Neuropsychological Characterization of Autosomal Recessive Intellectual Developmental Disorder 59 Associated with IMPA1 (MRT59)
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Sociodemographic and Clinical Variables
2.4. Wechsler Abbreviated Scale of Intelligence (WASI)
2.5. Functional Independence Measure (FIM)
3. Results
3.1. Description of Sample
3.2. Cognitive Profile
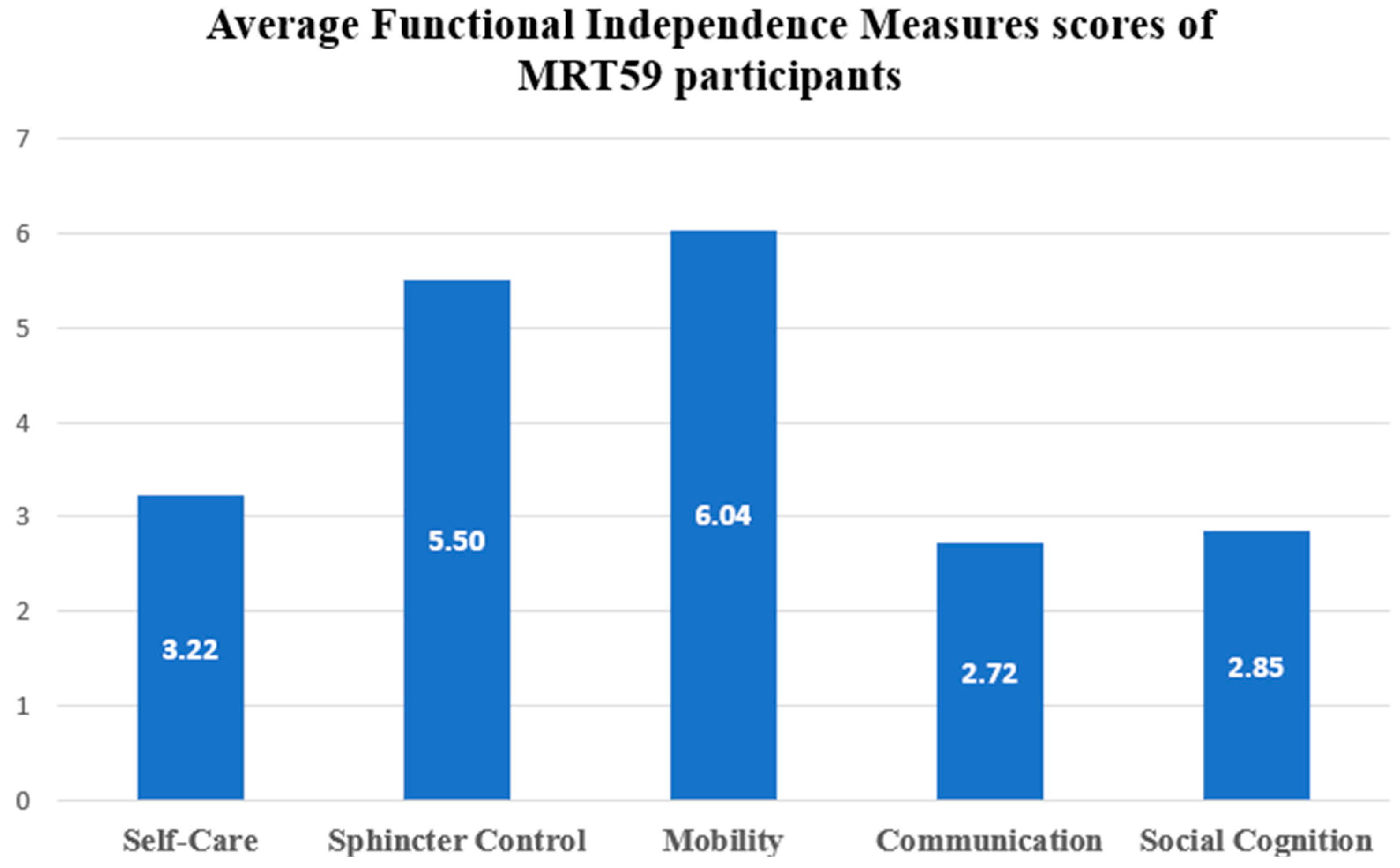
3.3. Functional Dependence
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hakim, S.; Bertucci, M.C.; Conduit, S.E.; Vuong, D.L.; Mitchell, C.A. Inositol Polyphosphate Phosphatases in Human Disease. Curr. Top. Microbiol. Immunol. 2012, 362, 247–314. [Google Scholar] [CrossRef]
- Atack, J.R.; Broughton, H.B.; Pollack, S.J. Inositol Monophosphatase—A Putative Target for Li+ in the Treatment of Bipolar Disorder. Trends Neurosci. 1995, 18, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Cryns, K.; Shamir, A.; Van Acker, N.; Levi, I.; Daneels, G.; Goris, I.; Bouwknecht, J.A.; Andries, L.; Kass, S.; Agam, G.; et al. IMPA1 Is Essential for Embryonic Development and Lithium-like Pilocarpine Sensitivity. Neuropsychopharmacology 2008, 33, 674–684. [Google Scholar] [CrossRef] [PubMed]
- Toker, L.; Bersudsky, Y.; Plaschkes, I.; Chalifa-Caspi, V.; Berry, G.T.; Buccafusca, R.; Moechars, D.; Belmaker, R.H.; Agam, G. Inositol-Related Gene Knockouts Mimic Lithium’s Effect on Mitochondrial Function. Neuropsychopharmacology 2014, 39, 319. