Dexmedetomidine Improves Anxiety-like Behaviors in Sleep-Deprived Mice by Inhibiting the p38/MSK1/NFκB Pathway and Reducing Inflammation and Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Induction of SD
2.2. Experimental Animals and Pharmacological Treatments
2.3. Open-Field Experiment
2.4. Elevated plus Maze Experiments
2.5. mRNA Sequencing
2.6. Immunohistochemical Assays
2.7. Western Blotting
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Superoxide Dismutase Activity
2.10. Data Analysis
3. Results
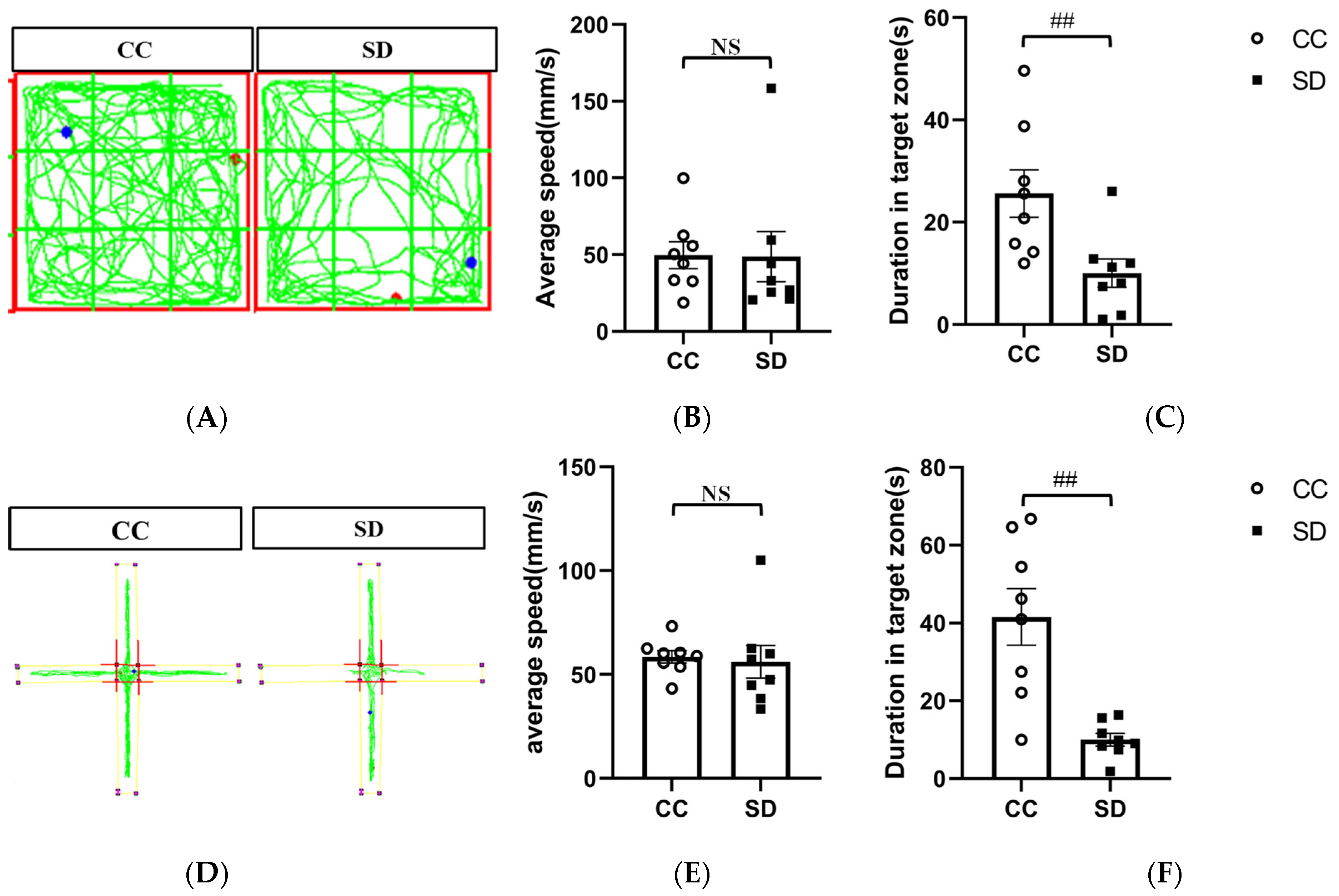
3.1. Effect of SD on Emotional Behavior in Mice
3.2. Effect of SD on the Transcriptome of the Prefrontal Cortex
3.3. Effect of SD on the Activation of the p38/MSK1/NFκB Pathway
3.4. SB203580 Can Effectively Inhibit the Activation of p38/MSK1/NFκB Pathway Induced by SD
3.5. SB203580 Ameliorates Oxidative Stress and Inflammatory Reactions in the Prefrontal Cortex of Sleep-Deprived Mice
3.6. Effect of Dex on Anxiety-like Behaviors in Sleep-Deprived Mice
3.7. Dex Acts by Inhibiting the Activation of the p38/MSK1/NFκBp65 Pathway
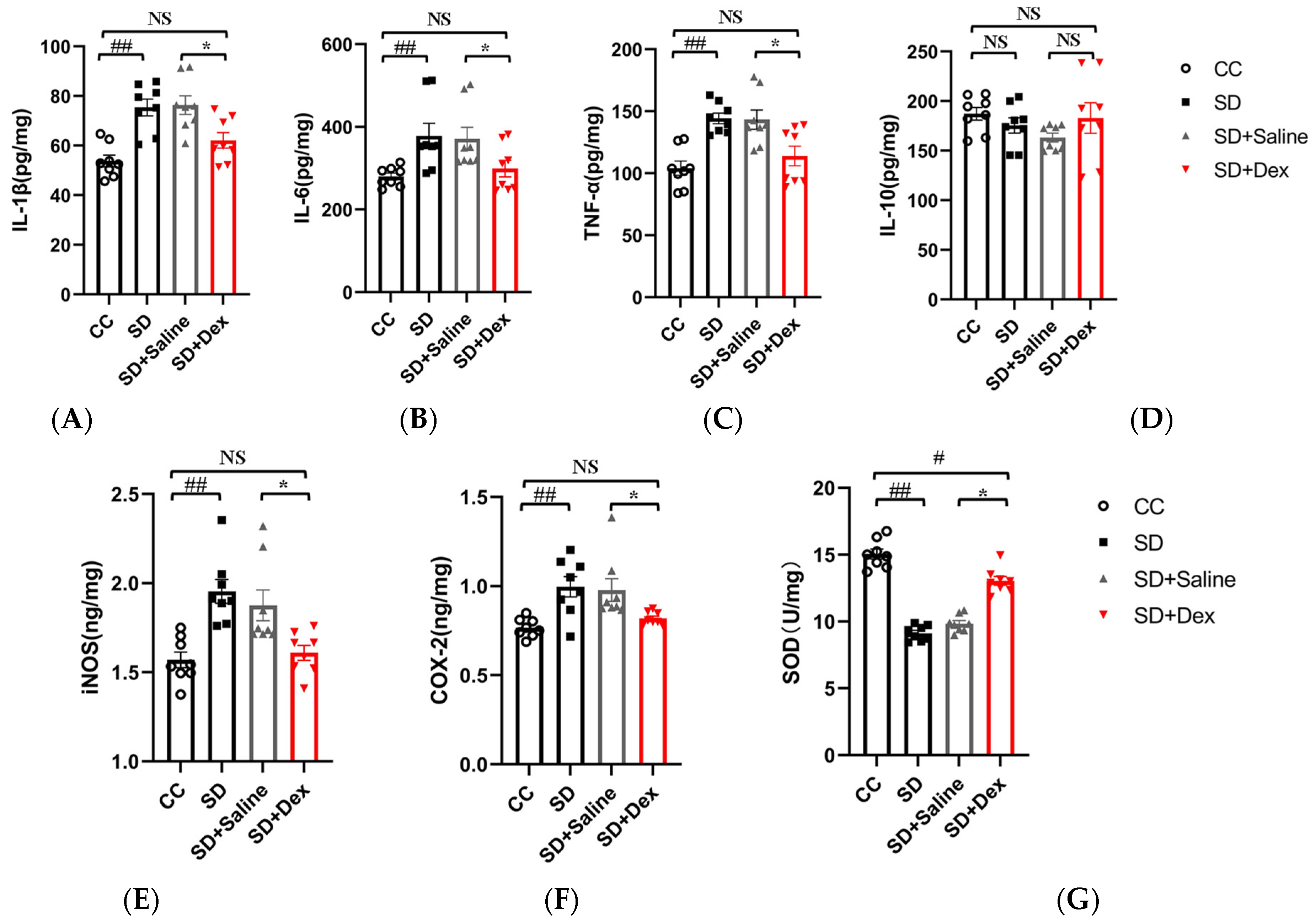
3.8. Dex Ameliorates Oxidative Stress and Inflammatory Reactions in the Prefrontal Cortex of Sleep-Deprived Mice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pires, G.N.; Bezerra, A.G.; Tufik, S.; Andersen, M.L. Effects of acute sleep deprivation on state anxiety levels: A systematic review and meta-analysis. Sleep Med. 2016, 24, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Chen, W.H.; Li, S.X.; He, Z.M.; Zhu, W.L.; Ji, Y.B.; Wang, Z.; Zhu, X.M.; Yuan, K.; Bao, Y.P.; et al. Gut microbiota modulates the inflammatory response and cognitive impairment induced by sleep deprivation. Mol. Psychiatry 2021, 26, 6277–6292. [Google Scholar] [CrossRef]
- Fullagar, H.H.; Skorski, S.; Duffield, R.; Hammes, D.; Coutts, A.J.; Meyer, T. Sleep and athletic performance: The effects of sleep loss on exercise performance, and physiological and cognitive responses to exercise. Sports Med. 2015, 45, 161–186. [Google Scholar] [CrossRef] [PubMed]
- Graves, L.; Pack, A.; Abel, T. Sleep and memory: A molecular perspective. Trends Neurosci. 2001, 24, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Leproult, R.; L’Hermite-Baleriaux, M.; Copinschi, G.; Penev, P.D.; Van Cauter, E. Leptin levels are dependent on sleep duration: Relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J. Clin. Endocrinol. Metab. 2004, 89, 5762–5771. [Google Scholar] [CrossRef] [Green Version]
