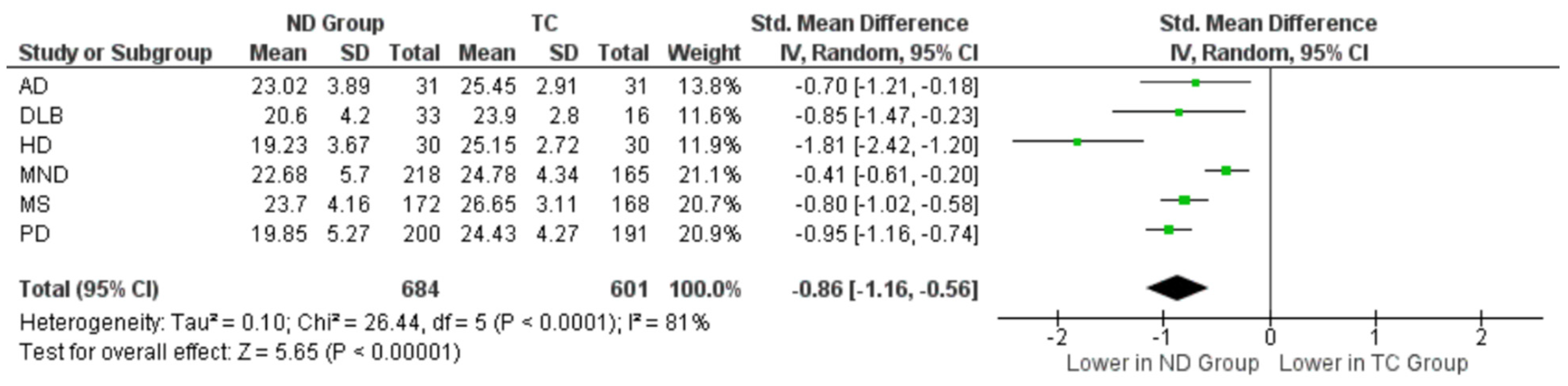
3.5. Specific Outcomes across Neurodegenerative Cohorts
3.5.1. Alzheimer’s Disease
3.5.2. Dementia with Lewy Bodies (DLB)
3.5.3. Huntington’s Disease (HD)
3.5.4. Motor Neuron Disease
3.5.5. Multiple Sclerosis (MS)
3.5.6. Parkinson’s Disease
4. Discussion
4.1. Summary of Main Findings
4.2. Strengths, Limitations, and Future Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bicks, L.K.; Koike, H.; Akbarian, S.; Morishita, H. Prefrontal Cortex and Social Cognition in Mouse and Man. Front. Psychol. 2015, 6, 1805. [Google Scholar] [CrossRef]
- Kilford, E.J.; Garrett, E.; Blakemore, S.-J. The development of social cognition in adolescence: An integrated perspective. Neurosci. Biobehav. Rev. 2016, 70, 106–120. [Google Scholar] [CrossRef]
- Sofologi, M.; Koutsouraki, E.; Tsolaki, M.; Tsolaki, A.; Koukoulidis, T.; Theofilidis, A.; Papantoniou, G.; Moraitou, D. Analyzing social cognition and understanding of social inferences in patients with multiple sclerosis. A comparative study. Hell. J. Nucl. Med. 2019, 22 (Suppl. 2), 15–26. [Google Scholar]
- Coundouris, S.P.; Adams, A.G.; Henry, J.D. Empathy and theory of mind in Parkinson’s disease: A meta-analysis. Neurosci. Biobehav. Rev. 2020, 109, 92–102. [Google Scholar] [CrossRef]
- Mitchell, R.L.; Phillips, L.H. The overlapping relationship between emotion perception and theory of mind. Neuropsychologia 2015, 70, 1–10. [Google Scholar] [CrossRef]
- Cotter, J.; Granger, K.; Backx, R.; Hobbs, M.; Looi, C.Y.; Barnett, J.H. Social cognitive dysfunction as a clinical marker: A systematic review of meta-analyses across 30 clinical conditions. Neurosci. Biobehav. Rev. 2018, 84, 92–99. [Google Scholar] [CrossRef]
- Altschuler, M.R.; Trevisan, D.A.; Wolf, J.M.; Naples, A.J.; Foss-Feig, J.H.; Srihari, V.H.; McPartland, J.C. Face perception predicts affective theory of mind in autism spectrum disorder but not schizophrenia or typical development. J. Abnorm. Psychol. 2021, 130, 413–422. [Google Scholar] [CrossRef]
- Péron, J.; Vicente, S.; Leray, E.; Drapier, S.; Drapier, D.; Cohen, R.; Biseul, I.; Rouaud, T.; Le Jeune, F.; Sauleau, P.; et al. Are dopaminergic pathways involved in theory of mind? A study in Parkinson’s disease. Neuropsychologia 2009, 47, 406–414. [Google Scholar] [CrossRef]
- Mckinlay, A.; Albicini, M.; Kavanagh, P.S. The effect of cognitive status and visuospatial performance on affective theory of mind in Parkinson’s disease. Neuropsychiatr. Dis. Treat. 2013, 9, 1071–1076. [Google Scholar] [CrossRef]
- Bodden, M.E.; Mollenhauer, B.; Trenkwalder, C.; Cabanel, N.; Eggert, K.M.; Unger, M.M.; Oertel, W.H.; Kessler, J.; Dodel, R.; Kalbe, E. Affective and cognitive theory of mind in patients with parkinson’s disease. Park. Relat. Disord. 2010, 16, 466–470. [Google Scholar] [CrossRef]
