Abstract
Background: chronic idiopathic demyelinating polyneuropathy (CIDP) is an acquired, immune-mediated neuropathy characterized by weakness, sensory symptoms and significant reduction or loss of deep tendon reflexes evolving over 2 months at least, associated with electrophysiological evidence of peripheral nerve demyelination. Recently, subcutaneous immunoglobulins (SCIg) have been introduced in clinical practice as a maintenance therapy for CIDP; nevertheless, electrophysiological and efficacy data are limited. Methods: to evaluate SCIg treatment efficacy, we retrospectively reviewed data from 15 CIDP patients referring to our clinic, receiving SCIg treatment and who performed electrophysiological studies (NCS) and clinical scores (MRC sumscore, INCAT disability score and ISS) before starting the treatment and at least one year after. Results: NCS showed no significant changes before and during treatment for all the nerves explored. Clinical scores did not significantly change between evaluations. Correlation analysis evidenced a positive correlation of cMAPs distal amplitude with MRC sumscore and a trend of negative correlation with the INCAT disability score. Conclusions: SCIg maintenance therapy preserves nerve function in CIDP with a good efficacy and safety. Treatment effectiveness can be assessed with ENG, which represents a useful instrument in the follow-up and prognostic assessment of CIDP.
1. Introduction
Chronic idiopathic demyelinating polyneuropathy (CIDP) is an acquired, immune-mediated polyradiculoneuropathy evolving over 2 months at least [1]. The typical form is characterized by sensory symptoms (e.g., paresthesia, sensory loss), distal muscle weakness and reduced or absent deep tendon reflexes, with a distal and symmetrical involvement that progresses proximally [2]. Cranial nerves and the autonomic system are usually spared in CIDP. However, there are also atypical forms in which some of the classical symptoms are absent (e.g., motor CIDP or sensory CIDP) or an asymmetrical or focal involvement is observed [3]. Electrophysiological findings play a key role in the diagnosis and monitoring of CIDP: according to the EAN/PNS (European Academy of Neurology/Peripheral Nerve Society) diagnostic criteria, the demonstration of peripheral nerve demyelination in two or more nerves is required for a defined diagnosis [4]. Electrophysiological variables have been also extensively used in clinical trials to evaluate the response to treatment and the progression of the disease [5,6].
There are several therapeutic options for CIDP, which include intravenous immunoglobulin (IVIG), plasma exchange (PEX) and glucocorticoids. After an induction therapy, most patients require a maintenance therapy with periodic IVIG administration, PEX procedures or immunodepressants (steroids, rituximab) to prevent relapses and progression [4,7,8]. Subcutaneous immunoglobulin therapy (SCIg) has been used as an alternative to IVIG in primary immunodeficiencies for over thirty years. Compared to IVIG, which are administered every 3–4 weeks, SCIg are administered in smaller doses; hence, the frequency of administration is higher (once or twice weekly). Evidence shows that, while the SCIg efficacy is similar to IVIG, patients usually report a lower incidence of side effects (e.g., headache, local reactions in injection site, renal and cardiac impairment) and a better quality of life; this is attributed to the lower peak serum dose reached by SCIg compared to IVIG (61%); another advantage is that SCIg therapy does not require an intravenous access. Hence, SCIg is commonly administered at home [9,10]. Recently, SCIg has been introduced in clinical practice as a maintenance therapy even for CIDP; nevertheless, data on the efficacy of SCIg and electrophysiological data are limited.
2. Materials and Methods
2.1. Study Procedures
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee Palermo I, University of Palermo (Protocol code 07/2020; 13 July 2020). In this study, we present a retrospective evaluation of the efficacy of SCIg treatment in a population of CIDP patients using electroneurography (ENG) and clinical scores.
2.2. Patient Demographics
We reviewed data from patients referring to our clinic (“Policlinico Paolo Giaccone di Palermo” –“Centro per la Diagnosi e Cura della Malattie Neuromuscolari Rare”) from January 2014 to September 2022.
