Effects of Early Adverse Life Events on Depression and Cognitive Performance from the Perspective of the Heart-Brain Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Psychological and Cognitive Function Measures
2.3. Heart Rate Variability
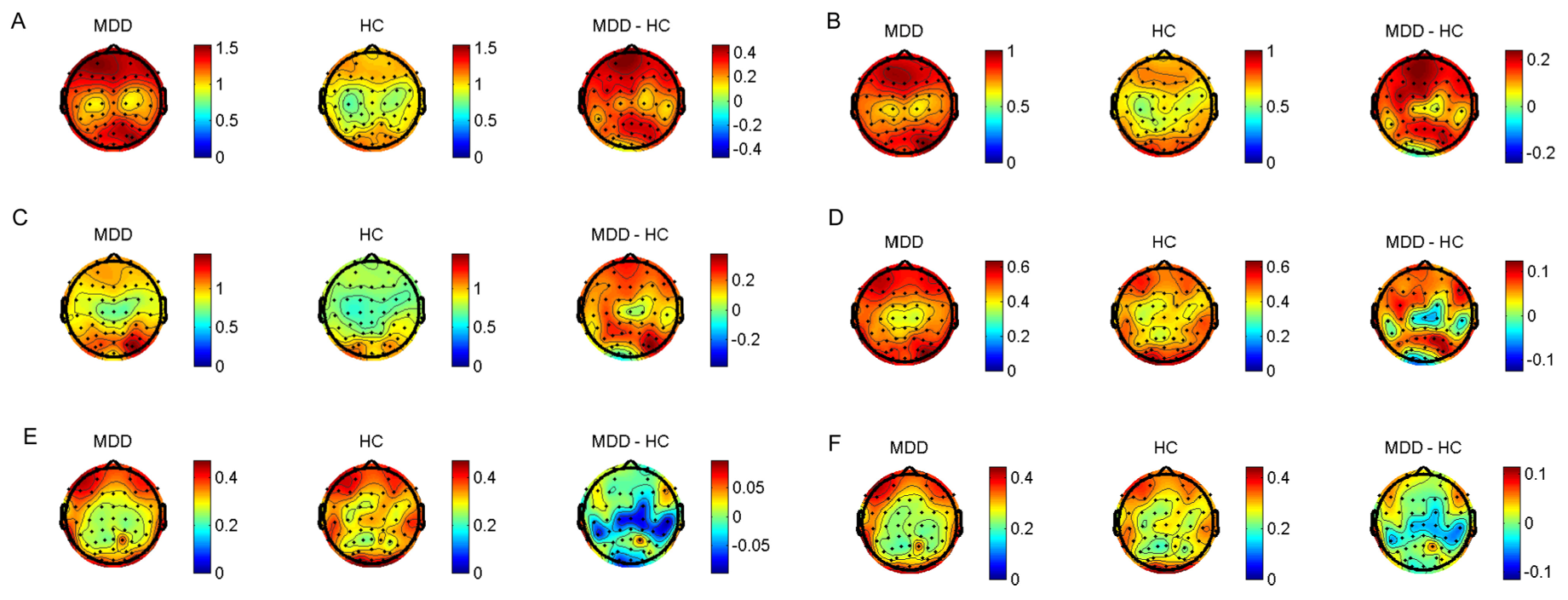
2.4. EEG Recording and Preprocessing
2.5. Data Analysis
3. Results
3.1. Demographic and Clinical Characteristics of the Two Groups
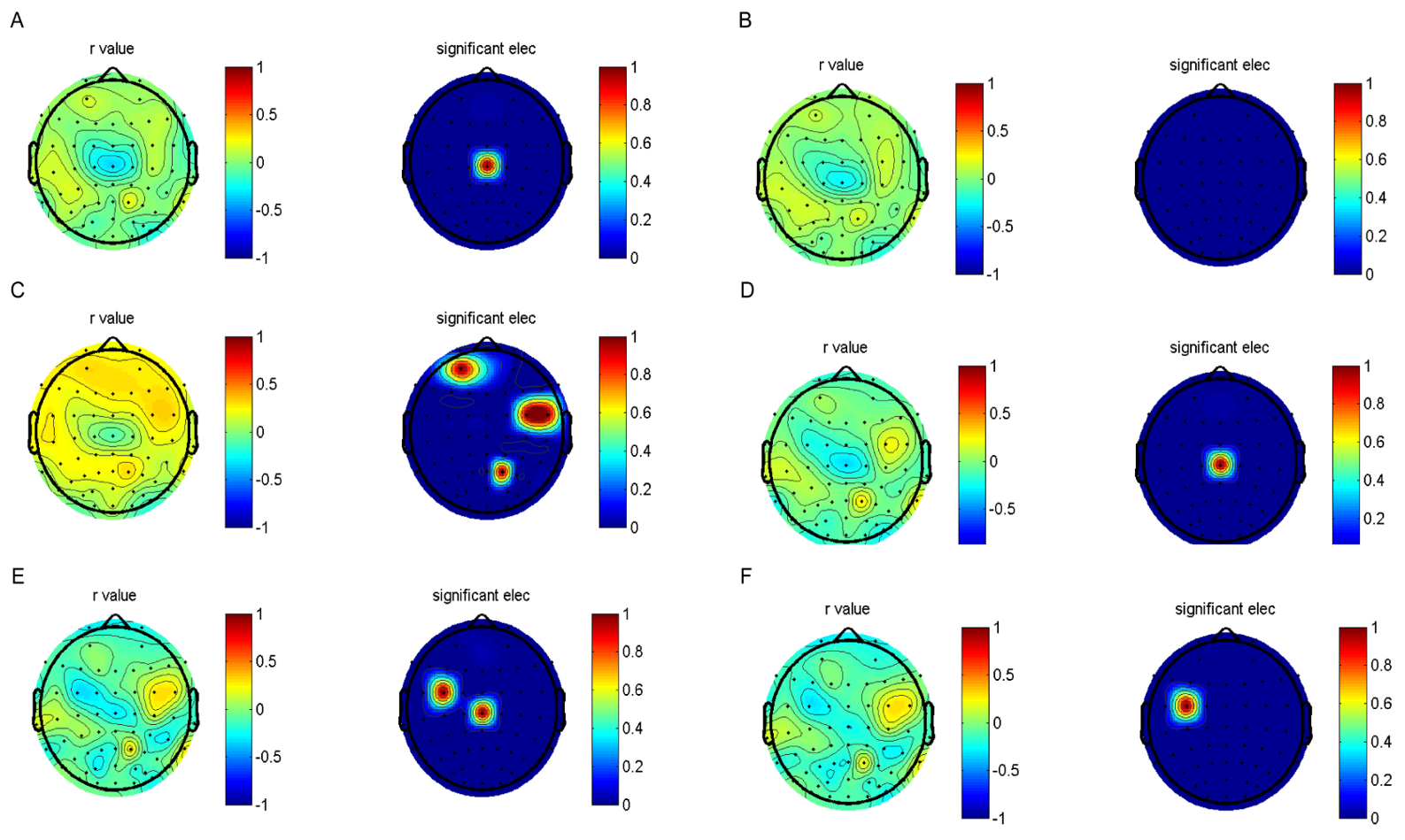
3.2. Analysis of the Correlation between EALs and Clinical Characteristics
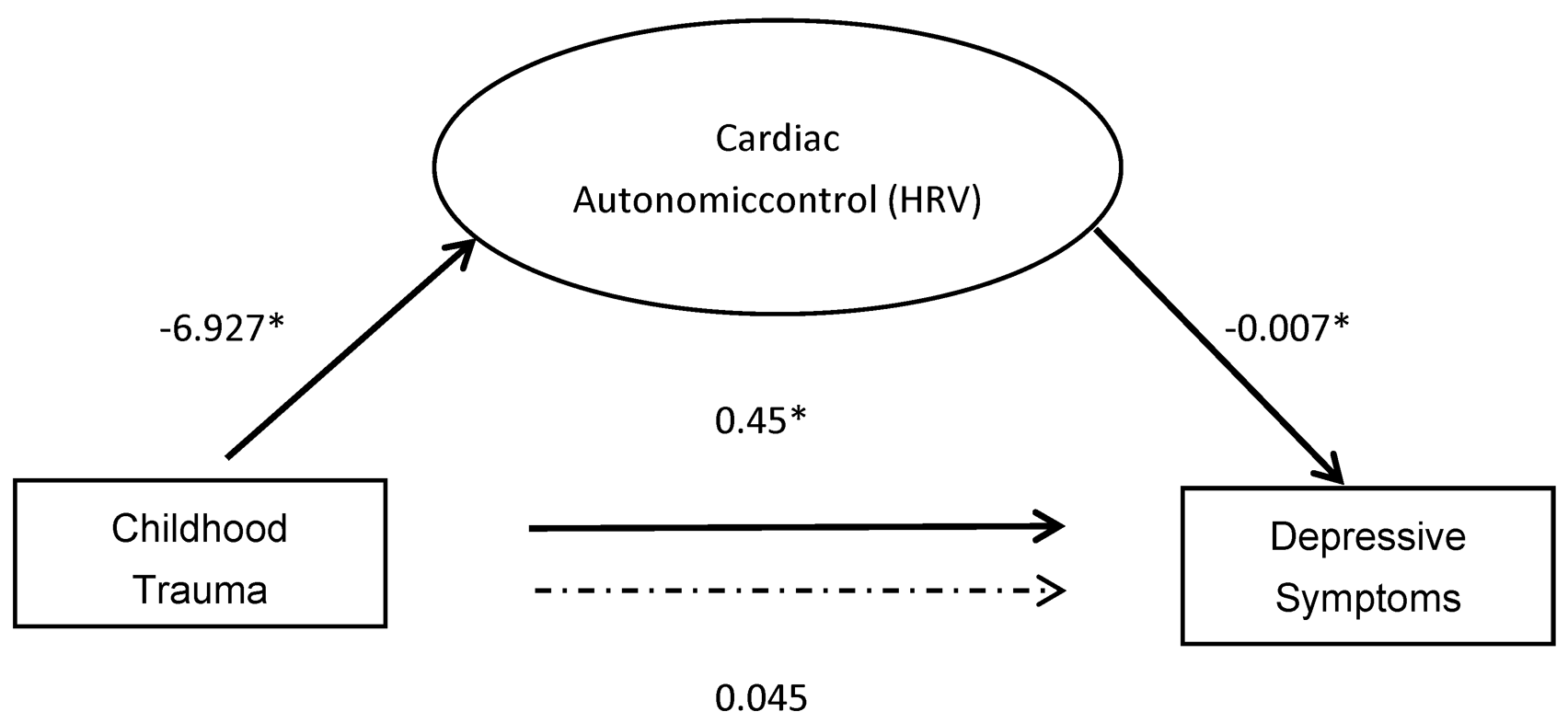
3.3. Mediation Analyses
4. Discussion
4.1. Effect of EALs on Depression
4.2. Effect of EALs on Cognitive Function
4.3. Effect of EALs on the Heart–Brain Axis
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Full Name | Abbreviations |
| Early adverse life events | EALs |
| Repeatable Battery for the Assessment of Neuropsychological Status | RBANS |
| Childhood Trauma Questionnaire | CTQ |
| Heart rate variability | HRV |
| Electroencephalograms | EEG |
| Hamilton Depression Scale | HAMD |
| Root mean square of the successive differences | RMSSD |
| Standard deviation of the RR intervals | SDNN |
| Low-frequency | LF |
| High-frequency | HF |
| Independent Component Analysis | ICA |
| Fast Fourier Transform | FFT |
| Major Depressive Disorder | MDD |
| Healthy control | HC |
| Analysis of variance | ANOVA |
| Standard deviation | SD |
| Confidence interval | CI |
| Standard error | SE |
| Prefrontal cortex | PFC |
| Autonomic Nervous System | ANS |
References
- Stanton, K.J.; Denietolis, B.; Goodwin, B.J.; Dvir, Y. Childhood Trauma and Psychosis: An Updated Review. Child Adolesc. Psychiatr. Clin. N. Am. 2020, 29, 115–129. [Google Scholar] [CrossRef] [PubMed]
- McKay, M.T.; Cannon, M.; Chambers, D.; Conroy, R.M.; Coughlan, H.; Dodd, P.; Healy, C.; O’Donnell, L.; Clarke, M.C. Childhood Trauma and Adult Mental Disorder: A Systematic Review and Meta-Analysis of Longitudinal Cohort Studies. Acta Psychiatr. Scand. 2021, 143, 189–205. [Google Scholar] [CrossRef]