- Sullivan, S.; Herzig, D.; Mohr, C.; Lewis, G.; Corcoran, R.; Drake, R.; Evans, J. Theory of mind and social functioning in first episode psychosis. Cogn. Neuropsychiatry 2013, 18, 219–242. [Google Scholar] [CrossRef]
- Shany-Ur, T.; Poorzand, P.; Grossman, S.N.; Growdon, M.E.; Jang, J.Y.; Ketelle, R.S.; Miller, B.L.; Rankin, K.P. Comprehension of insincere communication in neurodegenerative disease: Lies, sarcasm, and theory of mind. Cortex 2012, 48, 1329–1341. [Google Scholar] [CrossRef]
- Mason, S.L.; Zhang, J.; Begeti, F.; Guzman, N.V.; Lazar, A.S.; Rowe, J.B.; Barker, R.A.; Hampshire, A. The role of the amygdala during emotional processing in Huntington’s disease: From pre-manifest to late stage disease. Neuropsychologia 2015, 70, 80–89. [Google Scholar] [CrossRef]
- Raimo, S.; Trojano, L.; Pappacena, S.; Alaia, R.; Spitaleri, D.; Grossi, D.; Santangelo, G. Neuropsychological correlates of theory of mind deficits in patients with multiple sclerosis. Neuropsychology 2017, 31, 811–821. [Google Scholar] [CrossRef]
- Girardi, A.; MacPherson, S.E.; Abrahams, S. Deficits in emotional and social cognition in amyotrophic lateral sclerosis. Neuropsychology 2011, 25, 53–65. [Google Scholar] [CrossRef]
- Enrici, I.; Adenzato, M.; Ardito, R.B.; Mitkova, A.; Cavallo, M.; Zibetti, M.; Lopiano, L.; Castelli, L. Emotion Processing in Parkinson’s Disease: A Three-Level Study on Recognition, Representation, and Regulation. PLoS ONE 2015, 10, e0131470. [Google Scholar] [CrossRef]
- Hiser, J.; Koenigs, M. The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol. Psychiatry 2018, 83, 638–647. [Google Scholar] [CrossRef]
- Poletti, M.; Vergallo, A.; Ulivi, M.; Sonnoli, A.; Bonuccelli, U. Affective theory of mind in patients with Parkinson’s disease. Psychiatry Clin. Neurosci. 2013, 67, 273–276. [Google Scholar] [CrossRef]
- Henry, J.D.; von Hippel, W.; Molenberghs, P.; Lee, T.; Sachdev, P.S. Clinical assessment of social cognitive function in neurological disorders. Nat. Rev. Neurol. 2016, 12, 28–39. [Google Scholar] [CrossRef]
- Olderbak, S.; Wilhelm, O. Overarching Principles for the Organization of Socioemotional Constructs. Curr. Dir. Psychol. Sci. 2020, 29, 63–70. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S.; Hill, J.; Raste, Y.; Plumb, I. The “Reading the Mind in the Eyes” Test revised version: A study with normal adults, and adults with Asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry Allied Discip. 2001, 42, 241–251. [Google Scholar] [CrossRef]
- Wimmer, H.; Perner, J. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children’s understanding of deception. Cognition 1983, 13, 103–128. [Google Scholar] [CrossRef]
- Stone, V.E.; Baron-Cohen, S.; Knight, R.T. Frontal Lobe Contributions to Theory of Mind. J. Cogn. Neurosci. 1998, 10, 640–656. [Google Scholar] [CrossRef]
