Juvenile-Onset Recurrent Rhabdomyolysis Due to Compound Heterozygote Variants in the ACADVL Gene
Abstract
1. Introduction
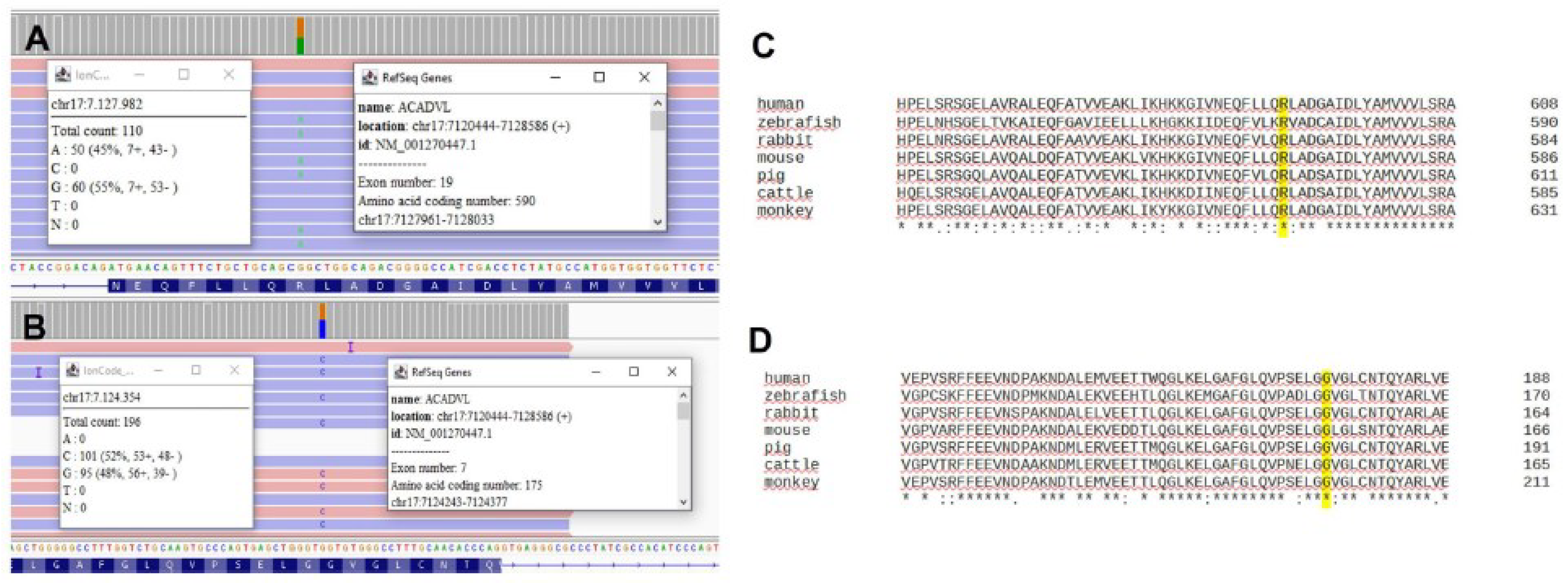
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Leslie, N.D.; Saenz-Ayala, S. Very Long-Chain Acyl-Coenzyme A Dehydrogenase Deficiency. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2009; pp. 1993–2023. [Google Scholar]
- Boneh, A.; Andresen, B.S.; Gregersen, N.; Ibrahim, M.; Tzanakos, N.; Peters, H.; Yaplito-Lee, J.; Pitt, J.J. VLCAD deficiency: Pitfalls in newborn screening and confirmation of diagnosis by mutation analysis. Mol. Genet. Metab. 2006, 88, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, J.C.; Kok, I.L.; Ferdinandusse, S.; Van der Pol, W.L.; Cuppen, I.; Bosch, A.M.; Langeveld, M.; Derks, T.G.J.; Williams, M.; De Vries, M.; et al. Impact of newborn screening for very-long-chain acyl-CoA dehydrogenase deficiency on genetic, enzymatic, and clinical outcomes. J. Inherit. Metab. Dis. 2019, 42, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Remec, Z.I.; Groselj, U.; Drole Torkar, A.; Zerjav Tansek, M.; Cuk, V.; Perko, D.; Ulaga, B.; Lipovec, N.; Debeljak, M.; Kovac, J.; et al. Very Long-Chain Acyl-CoA Dehydrogenase Deficiency: High Incidence of Detected Patients with Expanded Newborn Screening Program. Front. Genet. 2021, 12, 648493. [Google Scholar] [CrossRef]
- Andresen, B.S.; Olpin, S.; Poorthuis, B.J.; Scholte, H.R.; Vianey-Saban, C.; Wanders, R.; Ijlst, L.; Morris, A.; Pourfarzam, M.; Bartlett, K.; et al. Clear correlation of genotype with disease phenotype in very-long-chain acyl-CoA dehydrogenase deficiency. Am. J. Hum. Genet. 1999, 64, 479–494. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tong, F.; Wu, X.Y.; Zhu, L.; Yi, Q.Z.; Zheng, J.; Yang, R.L.; Zhao, Z.Y.; Cang, X.H.; Shu, Q.; et al. Novel ACADVL variants resulting in mitochondrial defects in long-chain acyl-CoA dehydrogenase deficiency. J. Zhejiang Univ. Sci. B 2020, 21, 885–896. [Google Scholar] [CrossRef]
- Miller, M.J.; Burrage, L.C.; Gibson, J.B.; Strenk, M.E.; Lose, E.J.; Bick, D.P.; Elsea, S.H.; Sutton, V.R.; Sun, Q.; Graham, B.H.; et al. Recurrent ACADVL molecular findings in individuals with a positive newborn screen for very long chain acyl-coA dehydrogenase (VLCAD) deficiency in the United States. Mol. Genet. Metab. 2015, 116, 139–145. [Google Scholar] [CrossRef]
- Van Calcar, S.C.; Sowa, M.; Rohr, F.; Beazer, J.; Setlock, T.; Weihe, T.U.; Pendyal, S. Nutrition management guideline for very-long chain acyl-CoA dehydrogenase deficiency (VLCAD): An evidence- and consensus-based approach. Mol. Genet. Metab. 2020, 131, 23–37. [Google Scholar] [CrossRef]