Inclusion criteria:
Patients accessing our clinic were enrolled in the presence of the following criteria:
- −
- Age >18 years;
- −
- Diagnosis of definite CIDP according to the EAN/PNS 2021 criteria;
- −
- Treatment with SCIg;
- −
- Evaluation with apposite clinical scales (INCAT, ISS, MRC) and nerve conduction studies.
- −
- Exclusion criteria:
- −
- Lack of infomed consent to participation;
- −
- Diagnosis of probable or possible CIDP according to the EAN/PNS 2021 criteria;
- −
- Lack of response to IVIg.
2.3. Clinical Assessment
The inflammatory neuropathy cause and treatment (INCAT) disability score is calculated by summing a score measuring arm impairment (0 = no upper limb problems; 5 = inability to use either arms for purposeful movements) and another measuring leg impairment (0 = walking unaffected; 5 = restricted to wheelchair). The INCAT score can range from 0 (no disability) to 10 (maximum disability) [11].
Similarly, the INCAT sensory sumscore (ISS) evaluates sensory impairment by measuring pinprick sensation, vibration sensation and two-point discrimination at arms and legs. It is calculated by adding scores obtained by all four limbs and it ranges from 0 (normal sensation) to 20 (severe sensory deficit) [12].
The Medical Research Council (MRC) sumscore measures strength by applying the MRC 5-point system (0 = no movement; 5 = movement completed against full resistance) to six muscle groups (abduction of the arm, flexion of the forearm, extension of the wrist, flexion of the leg, extension of the knee, dorsal flexion of the foot) of both sides. It ranges from 0 (minimum strength) to 60 (maximum strength) [13].
The INCAT disability score, the ISS and the MRC sumscore were developed specifically to evaluate patients affected by inflammatory polyneuropathies and have been used in several clinical trials to estimate the efficacy of treatments and the clinical progression of the disease over time.
2.4. Nerve Conduction Studies (NCS)
Sensory nerve action potentials (SNAPs) and compound muscle action potentials (CMAPs) were recorded, analyzing distal latencies (dL), negative peak amplitudes (dA) and conduction velocities (CV). We investigated median and ulnar nerves for the upper limbs and peroneal, tibial and sural nerves for the lower limbs, according to standard procedures (i.e., bipolar surface stimulating electrodes delivering rectangular pulses 0.1–0.5 ms in duration with recording electrodes placed over the recording site, with a ground electrode placed between the recording electrodes and stimulation site). In particular, the study protocol was defined as follows:
For upper-limb SNAPs: stimulation at wrist and registration from II digit (medial nerve) and V digit (ulnar nerve);
For upper-limb CMAPs: stimulation at the wrist and elbow and recording from abductor pollicis brevis (APB) for median nerve; stimulation at the wrist and elbow 4 cm distal from the medial epicondyle of the humerus and recording from the abductor digiti minimi (ADM) muscle for the ulnar nerve;
For lower-limb SNAPs: stimulation at the posterior-lateral calf, recording from the lateral malleolus for the sural nerve;
For lower-limb CMAPs: stimulation at the medial malleolus and popliteal fossa, recording from the abductor hallucis brevis (AHB) muscle for the tibial nerve; stimulation at the anterior ankle and popliteal fossa, recording from the extensor digitorum brevis (EDB) muscle for the peroneal nerve.
2.5. Statistical Analysis
Neurophysiological variables (continuous) and clinical scores (discrete) were compared using the Mann–Whitney test to detect changes between the evaluations before and during therapy. Correlations between clinical scores and neurophysiological variables (cMAPs and SAPs distal amplitude) were evaluated using Pearson’s r value. Significance was set at 0.05 for all the analyzed variables. Data are presented as a median ± interquartile range, except for correlations, which are presented as a mean value ± standard deviation. All the analyses were performed with JASP (version 0.16.2; computer software).