- Elamin, M.; Pender, N.; Hardiman, O.; Abrahams, S. Social cognition in neurodegenerative disorders: A systematic review. J. Neurol. Neurosurg. Psychiatry 2012, 83, 1071–1079. [Google Scholar] [CrossRef]
- Gallant, C.; Good, D. Examining the “reading the mind in the eyes test” as an assessment of subtle differences in affective theory of mind after concussion. Clin. Neuropsychol. 2020, 34, 296–317. [Google Scholar] [CrossRef]
- Burke, T.; Pinto-Grau, M.; Costello, E.; Peelo, C.; Lonergan, K.; Heverin, M.; Hardiman, O.; Pender, N. The reading the mind in the eyes test short form (A & B): Validation and outcomes in an amyotrophic lateral sclerosis cohort. Amyotroph. Lateral Scler. Front. Degener. 2020, 21, 380–388. [Google Scholar] [CrossRef]
- Carroll, G.A.; Montrose, V.T.; Burke, T. Correlates of Social Cognition and Psychopathic Traits in a Community-Based Sample of Males. Front. Psychol. 2021, 12, 656299. [Google Scholar] [CrossRef]
- Carey, E.; Gillan, D.; Burke, T.; Burns, A.; Murphy, T.M.; Kelleher, I.; Cannon, M. Social cognition and self-reported ASD traits in young adults who have reported psychotic experiences: A population-based, longitudinal study. Schizophr. Res. 2021, 237, 54–61. [Google Scholar] [CrossRef]
- Hudson, C.C.; Shamblaw, A.L.; Harkness, K.L.; Sabbagh, M.A. Valence in the Reading the Mind in the Eyes task. Psychol. Assess. 2020, 32, 623–634. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef]
- Hopewell, S.; Clarke, M.; Mallett, S. Grey literature and systematic reviews. In Publication Bias in Meta-Analysis: Prevention, Assessment and Adjustments; John Wiley & Sons: Hoboken, NJ, USA, 2005; pp. 48–72. [Google Scholar]
- Hawker, S.; Payne, S.; Kerr, C.; Hardey, M.; Powell, J. Appraising the Evidence: Reviewing Disparate Data Systematically. Qual. Health Res. 2002, 12, 1284–1299. [Google Scholar] [CrossRef]
- Huguet, A.; Hayden, J.A.; Stinson, J.; McGrath, P.J.; Chambers, C.T.; Tougas, M.E.; Wozney, L. Judging the quality of evidence in reviews of prognostic factor research: Adapting the GRADE framework. Syst. Rev. 2013, 2, 71. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Allain, P.; Havet-Thomassin, V.; Verny, C.; Gohier, B.; Lancelot, C.; Besnard, J.; Fasotti, L.; Le Gall, D. Evidence for deficits on different components of theory of mind in Huntington’s disease. Neuropsychology 2011, 25, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Laisney, M.; Bon, L.; Guiziou, C.; Daluzeau, N.; Eustache, F.; Desgranges, B. Cognitive and affective Theory of Mind in mild to moderate Alzheimer’s disease. J. Neuropsychol. 2013, 7, 107–120. [Google Scholar] [CrossRef]