- Stefanopoulou, E.; Manoharan, A.; Landau, S.; Geddes, J.R.; Goodwin, G.; Frangou, S. Cognitive Functioning in Patients with Affective Disorders and Schizophrenia: A Meta-Analysis. Int. Rev. Psychiatry 2009, 21, 336–356. [Google Scholar] [CrossRef] [PubMed]
- Snyder, H.R. Major Depressive Disorder Is Associated with Broad Impairments on Neuropsychological Measures of Executive Function: A Meta-Analysis and Review. Psychol. Bull. 2013, 139, 81–132. [Google Scholar] [CrossRef]
- Tahsili-Fahadan, P.; Geocadin, R.G. Heart-Brain Axis: Effects of Neurologic Injury on Cardiovascular Function. Circ. Res. 2017, 120, 559–572. [Google Scholar] [CrossRef]
- Wulsin, L.R.; Horn, P.S.; Perry, J.L.; Massaro, J.M.; D’Agostino, R.B. Autonomic Imbalance as a Predictor of Metabolic Risks, Cardiovascular Disease, Diabetes, and Mortality. J. Clin. Endocrinol. Metab. 2015, 100, 2443–2448. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Lane, R.D. A Model of Neurovisceral Integration in Emotion Regulation and Dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Kemp, A.H.; Koenig, J.; Thayer, J.F. From Psychological Moments to Mortality: A Multidisciplinary Synthesis on Heart Rate Variability Spanning the Continuum of Time. Neurosci. Biobehav. Rev. 2017, 83, 547–567. [Google Scholar] [CrossRef]
- Schiweck, C.; Piette, D.; Berckmans, D.; Claes, S.; Vrieze, E. Heart Rate and High Frequency Heart Rate Variability during Stress as Biomarker for Clinical Depression. A Systematic Review. Psychol. Med. 2019, 49, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Ahs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A Meta-Analysis of Heart Rate Variability and Neuroimaging Studies: Implications for Heart Rate Variability as a Marker of Stress and Health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Kimhy, D.; Crowley, O.V.; McKinley, P.S.; Burg, M.M.; Lachman, M.E.; Tun, P.A.; Ryff, C.D.; Seeman, T.E.; Sloan, R.P. The Association of Cardiac Vagal Control and Executive Functioning--Findings from the MIDUS Study. J. Psychiatr. Res. 2013, 47, 628–635. [Google Scholar] [CrossRef]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; Hillaire-Clarke, C.S.; Spruance, V.; et al. The Emerging Science of Interoception: Sensing, Integrating, Interpreting, and Regulating Signals within the Self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Freeman, W.J.; Quian Quiroga, R. Imaging Brain Function with EEG: Advanced Temporal and Spatial Analysis of Electroencephalographic Signals; Springer: New York, NY, USA, 2013. [Google Scholar]