3. Results
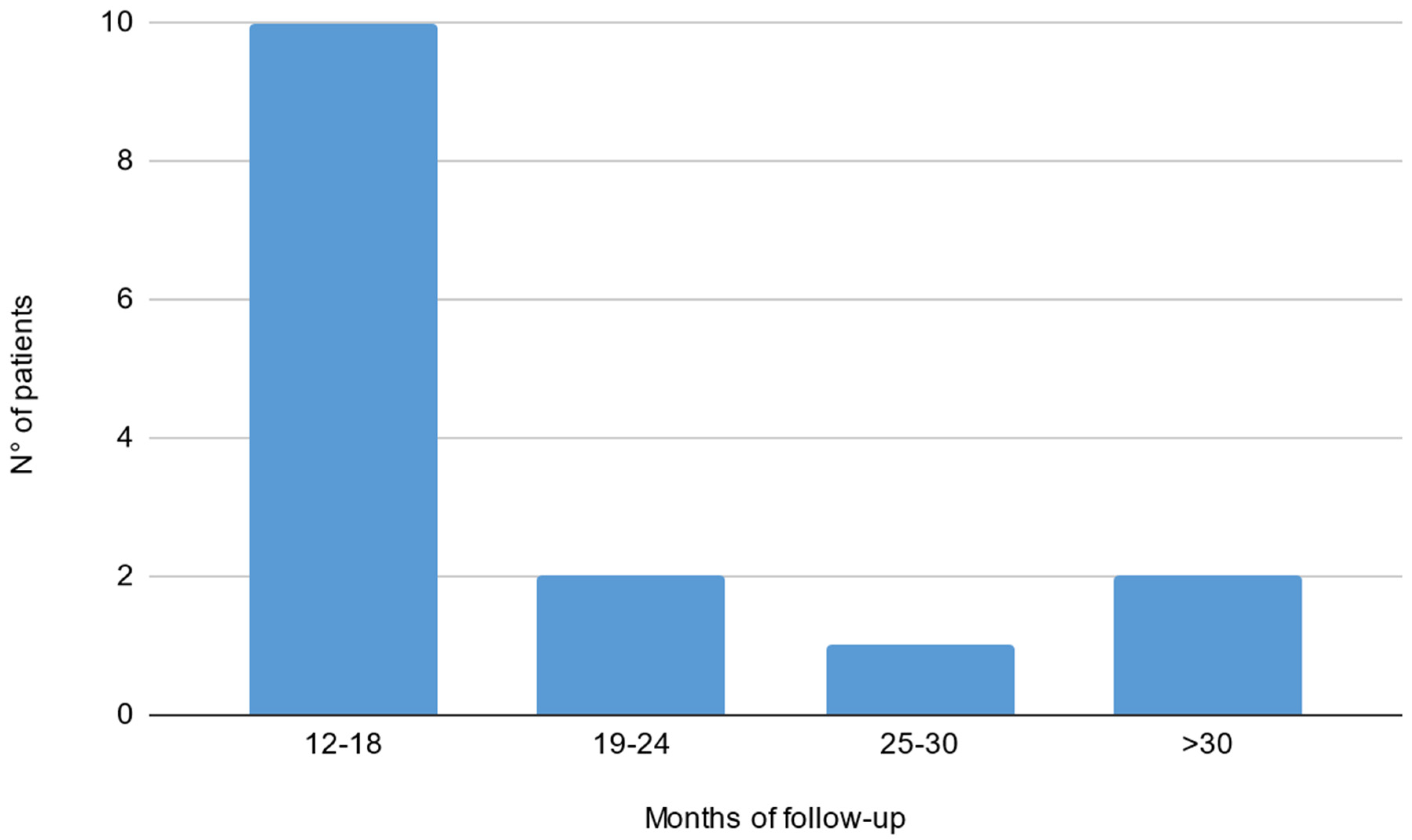
Out of 19 patients, 3 were lacking a baseline electrophysiological evaluation and 1 did not reach a 1-year follow-up. Fifteen patients were included in the final analysis (9 males, 56 ± 13 years; see Table 1 for population characteristics). Thirteen patients (87%) showed a typical CIDP phenotype, while two showed an atypical pattern (motor CIDP). Five patients out of fifteen did not have an evaluation with the mentioned clinical scales before and/or after treatment start; therefore, analysis on clinical scores and correlations were performed on the remaining 10 patients. Evaluations during SCIg treatment (both clinical and neurophysiological) were performed after a median interval of 16 months (IQ 13–19) from the start. Median time between ENG evaluations was 37 months (IQ range 29–42). The median time between clinical evaluations was 35 months (IQ range 10–20; see Figure 1 for histogram of follow-up time). Before starting SCIg as maintenance therapy, nine patients (60%) were treated with prednisone; nine patients (60%) received IVIg as a maintenance therapy; one patient (6%) was treated with cyclophosphamide; and one (6%) with azathioprine.