- Manchanda, S.; Singh, H.; Kaur, T.; Kaur, G. Low-grade neuroinflammation due to chronic sleep deprivation results in anxiety and learning and memory impairments. Mol. Cell. Biochem. 2018, 449, 63–72. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99, 1325–1380. [Google Scholar] [CrossRef] [Green Version]
- Chennaoui, M.; Gomez-Merino, D.; Drogou, C.; Geoffroy, H.; Dispersyn, G.; Langrume, C.; Ciret, S.; Gallopin, T.; Sauvet, F. Effects of exercise on brain and peripheral inflammatory biomarkers induced by total sleep deprivation in rats. J. Inflamm. 2015, 12, 56. [Google Scholar] [CrossRef] [Green Version]
- Bellesi, M.; de Vivo, L.; Chini, M.; Gilli, F.; Tononi, G.; Cirelli, C. Sleep Loss Promotes Astrocytic Phagocytosis and Microglial Activation in Mouse Cerebral Cortex. J. Neurosci. 2017, 37, 5263–5273. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Huang, X.; Li, Y.; Xi, K.; Han, Y.; Mao, H.; Ren, K.; Wang, W.; Wu, Z. TNF signaling pathway-mediated microglial activation in the PFC underlies acute paradoxical sleep deprivation-induced anxiety-like behaviors in mice. Brain Behav. Immun. 2022, 100, 254–266. [Google Scholar] [CrossRef]
- Xue, R.; Wan, Y.H.; Sun, X.Q.; Zhang, X.; Gao, W.; Wu, W. Nicotinic Mitigation of Neuroinflammation and Oxidative Stress After Chronic Sleep Deprivation. Front. Immunol. 2019, 10, 2546. [Google Scholar] [CrossRef] [Green Version]
- Mantz, J.; Josserand, J.; Hamada, S. Dexmedetomidine: New insights. Eur. J. Anaesthesiol. 2011, 28, 3–6. [Google Scholar] [CrossRef]
- Mei, B.; Li, J.; Zuo, Z. Dexmedetomidine attenuates sepsis-associated inflammation and encephalopathy via central α2A adrenoceptor. Brain Behav. Immun. 2021, 91, 296–314. [Google Scholar] [CrossRef]
- Unchiti, K.; Leurcharusmee, P.; Samerchua, A.; Pipanmekaporn, T.; Chattipakorn, N.; Chattipakorn, S.C. The potential role of dexmedetomidine on neuroprotection and its possible mechanisms: Evidence from in vitro and in vivo studies. Eur. J. Neurosci. 2021, 54, 7006–7047. [Google Scholar] [CrossRef]
- Liaquat, Z.; Xu, X.; Zilundu, P.L.M.; Fu, R.; Zhou, L. The Current Role of Dexmedetomidine as Neuroprotective Agent: An Updated Review. Brain Sci. 2021, 11, 846. [Google Scholar] [CrossRef]