- Abhang, P.A.; Gawali, B.W.; Mehrotra, S.C. Introduction to EEG- and Speech-Based Emotion Recognition; Academic Press: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Aftanas, L.I.; Golocheikine, S.A. Human Anterior and Frontal Midline Theta and Lower Alpha Reflect Emotionally Positive State and Internalized Attention: High-Resolution EEG Investigation of Meditation. Neurosci. Lett. 2001, 310, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Aftanas, L.I.; Varlamov, A.A.; Pavlov, S.V.; Makhnev, V.P.; Reva, N.V. Time-Dependent Cortical Asymmetries Induced by Emotional Arousal: EEG Analysis of Event-Related Synchronization and Desynchronization in Individually Defined Frequency Bands. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2002, 44, 67–82. [Google Scholar] [CrossRef]
- Lee, S.-H.; Park, Y.; Jin, M.J.; Lee, Y.J.; Hahn, S.W. Childhood Trauma Associated with Enhanced High Frequency Band Powers and Induced Subjective Inattention of Adults. Front. Behav. Neurosci. 2017, 11, 148. [Google Scholar] [CrossRef]
- Duschek, S.; Wörsching, J.; Reyes Del Paso, G.A. Autonomic Cardiovascular Regulation and Cortical Tone. Clin. Physiol. Funct. Imaging 2015, 35, 383–392. [Google Scholar] [CrossRef]
- Triggiani, A.I.; Valenzano, A.; Del Percio, C.; Marzano, N.; Soricelli, A.; Petito, A.; Bellomo, A.; Başar, E.; Mundi, C.; Cibelli, G.; et al. Resting State Rolandic Mu Rhythms Are Related to Activity of Sympathetic Component of Autonomic Nervous System in Healthy Humans. Int. J. Psychophysiol. 2016, 103, 79–87. [Google Scholar] [CrossRef]
- Ostwald, P.F. A Rating Scale for Depression. Am. J. Psychother. 1960, 14, 817–818. [Google Scholar] [CrossRef]
- Bernstein, D.P.; Ahluvalia, T.; Pogge, D.; Handelsman, L. Validity of the Childhood Trauma Questionnaire in an Adolescent Psychiatric Population. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 340–348. [Google Scholar] [CrossRef]