Table 1.
Clinical data in our cohort of CIDP patients treated with SCIg. Chronic inflammatory demyelinating polyneuropathy, CIDP; intravenous immunoglobuline, IVIg; subcutaneous immunoglobulins, SCIg.
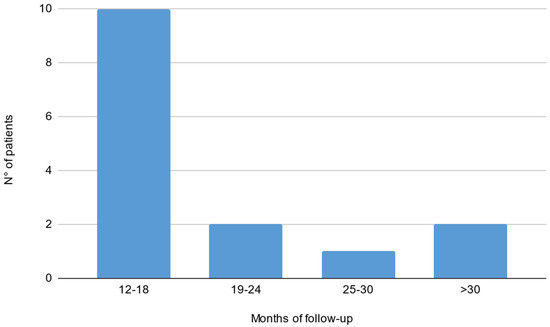
Figure 1.
Histogram of follow-up time reported in months in our cohort of CIDP patients treated with SCIg.
At baseline, the median nerve (both motor and sensitive) was tested in 60% of patients, the ulnar (both motor and sensitive) nerve in 60%, the peroneal motor nerve in 60%, the tibial nerve in 73.3% and the sural nerve in 20%. Repeat testing was conducted in 100% of patients for all the nerves, except for the sural nerve, which was tested in 66% of the patients at follow-up.
NCS showed no significant changes before and during treatment for all the nerves explored (Table 2); a trend of worsening was observed for dA (11.9 vs. 4.3 uV; p = 0.078) and CV (44.46 vs. 32.13 m/sec; p = 0.17) registered from the right sensitive median nerve, while CV registered from the right peroneal nerve improved at follow-up (29.82 vs. 44.78 m/sec; p= 0.15).

Table 2.
Electrophysiological data. Time 1: pre-SCIg; time 2: during SCIg; N: number of patients; Dx: right nerve; Sn: left nerve; dL: distal latency; dA: distal amplitude; SAP: sensory action potential; cMAP: compound motor action potential; CV: conduction velocity; SD: standard deviation.
The median scores before the start of the therapy were 3 (IQ range 3–4) for the INCAT disability score, 10 (IQ range 8–10) for ISS and 54 (IQ range 47–58) for the MRC sumscore. The scores did not significantly change at follow-up (Table 3).

Table 3.
Clinical scores. Time 1: pre-SCIg; time 2: during SCIg; MRC: Medical Research Council sumscore; ISS: INCAT sensory scale; SD: standard deviation.
Correlation analysis (Table 4) evidenced a positive correlation of cMAPs distal amplitude with an MRC sumscore (r = 0.2; p = 0.05) and a trend of negative correlation with the INCAT disability score (r = −0.156; p = 0.15).

Table 4.
Pearson’s correlations.
4. Discussion
Only a few studies reported electrophysiological data of CIDP patients undergoing SCIg treatment. The PATH study, which is the largest trial to evaluate SCIg efficacy in CIDP, reported no significant changes in nerve conduction variables after six months in 115 patients, equally divided in two treatment regimens (0.2 g/kg/week vs. 0.4 g/kg/week), while the placebo group (57 patients) showed a slight worsening of proximal motor latencies and conduction velocities in median, ulnar and peroneal motor nerves. Cirillo et al. reported how SCIg therapy is effective in preserving nerve function in the long term in a population of 14 patients, which also showed an improvement of CMAP amplitude and CVs after 48 months of treatment [14,15,16].