- Yamada, K. Management and diagnosis of mitochondrial fatty acid oxidation disorders: Focus on very-long-chain acyl-CoA dehydrogenase deficiency. J. Hum. Genet. 2019, 64, 73–85. [Google Scholar] [CrossRef]
- Shiraishi, H.; Yamada, K.; Egawa, K. Efficacy of bezafibrate for preventing myopathic attacks in patients with very long-chain acyl-CoA dehydrogenase deficiency. Brain Dev. 2021, 43, 214–219. [Google Scholar] [CrossRef]
- Zhou, C.; Blumberg, B. Overlapping gene structure of human VLCAD and DLG4. Gene 2003, 305, 161–166. [Google Scholar] [CrossRef]
- Aoyama, T.; Souri, M.; Ueno, I.; Kamijo, T.; Yamaguchi, S.; Rhead, W.J.; Tanaka, K.; Hashimoto, T. Cloning of human Very-Long-Chain Acyl-Coenzyme A Dehydrogenase and molecular characterization of its deficiency in two patients. Am. J. Hum. Genet. 1995, 57, 273–283. [Google Scholar]
- Prew, M.S.; Camara, C.M.; Botzanowski, T.; Moroco, J.A.; Bloch, N.B.; Levy, H.R.; Seo, H.; Dhe-Paganon, S.; Bird, G.H.; Herce, H.D.; et al. Structural basis for defective membrane targeting of mutant enzyme in human VLCAD deficiency. Nat. Commun. 2022, 13, 3669. [Google Scholar] [CrossRef]
- Schiff, M.; Mohsen, A.W.; Karunanidhi, A.; McCracken, E.; Yeasted, R.; Vockley, J. Molecular and cellular pathology of very-long-chain acyl-CoA dehydrogenase deficiency. Mol. Genet. Metab. 2013, 109, 21–27. [Google Scholar] [CrossRef]
- Chavez, L.O.; Leon, M.; Einav, S.; Varon, J. Beyond muscle destruction: A systematic review of rhabdomyolysis for clinical practice. Crit. Care 2016, 20, 135. [Google Scholar] [CrossRef]
- Harmelink, M. Uncommon Causes of Rhabdomyolysis. Crit. Care Clin. 2022, 38, 271–285. [Google Scholar] [CrossRef]
- Nance, J.R.; Mammen, A.L. Diagnostic evaluation of rhabdomyolysis. Muscle Nerve 2015, 51, 793–810. [Google Scholar] [CrossRef]
- Lilleker, J.B.; Keh, Y.S.; Roncaroli, F.; Sharma, R.; Roberts, M. Metabolic myopathies: A practical approach. Pract. Neurol. 2018, 18, 14–26. [Google Scholar] [CrossRef]
- Fatehi, F.; Okhovat, A.A.; Nilipour, Y.; Mroczek, M.; Straub, V.; Töpf, A.; Palibrk, A.; Peric, S.; Rakocevic Stojanovic, V.; Najmabadi, H.; et al. Adult-onset very-long-chain acyl-CoA dehydrogenase deficiency (VLCADD). Eur. J. Neurol. 2020, 27, 2257–2266. [Google Scholar] [CrossRef]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal Screening in Europe Revisited: An ISNS Perspective on the Current State and Developments Since 2010. Int. J. Neonatal. Screen. 2021, 7, 15. [Google Scholar] [CrossRef]