- Randolph, C.; Tierney, M.C.; Mohr, E.; Chase, T.N. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary Clinical Validity. J. Clin. Exp. Neuropsychol. 1998, 20, 310–319. [Google Scholar] [CrossRef]
- Camm, A.J.; Malik, M.; Bigger, J.T.; Breithardt, G.; Cerutti, S.; Cohen, R.J.; Coumel, P.; Fallen, E.L.; Kennedy, H.L.; Kleiger, R.E.; et al. Heart Rate Variability: Standards of Measurement, Physiological Interpretation, and Clinical Use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef]
- Williams, L.M.; Debattista, C.; Duchemin, A.-M.; Schatzberg, A.F.; Nemeroff, C.B. Childhood Trauma Predicts Antidepressant Response in Adults with Major Depression: Data from the Randomized International Study to Predict Optimized Treatment for Depression. Transl. Psychiatry 2016, 6, e799. [Google Scholar] [CrossRef] [PubMed]
- Nemeroff, C.B.; Heim, C.M.; Thase, M.E.; Klein, D.N.; Rush, A.J.; Schatzberg, A.F.; Ninan, P.T.; McCullough, J.P.; Weiss, P.M.; Dunner, D.L.; et al. Differential Responses to Psychotherapy versus Pharmacotherapy in Patients with Chronic Forms of Major Depression and Childhood Trauma. Proc. Natl. Acad. Sci. USA 2003, 100, 14293–14296. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Klimkiewicz, A.; Krasowska, A.; Kopera, M.; Sławińska-Ceran, A.; Brower, K.J.; Wojnar, M. History of Sexual Abuse and Suicide Attempts in Alcohol-Dependent Patients. Child Abuse Negl. 2014, 38, 1560–1568. [Google Scholar] [CrossRef]
- Luebbe, A.M.; Bell, D.J. Positive and Negative Family Emotional Climate Differentially Predict Youth Anxiety and Depression via Distinct Affective Pathways. J. Abnorm. Child Psychol. 2014, 42, 897–911. [Google Scholar] [CrossRef]
- Gershon, A.; Sudheimer, K.; Tirouvanziam, R.; Williams, L.M.; O’Hara, R. The Long-Term Impact of Early Adversity on Late-Life Psychiatric Disorders. Curr. Psychiatry Rep. 2013, 15, 352. [Google Scholar] [CrossRef] [PubMed]
- Baune, B.T.; Miller, R.; McAfoose, J.; Johnson, M.; Quirk, F.; Mitchell, D. The Role of Cognitive Impairment in General Functioning in Major Depression. Psychiatry Res. 2010, 176, 183–189. [Google Scholar] [CrossRef]