- Akeju, O.; Brown, E.N. Neural oscillations demonstrate that general anesthesia and sedative states are neurophysiologically distinct from sleep. Curr. Opin. Neurobiol. 2017, 44, 178–185. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Su, X.; Zhao, Y.; Zhong, C.L.; Mo, X.Q.; Zhang, R.; Wang, K.; Zhu, S.N.; Shen, Y.E.; Zhang, C.; et al. Effect of mini-dose dexmedetomidine supplemented intravenous analgesia on sleep structure in older patients after major noncardiac surgery: A randomized trial. Sleep Med. 2023, 102, 9–18. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, R.; Zhang, R.; Jiang, Y.; Liu, Y. Effects of dexmedetomidine administered for postoperative analgesia on sleep quality in patients undergoing abdominal hysterectomy. J. Clin. Anesth. 2017, 36, 118–122. [Google Scholar] [CrossRef]
- Zeng, W.; Chen, L.; Liu, X.; Deng, X.; Huang, K.; Zhong, M.; Zhou, S.; Zhan, L.; Jiang, Y.; Liang, W. Intranasal Dexmedetomidine for the Treatment of Pre-operative Anxiety and Insomnia: A Prospective, Randomized, Controlled, and Clinical Trial. Front. Psychiatry 2022, 13, 816893. [Google Scholar] [CrossRef]
- Alexopoulou, C.; Kondili, E.; Diamantaki, E.; Psarologakis, C.; Kokkini, S.; Bolaki, M.; Georgopoulos, D. Effects of dexmedetomidine on sleep quality in critically ill patients: A pilot study. Anesthesiology 2014, 121, 801–807. [Google Scholar] [CrossRef] [Green Version]
- Oto, J.; Yamamoto, K.; Koike, S.; Onodera, M.; Imanaka, H.; Nishimura, M. Sleep quality of mechanically ventilated patients sedated with dexmedetomidine. Intensive Care Med. 2012, 38, 1982–1989. [Google Scholar] [CrossRef] [PubMed]
- Moon, E.J.; Ko, I.G.; Kim, S.E.; Jin, J.J.; Hwang, L.; Kim, C.J.; An, H.; Lee, B.J.; Yi, J.W. Dexmedetomidine Ameliorates Sleep Deprivation-Induced Depressive Behaviors in Mice. Int. Neurourol. J. 2018, 22 (Suppl. 3), S139–S146. [Google Scholar] [CrossRef] [Green Version]
- Hwang, L.; Ko, I.G.; Jin, J.J.; Kim, S.H.; Kim, C.J.; Chang, B.; Rho, J.H.; Moon, E.J.; Yi, J.W. Dexmedetomidine ameliorates memory impairment in sleep-deprived mice. Anim. Cells Syst. 2019, 23, 371–379. [Google Scholar] [CrossRef]
- Cui, L.; Xue, R.; Zhang, X.; Chen, S.; Wan, Y.; Wu, W. Sleep deprivation inhibits proliferation of adult hippocampal neural progenitor cells by a mechanism involving IL-17 and p38 MAPK. Brain Res. 2019, 1714, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Yang, J.; Gong, W.Y.; Pan, Y.C.; Zheng, P.; Yue, X.F. NLRP3 inflammasome activation mediates sleep deprivation-induced pyroptosis in mice. PeerJ 2021, 9, e11609. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Yi, Y.; Liu, G.; Guo, R.; Wang, L.; Lan, T.; Wang, W.; Chen, X.; Chen, S.; et al. Hippocampal microRNA-26a-3p deficit contributes to neuroinflammation and behavioral disorders via p38 MAPK signaling pathway in rats. J. Neuroinflamm. 2022, 19, 283. [Google Scholar] [CrossRef]