- Eddy, C.M.; Mahalingappa, S.S.; Rickards, H.E. Putting things into perspective: The nature and impact of theory of mind impairment in Huntington’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2014, 264, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Di Rosa, E.; Sorarù, G.; Kleinbub, J.R.; Calvo, V.; Vallesi, A.; Querin, G.; Marcato, S.; Grasso, I.; Palmieri, A. Theory of mind, empathy and neuropsychological functioning in X-linked Spinal and Bulbar Muscular Atrophy: A controlled study of 20 patients. J. Neurol. 2015, 262, 394–401. [Google Scholar] [CrossRef]
- Jelsone-Swain, L.; Persad, C.; Burkard, D.; Welsh, R.C. Action Processing and Mirror Neuron Function in Patients with Amyotrophic Lateral Sclerosis: An fMRI Study. PLoS ONE 2015, 10, e0119862. [Google Scholar] [CrossRef]
- Eddy, C.M.; Rickards, H.E. Theory of mind can be impaired prior to motor onset in Huntington’s disease. Neuropsychology 2015, 29, 792–798. [Google Scholar] [CrossRef]
- Heitz, C.; Noblet, V.; Phillipps, C.; Cretin, B.; Vogt, N.; Philippi, N.; Kemp, J.; de Petigny, X.; Bilger, M.; Demuynck, C.; et al. Cognitive and affective theory of mind in dementia with Lewy bodies and Alzheimer’s disease. Alzheimer’s Res. Ther. 2016, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Burke, T.; Elamin, M.; Bede, P.; Pinto-Grau, M.; Lonergan, K.; Hardiman, O.; Pender, N. Discordant performance on the ‘Reading the Mind in the Eyes’ Test, based on disease onset in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Front. Degener. 2016, 17, 467–472. [Google Scholar] [CrossRef]
- Burke, T.; Pinto-Grau, M.; Lonergan, K.; Elamin, M.; Bede, P.; Costello, E.; Hardiman, O.; Pender, N. Measurement of Social Cognition in Amyotrophic Lateral Sclerosis: A Population Based Study. PLoS ONE 2016, 11, e0160850. [Google Scholar] [CrossRef] [PubMed]
- Dulau, C.; Deloire, M.; Diaz, H.; Saubusse, A.; Charre-Morin, J.; Prouteau, A.; Brochet, B. Social cognition according to cognitive impairment in different clinical phenotypes of multiple sclerosis. J. Neurol. 2017, 264, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Enrici, I.; Mitkova, A.; Castelli, L.; Lanotte, M.; Lopiano, L.; Adenzato, M. Deep Brain Stimulation of the subthalamic nucleus does not negatively affect social cognitive abilities of patients with Parkinson’s disease. Sci. Rep. 2017, 7, 9413. [Google Scholar] [CrossRef]
- Pitteri, M.; Genova, H.; Lengenfelder, J.; DeLuca, J.; Ziccardi, S.; Rossi, V.; Calabrese, M. Social cognition deficits and the role of amygdala in relapsing remitting multiple sclerosis patients without cognitive impairment. Mult. Scler. Relat. Disord. 2019, 29, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Realmuto, S.; Dodich, A.; Meli, R.; Canessa, N.; Ragonese, P.; Salemi, G.; Cerami, C. Moral Cognition and Multiple Sclerosis: A Neuropsychological Study. Arch. Clin. Neuropsychol. 2019, 34, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Romosan, A.-M.; Dehelean, L.; Romosan, R.-S.; Andor, M.; Bredicean, A.C.; Simu, M.A. Affective theory of mind in Parkinson’s disease: The effect of cognitive performance. Neuropsychiatr. Dis. Treat. 2019, 15, 2521–2535. [Google Scholar] [CrossRef] [PubMed]