- McDermott, L.M.; Ebmeier, K.P. A Meta-Analysis of Depression Severity and Cognitive Function. J. Affect. Disord. 2009, 119, 1–8. [Google Scholar] [CrossRef]
- Barry, T.J.; Lenaert, B.; Hermans, D.; Raes, F.; Griffith, J.W. Meta-Analysis of the Association Between Autobiographical Memory Specificity and Exposure to Trauma. J. Trauma. Stress 2018, 31, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Djonlagic, I.; Mariani, S.; Fitzpatrick, A.L.; Van Der Klei, V.M.G.T.H.; Johnson, D.A.; Wood, A.C.; Seeman, T.; Nguyen, H.T.; Prerau, M.J.; Luchsinger, J.A.; et al. Macro and Micro Sleep Architecture and Cognitive Performance in Older Adults. Nat. Hum. Behav. 2020, 5, 123–145. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Devilly, G.J.; Shum, D.H.K. A Meta-Analytic Review of Overgeneral Memory: The Role of Trauma History, Mood, and the Presence of Posttraumatic Stress Disorder. Psychol. Trauma Theory Res. Pract. Policy 2016, 8, 157–164. [Google Scholar] [CrossRef]
- Popovic, D.; Schmitt, A.; Kaurani, L.; Senner, F.; Papiol, S.; Malchow, B.; Fischer, A.; Schulze, T.G.; Koutsouleris, N.; Falkai, P. Childhood Trauma in Schizophrenia: Current Findings and Research Perspectives. Front. Neurosci. 2019, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Billman, G.E. The LF/HF Ratio Does Not Accurately Measure Cardiac Sympatho-Vagal Balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef]
- Eikeseth, F.F.; Sætren, S.S.; Benjamin, B.R.; Ulltveit-Moe Eikenæs, I.; Sütterlin, S.; Hummelen, B. The Test-Retest Reliability of Heart Rate Variability and Its Association with Personality Functioning. Front. Psychiatry 2020, 11, 558145. [Google Scholar] [CrossRef]
- Park, G.; Thayer, J.F. From the Heart to the Mind: Cardiac Vagal Tone Modulates Top-down and Bottom-up Visual Perception and Attention to Emotional Stimuli. Front. Psychol. 2014, 5, 278. [Google Scholar] [CrossRef]
- Hosseinifard, B.; Moradi, M.H.; Rostami, R. Classifying Depression Patients and Normal Subjects Using Machine Learning Techniques and Nonlinear Features from EEG Signal. Comput. Methods Programs Biomed. 2013, 109, 339–345. [Google Scholar] [CrossRef]