- Huang, H.; Liu, A.; Wu, H.; Ansari, A.R.; Wang, J.; Huang, X.; Zhao, X.; Peng, K.; Zhong, J.; Liu, H. Transcriptome analysis indicated that Salmonella lipopolysaccharide-induced thymocyte death and thymic atrophy were related to TLR4-FOS/JUN pathway in chicks. BMC Genom. 2016, 17, 322. [Google Scholar] [CrossRef] [Green Version]
- Villafuerte, G.; Miguel-Puga, A.; Rodriguez, E.M.; Machado, S.; Manjarrez, E.; Arias-Carrion, O. Sleep deprivation and oxidative stress in animal models: A systematic review. Oxidative Med. Cell. Longev. 2015, 2015, 234952. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Wu, X.; Zhu, G.; Han, S.; Zhang, J. Dexmedetomidine alleviates sleep-restriction-mediated exaggeration of postoperative immunosuppression via splenic TFF2 in aged mice. Aging 2020, 12, 5318–5335. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Melatonin Alleviates Acute Sleep Deprivation-Induced Memory Loss in Mice by Suppressing Hippocampal Ferroptosis. Front. Pharmacol. 2021, 12, 708645. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, Z.Z.; Li, P.; Zhu, C.L.; Guo, Y.; Wang, J.; Deng, X.M.; Wang, J.F. Senkyunolide I Protects against Sepsis-Associated Encephalopathy by Attenuating Sleep Deprivation in a Murine Model of Cecal Ligation and Puncture. Oxid. Med. Cell. Longev. 2021, 2021, 6647258. [Google Scholar] [CrossRef]
- Tai, F.; Wang, C.; Deng, X.; Li, R.; Guo, Z.; Quan, H.; Li, S. Treadmill exercise ameliorates chronic REM sleep deprivation-induced anxiety-like behavior and cognitive impairment in C57BL/6J mice. Brain Res. Bull. 2020, 164, 198–207. [Google Scholar] [CrossRef]
- Mashour, G.A.; Pal, D.; Brown, E.N. Prefrontal cortex as a key node in arousal circuitry. Trends Neurosci. 2022, 45, 722–732. [Google Scholar] [CrossRef]
- Kenwood, M.M.; Kalin, N.H.; Barbas, H. The prefrontal cortex, pathological anxiety, and anxiety disorders. Neuropsychopharmacology 2022, 47, 260–275. [Google Scholar] [CrossRef]
- Ben Simon, E.; Rossi, A.; Harvey, A.G.; Walker, M.P. Overanxious and underslept. Nat. Hum. Behav. 2020, 4, 100–110. [Google Scholar] [CrossRef]
- Hotamisligil, G.S.; Davis, R.J. Cell Signaling and Stress Responses. Cold. Spring Harb. Perspect. Biol. 2016, 8, a006072. [Google Scholar] [CrossRef] [Green Version]