- Bayliss, L.; Galvez, V.; Ochoa-Morales, A.; Chávez-Oliveros, M.; Rodríguez-Agudelo, Y.; Delgado-García, G.; Boll, M.C. Theory of mind impairment in Huntington’s disease patients and their relatives. Arq. Neuro Psiquiatr. 2019, 77, 574–578. [Google Scholar] [CrossRef]
- Bisecco, A.; Altieri, M.; Santangelo, G.; Di Nardo, F.; Docimo, R.; Caiazzo, G.; Capuano, R.; Pappacena, S.; D’ambrosio, A.; Bonavita, S.; et al. Resting-State Functional Correlates of Social Cognition in Multiple Sclerosis: An Explorative Study. Front. Behav. Neurosci. 2020, 13, 276. [Google Scholar] [CrossRef]
- Orso, B.; Arnaldi, D.; Famà, F.; Girtler, N.; Brugnolo, A.; Doglione, E.; Filippi, L.; Massa, F.; Peira, E.; Bauckneht, M.; et al. Anatomical and neurochemical bases of theory of mind in de novo Parkinson’s Disease. Cortex 2020, 130, 401–412. [Google Scholar] [CrossRef]
- Bathgate, D.; Snowden, J.S.; Varma, A.; Blackshaw, A.; Neary, D. Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol. Scand. 2001, 103, 367–378. [Google Scholar] [CrossRef]
- Bozeat, S.; Gregory, C.A.; Ralph, M.A.L.; Hodges, J.R. Which neuropsychiatric and behavioural features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer’s disease? J. Neurol. Neurosurg. Psychiatry 2000, 69, 178–186. [Google Scholar] [CrossRef]
- Grace, J.; Malloy, P. Frontal Systems Behavior Scale; Psychological Assessment Resources, Inc.: Lutz, FL, USA, 2001. [Google Scholar]
- Kennedy, W.R.; Alter, M.; Sung, J.H. Progressive proximal spinal and bulbar muscular atrophy of late onset: A sex-linked recessive trait. Neurology 1998, 50, 583. [Google Scholar] [CrossRef] [PubMed]
- Bora, E. Meta-analysis of social cognition in amyotrophic lateral sclerosis. Cortex 2017, 88, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Turner, K.; Bartlett, D.; Grainger, S.A.; Eddy, C.; Reyes, A.; Kordsachia, C.; Turner, M.; Stout, J.C.; Georgiou-Karistianis, N.; Henry, J.D.; et al. Profiling Social Cognition in Premanifest Huntington’s Disease. J. Int. Neuropsychol. Soc. 2022, 28, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Batista, S.; Alves, C.; D’almeida, O.C.; Afonso, A.; Félix-Morais, R.; Pereira, J.; Macário, C.; Sousa, L.; Castelo-Branco, M.; Santana, I.; et al. Disconnection as a mechanism for social cognition impairment in multiple sclerosis. Neurology 2017, 89, 38–45. [Google Scholar] [CrossRef]