- Domingos, C.; Silva, C.M.D.; Antunes, A.; Prazeres, P.; Esteves, I.; Rosa, A.C. The Influence of an Alpha Band Neurofeedback Training in Heart Rate Variability in Athletes. Int. J. Environ. Res. Public Health 2021, 18, 12579. [Google Scholar] [CrossRef]
- Santos, J.; Ihle, A.; Peralta, M.; Domingos, C.; Gouveia, É.R.; Ferrari, G.; Werneck, A.; Rodrigues, F.; Marques, A. Associations of Physical Activity and Television Viewing With Depressive Symptoms of the European Adults. Front. Public Health 2022, 9, 799870. [Google Scholar] [CrossRef]
- Ionson, E.; Limbachia, J.; Rej, S.; Puka, K.; Newman, R.I.; Wetmore, S.; Burhan, A.M.; Vasudev, A. Effects of Sahaj Samadhi meditation on heart rate variability and depressive symptoms in patients with late-life depression. Br. J. Psychiatry J. Ment. Sci. 2019, 214, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwan, J.E.; de Vente, W.; Huizink, A.C.; Bögels, S.M.; de Bruin, E.I. Physical activity, mindfulness meditation, or heart rate variability biofeedback for stress reduction: A randomized controlled trial. Appl. Psychophysiol. Biofeedback 2015, 40, 257–268. [Google Scholar] [CrossRef] [PubMed]
| Variables | Depressed Subjects (n = 48) | Healthy Controls (n = 49) | F or X 2 | p |
|---|---|---|---|---|
| Gender (male/female) | 15/33 | 23/26 | 1.768 | 0.184 |
| Age (years) | 21.06 ± 2.60 | 21.53 ± 2.18 | 0.432 | 0.513 |
| Education (years) | 14.69 ± 1.80 | 15.27 ± 1.55 | 2.458 | 0.120 |
| BMI (kg/m2) | 21.36 ± 3.43 | 20.51 ± 2.59 | 1.918 | 0.169 |
| Drinking (drinker/nondrinker) | 5/44 | 3/45 | 0.501 | 0.479 |
| Smoking (smoker/nonsmoker) | 3/45 | 4/45 | 0.133 | 0.716 |
| emotional abuse | 10.09 ± 4.31 | 6.45 ± 1.99 | 6.54 | 0.000 |
| physical abuse | 7.58 ± 3.57 | 5.80 ± 2.08 | 2.76 | 0.003 |
| sexual abuse | 6.52 ± 2.32 | 5.43 ± 1.15 | 2.00 | 0.004 |
| emotional neglect | 14.13 ± 4.63 | 9.22 ± 4.87 | 5.07 | 0.000 |
| physical neglect | 9.50 ± 3.93 | 6.82 ± 2.65 | 3.94 | 0.000 |
| CTQ sum score | 48.40 ± 13.20 | 33.96 ± 9.36 | 6.23 | 0.000 |
| RBANS Score | Depressed Subjects (n = 48) | Healthy Controls (n = 49) | F | p |
|---|---|---|---|---|