- Bachstetter, A.D.; Van Eldik, L.J. The p38 MAP Kinase Family as Regulators of Proinflammatory Cytokine Production in Degenerative Diseases of the CNS. Aging Dis. 2010, 1, 199–211. [Google Scholar]
- Arthur, J.S. MSK activation and physiological roles. Front. Biosci. 2008, 13, 5866–5879. [Google Scholar] [CrossRef]
- Gorska, M.M.; Liang, Q.; Stafford, S.J.; Goplen, N.; Dharajiya, N.; Guo, L.; Sur, S.; Gaestel, M.; Alam, R. MK2 controls the level of negative feedback in the NF-kappaB pathway and is essential for vascular permeability and airway inflammation. J. Exp. Med. 2007, 204, 1637–1652. [Google Scholar] [CrossRef] [Green Version]
- Shao, Y.; Chen, X.; Liu, Y.; Chen, X.; Li, C.; Wang, L.; Zhao, W. Dexmedetomidine alleviates lung injury in sepsis mice through regulating P38 MAPK signaling pathway. Panminerva Med. 2021, 63, 563–564. [Google Scholar] [CrossRef]
- Chen, Y.; Li, L.; Zhang, J.; Cui, H.; Wang, J.; Wang, C.; Shi, M.; Fan, H. Dexmedetomidine Alleviates Lipopolysaccharide-Induced Hippocampal Neuronal Apoptosis via Inhibiting the p38 MAPK/c-Myc/CLIC4 Signaling Pathway in Rats. Mol. Neurobiol. 2021, 58, 5533–5547. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J.M.; Majde, J.A. Cytokines and sleep. Int. Arch. Allergy Immunol. 1995, 106, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Opp, M.R. Cytokines and sleep. Sleep Med. Rev. 2005, 9, 355–364. [Google Scholar] [CrossRef]
- Kang, X.; Jiang, L.; Lan, F.; Tang, Y.Y.; Zhang, P.; Zou, W.; Chen, Y.J.; Tang, X.Q. Hydrogen sulfide antagonizes sleep deprivation-induced depression- and anxiety-like behaviors by inhibiting neuroinflammation in a hippocampal Sirt1-dependent manner. Brain Res. Bull. 2021, 177, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Guo, Y.; Zhou, G.; Wang, Y.; Zhang, J.; Wang, Z.; Wang, Q. Dexmedetomidine protects against high mobility group box 1-induced cellular injury by inhibiting pyroptosis. Cell Biol. Int. 2019, 43, 651–657. [Google Scholar] [CrossRef]
- Grubac, Z.; Sutulovic, N.; Suvakov, S.; Jerotic, D.; Puskas, N.; Macut, D.; Rasic-Markovic, A.; Simic, T.; Stanojlovic, O.; Hrncic, D. Anxiogenic Potential of Experimental Sleep Fragmentation Is Duration-Dependent and Mediated via Oxidative Stress State. Oxid. Med. Cell. Longev. 2021, 2021, 2262913. [Google Scholar] [CrossRef]
- Ramanathan, L.; Gulyani, S.; Nienhuis, R.; Siegel, J.M. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport 2002, 13, 1387–1390. [Google Scholar] [CrossRef]