- Gregory, C.; Lough, S.; Stone, V.; Erzinclioglu, S.; Martin, L.; Baron-Cohen, S.; Hodges, J.R. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: Theoretical and practical implications. Brain 2002, 125, 752–764. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Pawelke, S.; Bisenius, S.; Kynast, J.; Schuemberg, K.; Polyakova, M.; Anderl-Straub, S.; Danek, A.; Fassbender, K.; Jahn, H.; et al. A mod-ified reading the mind in the eyes test predicts behavioral variant frontotemporal dementia better than executive function tests. Front. Aging Neurosci. 2018, 10, 11. [Google Scholar] [CrossRef]
- Tabernero, M.E.; Musich, F.; Cossini, F.C.; Politis, D.G. Social cognition in Parkinson’s disease dementia and be-havioral variant of frontotemporal dementia. Rev. Neurol. 2017, 65, 539–545. [Google Scholar]
- Torralva, T.; Kipps, C.M.; Hodges, J.R.; Clark, L.; Bekinschtein, T.; Roca, M.; Calcagno, M.L.; Manes, F. The relationship be-tween affective decision-making and theory of mind in the frontal variant of fronto-temporal dementia. Neuropsychologia 2007, 45, 342–349. [Google Scholar] [CrossRef]
| Authors (Year) | Country | Design | Sample, n (% Female) | Age in Years, Mean ± SD | Mean Years of Education ± SD | Mean Time Since Disease Onset ± SD (Time) | Mean Disease Duration ± SD (Years) |
|---|---|---|---|---|---|---|---|
| Bodden et al. [10] * | Germany | Cross-sectional | 21 PD (29%) 21 TC (29%) | 63.7 ± 10 PD 58.5 ± 10.2 TC | 14.6 ± 3.0 PD 14.8 ± 2.9 TC | - | 5.1 ± 2.8 |
| Girardi et al. [15] | Scotland | Cross-sectional | 14 ALS (29%) 20 TC (25%) | 57.4 ± 16 ALS 54.8 ± 11.5 TC | 14.4 ± 5.4 ALS 15.1 ± 3.2 TC | 38.1 ± 29.6 (months) | - |
| Allain et al. [35] | France | Cross-sectional | 18 HD (44%) 18 TC (39%) | 50.7 ± 8.8 HD 47.7 ± 8.9 TC | 11.8 ± 2.5 HD 11.1 ± 2.4 TC | - | 4.5 ± 2.9 |
| Laisney et al. [36] | France | Cross-sectional | 16 AD (69%) 15 TC (80%) | 78.1 ± 2.6 AD 76.4 ± 3.2 TC | 7.7 ± 2.5 AD 7.5 ± 1.6 TC | - | - |
| Poletti et al. [18] | Italy | Cross-sectional | 35 PD (37%) 35 TC (57%) | 68.5 ± 6.7 PD 67 ± 10 TC | 9.1 ± 3.9 PD 10.8 ± 3.8 TC | - | 6.31 ± 4 |
| Eddy et al. [37] * | UK | Cross-sectional | 30 HD (40%) 20 TC (50%) | 54.9 HD 47.3 TC | 13 ± 2.3 HD 13.9 ± 2.1 TC | - | 7.03 ± 4.9 |
| Di rosa et al. [38] | Italy | Cross-sectional | 20 SBMA (0%) 18 TC (0%) | 55.4 ± 9.4 SBMA 55.1 ± 10.3 TC | 11.4 ± 4.3 SBMA 10.3 ± 3.9 TC | - | - |
| Jelsone-swain et al. [39] | USA | Cross-sectional | 19 ALS (32%) 18 TC (39%) | 57.2 ± 8.7 ALS 59.9 ± 8.6 TC | 13.6 ± 2.1 ALS 16.3 ± 2.4 TC | 47 ± 33.7 (months) | - |
| Enrici et al. [16] | Italy | Cross-sectional | 32 PD (47%) 25 TC (56%) | 58 ± 7.2 PD 56.3 ± 6.4 TC | 9.5 ± 4 PD 10.8 ±3.8 TC | - | 10.6 ± 3.9 |
| Eddy et al. [40] * | UK | Cross-sectional | 20 HD (70%) 26 TC (69%) | 45 ± 14 HD 45.7 ± 14.4 TC | 14 ± 2.5 HD 14 ± 1.9 TC | - | - |