| Immediate memory | 86.95 ± 10.98 | 92.80 ± 10.73 | 6.99 | 0.010 |
| Visuospatial/constructional | 93.98 ± 15.53 | 98.78 ± 13.11 | 2.68 | 0.105 |
| Language | 89.32 ± 14.07 | 100.29 ± 13.10 | 15.62 | 0.000 |
| Attention | 110.60 ± 15.94 | 117.27 ± 10.85 | 5.78 | 0.018 |
| Delayed memory | 89.87 ± 14.14 | 94.35 ± 10.79 | 3.05 | 0.084 |
| RBANS total score | 91.68 ± 9.87 | 100.43 ± 9.97 | 18.63 | 0.000 |
| Depressed Subjects (n = 48) | Healthy Controls (n = 49) | t | p | |
|---|---|---|---|---|
| SDNN (ms) | 40.00 (33.00, 52.00) | 49.00 (40.00, 63.00) | 2.72 | 0.003 |
| RMSSD (ms) | 28.00 (19.00, 39.00) | 33.00 (27.00, 45.00) | 2.10 | 0.026 |
| LF (ms2) | 302.40 (171.91, 562.27) | 581.62 (396.71, 834.34) | 3.85 | 0.000 |
| HF (ms2) | 230.93 (129.32, 552.77) | 301.95 (243.78, 630.03) | 2.02 | 0.038 |
| LF/HF | 2.40 (1.55, 3.25) | 3.47 (1.59, 5.34) | 1.07 | 0.30 |
| SD1 | 19.79 (13.52, 27.35) | 24.35 (19.16, 33.45) | 2.20 | 0.028 |
| SD2 | 50.49 (41.43, 70.01) | 66.48 (54.04, 84.18) | 3.09 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Wang, G.; Xiao, L.; Du, Y.; Lin, S.; Nan, C.; Weng, S. Effects of Early Adverse Life Events on Depression and Cognitive Performance from the Perspective of the Heart-Brain Axis. Brain Sci. 2023, 13, 1174. https://doi.org/10.3390/brainsci13081174
Xia Y, Wang G, Xiao L, Du Y, Lin S, Nan C, Weng S. Effects of Early Adverse Life Events on Depression and Cognitive Performance from the Perspective of the Heart-Brain Axis. Brain Sciences. 2023; 13(8):1174. https://doi.org/10.3390/brainsci13081174
Chicago/Turabian StyleXia, Yujie, Gaohua Wang, Ling Xiao, Yiwei Du, Shanshan Lin, Cai Nan, and Shenhong Weng. 2023. "Effects of Early Adverse Life Events on Depression and Cognitive Performance from the Perspective of the Heart-Brain Axis" Brain Sciences 13, no. 8: 1174. https://doi.org/10.3390/brainsci13081174
APA StyleXia, Y., Wang, G., Xiao, L., Du, Y., Lin, S., Nan, C., & Weng, S. (2023). Effects of Early Adverse Life Events on Depression and Cognitive Performance from the Perspective of the Heart-Brain Axis. Brain Sciences, 13(8), 1174. https://doi.org/10.3390/brainsci13081174