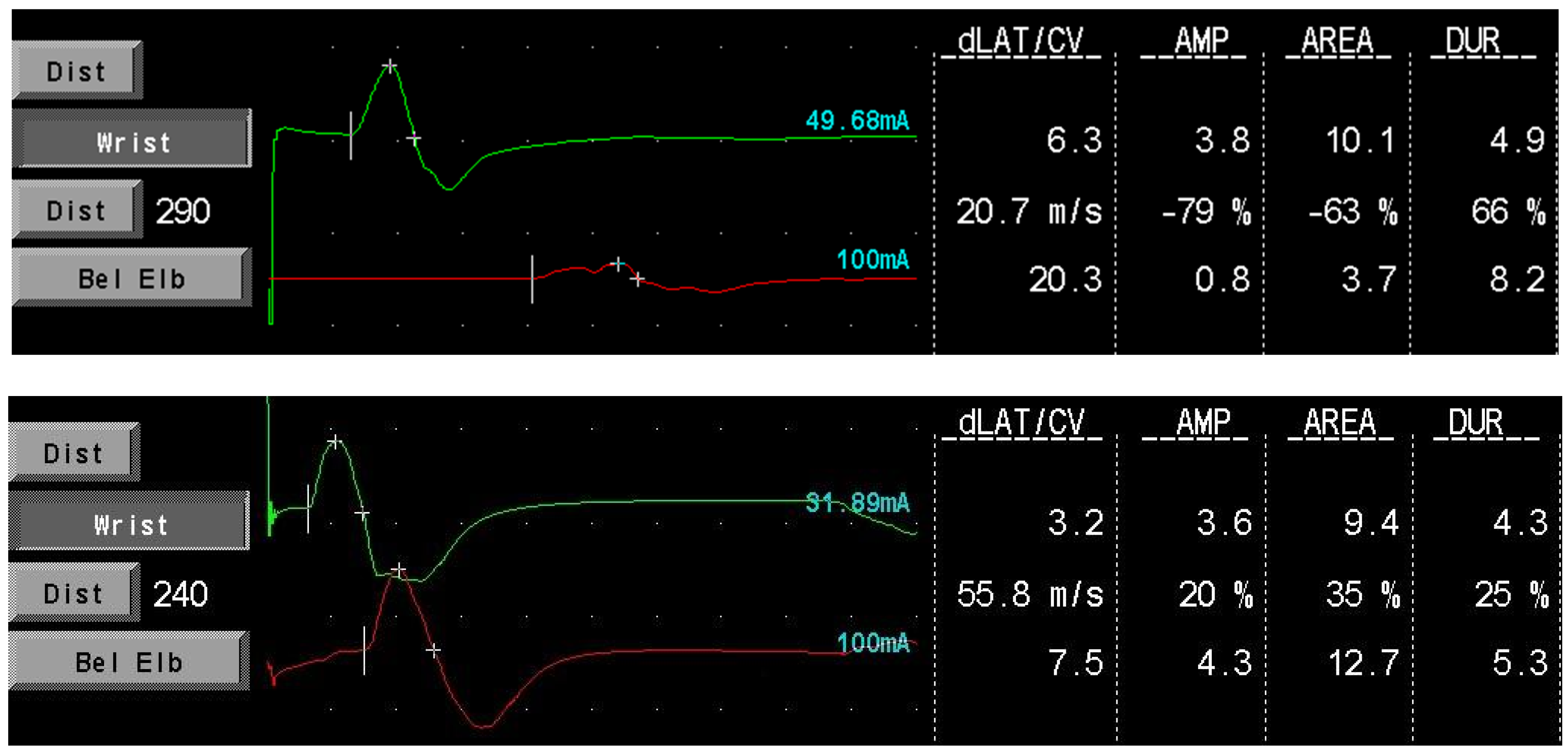
Our data provide additional support that SCIg maintenance therapy is effective in preventing nerve function deterioration in CIDP patients, confirming the findings of the aforementioned studies. The absence of cMAP amplitude improvement in our population could be due to the shorter median follow-up time after the start of therapy compared to the study of Cirillo et al. However, an improvement was seen in single patients (see Figure 2; a block conduction on motor median nerve resolved after treatment in patient 001). Furthermore, we confirmed the presence of a positive correlation between cMAPs amplitude and MRC sumscore, suggesting that ENG variables could hold a role as prognostic factors to estimate treatment efficacy and duration time.