| Heitz et al. [41] | France | Longitudinal | 33 DLB (52%) 15 AD (47%) 16 TC (56%) | 68 ± 8.4 DLB 70.9 ± 11.1 AD 68.3 ± 10.5 TC | 12.4 ± 3.2 DLB 13.5 ± 3.6 AD 11.8 ± 3.2 TC | 4.6 ± 4.2 DLB 3.6 ± 1.8 AD | |
| Burke et al. [42] | Ireland | Cross-sectional | 106 ALS (28%) 50 TC (40%) | 60.4 ±10.8 ALS 61.4 ±9.1 TC | 12.8 ± 3.3 ALS 12.7 ± 2.9 TC | - | - |
| Burke et al. [43] | Ireland | Cross-sectional | 59 ALS (37%) (bulbar n = 20 (65%), spinal n = 39 (23%) 59 TC (56%) | 66.1 ± 8.8 bulbar-onset ALS 61.2 ± 9.1 spinal onset ALS 64.7 ± 9 TC | 12.40 ± 2.58 bulbar-onset ALS 13.46 years ± 3.49 spinal onset ALS 12.64 ± 3.07 TC | 10.8 ± 9.2 bulbar-onset ALS (months) 14.4 ± 12.7 spinal onset ALS (months) | - |
| Dulau et al. [44] | France | Longitudinal | 60 MS (NR) 65 TC (NR) | 46.5 ± 10.6 MS (40.1 ± 10.4 RRMS; 54.8 ± 11.5 PPMS; 51 ± 7.3 SPMS) 43.2 ± 9.3 TC | 12.9 ± 3.3 MS (14.2 ± 3.7 RRMS; 10.9 ± 4.6 PPMS; 12.5 ± 5.3 SPMS) 12.5 ± 2.8 TC | - | 14.4 ± 9.4 MS (11.5 ± 5.6 RRMS; 10 ± 6.5 PPMS; 18.8 ± 10 SPMS) |
| Enrici et al. [45] | Italy | Cross-sectional | 20 DRT-PD (50%) 18 STN-DBS-PD (50%) 20 TC (50%) | 59.8 ± 5.8 DRT-PD 60.9 ± 6.3 STN-DBS-PD 60 ± 7.5 TC | 7.7 ± 2.5 DRT-PD 7.4 ± 3.9 STN-DBS-PD 9.4 ± 3.3 TC | - | 11.5 ± 3 DRT-PD 12.6 ± 3 STN-DBS-PD |
| Raimo et al. [14] | Italy | Cross-sectional | 40 MS (73%) 40 TC (78%) | 40.6 ± 11.5 MS 40.2 ± 11.4 TC | 13.1 ± 3.8 MS 13 ± 3.6 TC | - | 8.2 ± 7.5 |
| Pitteri et al. [46] | Italy | Cross-sectional | 31 RRMS (77%) 38 TC (74%) | 36.3 ± 7.6 RRMS 37.1 ± 8.9 TC | 13.4 ± 3.4 RRMS 14.6 ± 3.4 TC | 7 ± 4.5 | |
| Realmuto et al. [47] * | Italy | Cross-sectional | 45 RRMS (69%) 45 TC (71%) | 34.2 ± 7.7 RRMS 33 ± 7.7 TC | RRMS 13.4 ± 2.6 TC 13.3 ± 3.1 | 9.7 ± 6.2 | |
| Romosan et al. [48] | Romania | Cross-sectional | 65 PD (42%) 51 TC (39%) | 58.1 ± 5.3 PD 56.5 ± 6 TC | NR | 6.4 ± 3.2 | |
| Bayliss et al. [49] | Spain | Cross-sectional | 12 HD (67%) 12 relatives (58%) (spouse or gene-negative relative) 12 TC (75%) | Median and IQR: 42.7 (11.3) HD; 44.7 (22.3) HD relatives; 37.1 (15.8) TC | Median and IQR: 16.0 (5.0) HD 16 (6.2) HD relatives 14.5 (7.5) TC | - | - |
| Bisecco et al. [50] | Italy | Cross-sectional | 41 MS (66%) 25 TC (72%) | 34.2 ± 10.3 MS 37.8 ± 12 TC | NR | - | 8.8 ± 8.2 |
| Orso et al. [51] | Italy | Cross-sectional | 30 PD (36%) 60 TC (32%) | 73.39 ±8.93 70.1 ± 10.9 | 11.0 ±3.54 PD 9.5 ±5.4 TC | - | - |
| Study | Title and Abstract | Introduction and Aims | Method and Data | Sampling | Data Analysis | Ethics and Bias | Findings and Results | Transferability/Generalizability | Implications/ Usefulness | Overall |
|---|---|---|---|---|---|---|---|---|---|---|
| Bodden et al. [10] * | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 4 | 35 |
| Girardi et al. [15] | 4 | 4 | 3 | 4 | 3 | 4 | 4 | 4 | 4 | 34 |
| Allain et al. [35] | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 36 |