- Ramanathan, L.; Hu, S.; Frautschy, S.A.; Siegel, J.M. Short-term total sleep deprivation in the rat increases antioxidant responses in multiple brain regions without impairing spontaneous alternation behavior. Behav. Brain Res. 2010, 207, 305–309. [Google Scholar] [CrossRef] [Green Version]
- D’Almeida, V.; Hipolide, D.C.; Azzalis, L.A.; Lobo, L.L.; Junqueira, V.B.; Tufik, S. Absence of oxidative stress following paradoxical sleep deprivation in rats. Neurosci. Lett. 1997, 235, 25–28. [Google Scholar] [CrossRef]
- Gopalakrishnan, A.; Ji, L.L.; Cirelli, C. Sleep deprivation and cellular responses to oxidative stress. Sleep 2004, 27, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Vutskits, L.; Clark, J.D.; Kharasch, E.D. Reporting Laboratory and Animal Research in ANESTHESIOLOGY: The Importance of Sex as a Biologic Variable. Anesthesiology 2019, 131, 949–952. [Google Scholar] [CrossRef]
- Dolsen, E.A.; Crosswell, A.D.; Prather, A.A. Links Between Stress, Sleep, and Inflammation: Are there Sex Differences? Curr. Psychiatry Rep. 2019, 21, 8. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, H.; Deng, B.; Wang, X.; Liang, P.; Xu, S.; Jing, Z.; Xiao, Z.; Sun, L.; Gao, C.; et al. Dexmedetomidine Improves Anxiety-like Behaviors in Sleep-Deprived Mice by Inhibiting the p38/MSK1/NFκB Pathway and Reducing Inflammation and Oxidative Stress. Brain Sci. 2023, 13, 1058. https://doi.org/10.3390/brainsci13071058
Li J, Zhang H, Deng B, Wang X, Liang P, Xu S, Jing Z, Xiao Z, Sun L, Gao C, et al. Dexmedetomidine Improves Anxiety-like Behaviors in Sleep-Deprived Mice by Inhibiting the p38/MSK1/NFκB Pathway and Reducing Inflammation and Oxidative Stress. Brain Sciences. 2023; 13(7):1058. https://doi.org/10.3390/brainsci13071058
Chicago/Turabian StyleLi, Jiangjing, Heming Zhang, Bin Deng, Xin Wang, Peng Liang, Shenglong Xu, Ziwei Jing, Zhibin Xiao, Li Sun, Changjun Gao, and et al. 2023. "Dexmedetomidine Improves Anxiety-like Behaviors in Sleep-Deprived Mice by Inhibiting the p38/MSK1/NFκB Pathway and Reducing Inflammation and Oxidative Stress" Brain Sciences 13, no. 7: 1058. https://doi.org/10.3390/brainsci13071058
APA StyleLi, J., Zhang, H., Deng, B., Wang, X., Liang, P., Xu, S., Jing, Z., Xiao, Z., Sun, L., Gao, C., Wang, J., & Sun, X. (2023). Dexmedetomidine Improves Anxiety-like Behaviors in Sleep-Deprived Mice by Inhibiting the p38/MSK1/NFκB Pathway and Reducing Inflammation and Oxidative Stress. Brain Sciences, 13(7), 1058. https://doi.org/10.3390/brainsci13071058