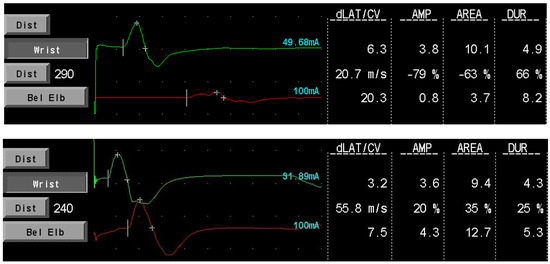
Figure 2.
Patient 001 motor medial nerve conduction before (upper image) and during (lower image) SCIg therapy. The conduction block resolves with treatment. dLAT: distal latency; CV: conduction velocity; AMP: amplitude (mV); DUR: duration (msec).
There were no differences between patients with typical and atypical CIDP phenotype in our population. However, the number of patients with atypical characteristics in our population was too small to draw conclusions from our findings. Considering that atypical phenotypes are reported to respond poorly to immunoglobulin treatment [17], further studies are required to investigate whether the efficacy of SCIg treatment changes in atypical CIDP.
A relevant strength of this study is the long follow-up of our cohort of CIDP patients compared to previous studies. Indeed, prolonged therapy with SCIg was safe and provided stable disease burden and neurophysiological data. Moreover, an improvement in the MCV of peroneal nerves support the idea that the demyelinating process and inflammation in CIDP recede from this treatment; also, stable CMAP amplitude on motor nerves confirm that no more significant axonal loss happens during SCIg treatment in CIDP. Another relevant point is the use of neurophysiological variables to assess treatment efficacy. Despite being cheap, easily reproducible and easy to perform, ENG has been seldom used as a long-term follow-up technique in clinical studies. We suggest that neurophysiological examination could provide more detailed information on SCIg treatment efficacy compared to clinical scores.
Our study has some limitations; first, the small size of the population analyzed; second, the lack of a homogenous protocol of ENG testing and clinical follow-up among patients, which reduces the significance of our data; and third, the absence of a control group (i.e., with IVIg administration).
5. Conclusions
Our data strengthen the evidence on the efficacy of SCIg maintenance therapy in CIDP. Indeed, no patients presented a worsening of symptoms during maintenance treatment with SCIg and there was good safety. Nerve conduction studies are a useful instrument not only in the diagnostic process, but also in the follow-up and prognostic assessment of CIDP.
Author Contributions
Conceptualization, V.D.S. and F.B.; methodology, V.D.S. and F.B.; software, P.A.; formal analysis, P.A.; investigation, A.T., A.L., P.A. and S.I.; data curation, B.M., A.P. and F.G.; writing—original draft preparation, P.A.; writing—review and editing, V.D.S., A.T. and M.G.; supervision, F.B and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee Palermo I, University of Palermo (Protocol code 07/2020; 13 July 2020).
Informed Consent Statement
Informed consent was obtained from all the subjects involved in the study.