| Laisney et al. [36] | 4 | 4 | 3 | 3 | 4 | 1 | 4 | 3 | 3 | 29 |
| Poletti et al. [18] | 4 | 2 | 3 | 2 | 4 | 4 | 4 | 2 | 3 | 28 |
| Eddy et al. [37] * | 4 | 3 | 4 | 4 | 3 | 4 | 4 | 4 | 4 | 34 |
| Di rosa et al. [38] | 3 | 4 | 4 | 4 | 4 | 1 | 4 | 4 | 3 | 31 |
| Jelsone-swain et al. [39] | 4 | 4 | 4 | 3 | 3 | 4 | 4 | 4 | 3 | 33 |
| Enrici et al. [16] | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 36 |
| Eddy et al. [40] * | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 36 |
| Heitz et al. [41] | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 36 |
| Burke et al. [42] | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 35 |
| Burke et al. [43] | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 36 |
| Dulau et al. [44] | 3 | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 3 | 32 |
| Enrici et al. [45] | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 35 |
| Raimo et al. [14] | 4 | 4 | 4 | 4 | 4 | 1 | 4 | 4 | 4 | 33 |
| Pitteri et al. [46] | 4 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 35 |
| Realmuto et al. [47] * | 4 | 3 | 3 | 4 | 4 | 1 | 3 | 4 | 3 | 29 |
| Romosan et al. [48] | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 3 | 35 |
| Bayliss et al. [49] | 4 | 2 | 3 | 3 | 4 | 4 | 3 | 3 | 3 | 29 |
| Bisecco et al. [50] | 3 | 4 | 4 | 4 | 4 | 4 | 3 | 4 | 4 | 34 |
| Orso et al. [51] | 3 | 3 | 4 | 4 | 4 | 4 | 4 | 4 | 2 | 32 |
| Average | 3.81 | 3.54 | 3.72 | 3.77 | 3.86 | 3.45 | 3.86 | 3.77 | 3.5 | 33.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stafford, O.; Gleeson, C.; Egan, C.; Tunney, C.; Rooney, B.; O’Keeffe, F.; McDermott, G.; Baron-Cohen, S.; Burke, T. A 20-Year Systematic Review of the ‘Reading the Mind in the Eyes’ Test across Neurodegenerative Conditions. Brain Sci. 2023, 13, 1268. https://doi.org/10.3390/brainsci13091268
Stafford O, Gleeson C, Egan C, Tunney C, Rooney B, O’Keeffe F, McDermott G, Baron-Cohen S, Burke T. A 20-Year Systematic Review of the ‘Reading the Mind in the Eyes’ Test across Neurodegenerative Conditions. Brain Sciences. 2023; 13(9):1268. https://doi.org/10.3390/brainsci13091268
Chicago/Turabian StyleStafford, Owen, Christina Gleeson, Ciara Egan, Conall Tunney, Brendan Rooney, Fiadhnait O’Keeffe, Garret McDermott, Simon Baron-Cohen, and Tom Burke. 2023. "A 20-Year Systematic Review of the ‘Reading the Mind in the Eyes’ Test across Neurodegenerative Conditions" Brain Sciences 13, no. 9: 1268. https://doi.org/10.3390/brainsci13091268
APA StyleStafford, O., Gleeson, C., Egan, C., Tunney, C., Rooney, B., O’Keeffe, F., McDermott, G., Baron-Cohen, S., & Burke, T. (2023). A 20-Year Systematic Review of the ‘Reading the Mind in the Eyes’ Test across Neurodegenerative Conditions. Brain Sciences, 13(9), 1268. https://doi.org/10.3390/brainsci13091268