- Evans, M.; Andresen, B.S.; Nation, J.; Boneh, A. VLCAD deficiency: Follow-up and outcome of patients diagnosed through newborn screening in Victoria. Mol. Genet. Metab. 2016, 118, 282–287. [Google Scholar] [CrossRef]
- Roe, C.R.; Brunengraber, H. Anaplerotic treatment of long-chain fat oxidation disorders with triheptanoin: Review of 15 years experience. Mol. Genet. Metab. 2015, 116, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Tucci, S.; Floegel, U.; Beermann, F.; Behringer, S.; Spiekerkoetter, U. Triheptanoin: Long-term effects in the very long-chain acyl-CoA dehydrogenase-deficient mouse. J. Lipid. Res. 2017, 58, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Triheptanoin: First Approval. Drugs 2020, 80, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Vockley, J.; Burton, B.; Berry, G.; Longo, N.; Phillips, J.; Sanchez-Valle, A.; Chapman, K.; Tanpaiboon, P.; Grunewald, S.; Murphy, E.; et al. Effects of triheptanoin (UX007) in patients with long-chain fatty acid oxidation disorders: Results from an open-label, long-term extension study. J. Inherit. Metab. Dis. 2021, 44, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Djouadi, F.; Aubey, F.; Schlemmer, D.; Ruiter, J.P.; Wanders, R.J.; Strauss, A.W.; Bastin, J. Bezafibrate increases very-long-chain acyl-CoA dehydrogenase protein and mRNA expression in deficient fibroblasts and is a potential therapy for fatty acid oxidation disorders. Hum. Mol. Genet. 2005, 14, 2695–2703. [Google Scholar] [CrossRef]
- Gobin-Limballe, S.; Djouadi, F.; Aubey, F.; Olpin, S.; Andresen, B.S.; Yamaguchi, S.; Mandel, H.; Fukao, T.; Ruiter, J.P.; Wanders, R.J.; et al. Genetic basis for correction of very-long-chain acyl-coenzyme A dehydrogenase deficiency by bezafibrate in patient fibroblasts: Toward a genotype-based therapy. Am. J. Hum. Genet. 2007, 81, 1133–1143. [Google Scholar] [CrossRef]
- Ørngreen, M.C.; Vissing, J.; Laforét, P. No effect of bezafibrate in patients with CPTII and VLCAD deficiencies. J. Inherit. Metab. Dis. 2015, 38, 373–374. [Google Scholar] [CrossRef]
- Ørngreen, M.C.; Madsen, K.L.; Preisler, N.; Andersen, G.; Vissing, J.; Laforêt, P. Bezafibrate in skeletal muscle fatty acid oxidation disorders: A randomized clinical trial. Neurology 2014, 82, 607–613. [Google Scholar] [CrossRef]
- Shiraishi, H.; Yamada, K.; Oki, E.; Ishige, M.; Fukao, T.; Hamada, Y.; Sakai, N.; Ochi, F.; Watanabe, A.; Kawakami, S.; et al. Open-label clinical trial of bezafibrate treatment in patients with fatty acid oxidation disorders in Japan; 2nd report QOL survey. Mol. Genet. Metab. Rep. 2019, 20, 100496. [Google Scholar] [CrossRef]