Data Availability Statement
Data are available from the corresponding author upon a reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuwabara, S.; Misawa, S. Chronic Inflammatory Demyelinating Polyneuropathy; Springer: Singapore, 2019; pp. 333–343. [Google Scholar] [CrossRef]
- Di Stefano, V.; Barbone, F.; Ferrante, C.; Telese, R.; Vitale, M.; Onofrj, M.; Di Muzio, A. Inflammatory polyradiculoneuropathies: Clinical and immunological aspects, current therapies, and future perspectives. Eur. J. Inflamm. 2020, 18, 2058739220942340. [Google Scholar] [CrossRef]
- Nobile-Orazio, E. Chronic inflammatory demyelinating polyradiculoneuropathy and variants: Where we are and where we should go. J. Peripher. Nerv. Syst. 2014, 19, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Bergh, P.Y.K.V.D.; Doorn, P.A.; Hadden, R.D.M.; Avau, B.; Vankrunkelsven, P.; Allen, J.A.; Attarian, S.; Blomkwist-Markens, P.H.; Cornblath, D.R.; Eftimov, F.; et al. European Academy of Neurology/Peripheral Nerve Society guideline on diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy: Report of a joint Task Force—Second revision. Eur. J. Neurol. 2021, 28, 3556–3583. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, P.D.; Bril, V.; Dalakas, M.C.; Deng, C.; Hanna, K.; Hartung, H.-P.; Hughes, R.; Latov, N.; Merkies, I.; Van Doorn, P. Safety and Tolerability of Immune Globulin Intravenous in Chronic Inflammatory Demyelinating Polyradiculoneuropathy. Arch. Neurol. 2010, 67, 1082–1088. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, M.; Hartung, H.; Lawo, J.; Durn, B.L.; Mielke, O.; Bril, V. Electrophysiological predictors of response to subcutaneous immunoglobulin therapy in chronic inflammatory demyelinating polyneuropathy. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2021, 132, 2184–2190. [Google Scholar] [CrossRef]
- Doneddu, P.E.; Nobile-Orazio, E. Management of chronic inflammatory demyelinating polyradiculopathy. Curr. Opin. Neurol. 2018, 31, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Bunschoten, C.; Jacobs, B.C.; van den Bergh, P.Y.K.; Cornblath, D.R.; van Doorn, P.A. Progress in diagnosis and treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Lancet Neurol. 2019, 18, 784–794. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.; Allen, J.A. Optimizing IgG therapy in chronic autoimmune neuropathies: A hypothesis driven approach. Muscle Nerve 2015, 51, 315–326. [Google Scholar] [CrossRef] [PubMed]
- Gentile, L.; Mazzeo, A.; Russo, M.; Arimatea, I.; Vita, G.; Toscano, A. Long-term treatment with subcutaneous immunoglobulin in patients with chronic inflammatory demyelinating polyradiculoneuropathy: A follow-up period up to 7 years. Sci. Rep. 2020, 10, 7910. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.; Bensa, S.; Willison, H.; van den Bergh, P.; Comi, G.; Illa, I.; Nobile-Orazio, E.; van Doorn, P.; Dalakas, M.; Bojar, M.; et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Ann. Neurol. 2001, 50, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Merkies IS, J.; Schmitz PI, M.; Van Der, M.e.c.h.é.; FG, A.; Samijn JP, A.; Van Doorn, P.A. Psychometric evaluation of a new handicap scale in immune-mediated polyneuropathies. Muscle Nerve 2002, 25, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Syndrome, G.-B. Interobserver agreement in the assessment of muscle strength Guillain-Barre Syndrome. Muscle Nerve 1991, 14, 1103–1109. [Google Scholar]
- Lamb, Y.N.; Syed, Y.Y.; Dhillon, S. Immune Globulin Subcutaneous (Human) 20% (Hizentra®): A Review in Chronic Inflammatory Demyelinating Polyneuropathy. CNS Drugs 2019, 33, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Todisco, V.; Tedeschi, G. Long-term neurophysiological and clinical response in patients with chronic inflammatory demyelinating polyradiculoneuropathy treated with subcutaneous immunoglobulin. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2018, 129, 967–973. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Todisco, V.; Ricciardi, D.; Tedeschi, G. Clinical-neurophysiological correlations in chronic inflammatory demyelinating polyradiculoneuropathy patients treated with subcutaneous immunoglobulin. Muscle Nerve 2019, 60, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Dziadkowiak, E.; Waliszewska-Prosół, M.; Nowakowska-Kotas, M.; Budrewicz, S.; Koszewicz, Z.; Koszewicz, M. Pathophysiology of the Different Clinical Phenotypes of Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP). Int. J. Mol. Sci. 2021, 23, 179. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).