- Testai, L.; Martelli, A.; Flori, L.; Cicero, A.F.G.; Colletti, A. Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases. Nutrients 2021, 13, 1697. [Google Scholar] [CrossRef]
- Mosegaard, S.; Dipace, G.; Bross, P.; Carlsen, J.; Gregersen, N.; Olsen, R.K.J. Riboflavin Deficiency-Implications for General Human Health and Inborn Errors of Metabolism. Int. J. Mol. Sci. 2020, 21, 3847. [Google Scholar] [CrossRef]
- Laforêt, P.; Acquaviva-Bourdain, C.; Rigal, O.; Brivet, M.; Penisson-Besnier, I.; Chabrol, B.; Chaigne, D.; Boespflug-Tanguy, O.; Laroche, C.; Bedat-Millet, A.L.; et al. Diagnostic assessment and long-term follow-up of 13 patients with Very Long-Chain Acyl-Coenzyme A dehydrogenase (VLCAD) deficiency. Neuromuscul. Disord. 2009, 19, 324–329. [Google Scholar] [CrossRef]
- Scalais, E.; Bottu, J.; Wanders, R.J.; Ferdinandusse, S.; Waterham, H.R.; De Meirleir, L. Familial very long chain acyl-CoA dehydrogenase deficiency as a cause of neonatal sudden infant death: Improved survival by prompt diagnosis. Am. J. Med. Genet. A 2015, 167A, 211–214. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labella, B.; Lanzi, G.; Cotti Piccinelli, S.; Caria, F.; Damioli, S.; Risi, B.; Bertella, E.; Poli, L.; Padovani, A.; Filosto, M. Juvenile-Onset Recurrent Rhabdomyolysis Due to Compound Heterozygote Variants in the ACADVL Gene. Brain Sci. 2023, 13, 1178. https://doi.org/10.3390/brainsci13081178
Labella B, Lanzi G, Cotti Piccinelli S, Caria F, Damioli S, Risi B, Bertella E, Poli L, Padovani A, Filosto M. Juvenile-Onset Recurrent Rhabdomyolysis Due to Compound Heterozygote Variants in the ACADVL Gene. Brain Sciences. 2023; 13(8):1178. https://doi.org/10.3390/brainsci13081178
Chicago/Turabian StyleLabella, Beatrice, Gaetana Lanzi, Stefano Cotti Piccinelli, Filomena Caria, Simona Damioli, Barbara Risi, Enrica Bertella, Loris Poli, Alessandro Padovani, and Massimiliano Filosto. 2023. "Juvenile-Onset Recurrent Rhabdomyolysis Due to Compound Heterozygote Variants in the ACADVL Gene" Brain Sciences 13, no. 8: 1178. https://doi.org/10.3390/brainsci13081178
APA StyleLabella, B., Lanzi, G., Cotti Piccinelli, S., Caria, F., Damioli, S., Risi, B., Bertella, E., Poli, L., Padovani, A., & Filosto, M. (2023). Juvenile-Onset Recurrent Rhabdomyolysis Due to Compound Heterozygote Variants in the ACADVL Gene. Brain Sciences, 13(8), 1178. https://doi.org/10.3390/brainsci13081178