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.; Melo, U.S.; Pessoa, A.L.S.; Nobrega, P.R.; Kitajima, J.P.; Rusch, H.; Vaz, F.; Lucato, L.T.; Zatz, M.; Kok, F.; et al. A Homozygous Loss-of-Function Mutation in Inositol Monophosphatase 1 (IMPA1) Causes Severe Intellectual Disability. Mol. Psychiatry 2016, 21, 1125–1129. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.P.; Pessoa, A.L.S.; Figueiredo, T.; Rafferty, M.; Melo, U.S.; Nóbrega, P.R.; Murphy, N.; Kok, F.; Zatz, M.; Santos, S.; et al. Loss-of-Function Mutation in Inositol Monophosphatase 1 (IMPA1) Results in Abnormal Synchrony in Resting-State EEG. Orphanet. J. Rare Dis. 2019, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.; Mendes, A.P.D.; Moreira, D.P.; Goulart, E.; Oliveira, D.; Kobayashi, G.S.; Stern, S.; Kok, F.; Marchetto, M.C.; Santos, R.; et al. Inositol Monophosphatase 1 (IMPA1) Mutation in Intellectual Disability Patients Impairs Neurogenesis but Not Gliogenesis. Mol. Psychiatry 2021, 26, 3558–3571. [Google Scholar] [CrossRef]
- Wechsler, D. WASI—Escala Wechsler Abreviada de Inteligência; Pearson: London, UK, 2014; ISBN 978-85-8040-375-6. [Google Scholar]
- Wechsler, D. Wechsler Abbreviated Scale of Intelligence WASI: Manual; Pearson: London, UK, 1999; ISBN 9780158979267. [Google Scholar]
- Weiss, L.; Saklofske, D.; Prifitera, A.; Holdnack, J. WISC-IV—Interpretação Clínica Avançada; Editora Pearson Clinical: São Paulo, Brazil, 2016; ISBN 978-85-8040-754-9. [Google Scholar]
- Granger, C.V.; Hamilton, B.B.; Keith, R.A.; Zielezny, M.; Sherwin, F.S. Advances in Functional Assessment for Medical Rehabilitation. Top Geriatr. Rehabil. 1986, 1, 59–74. [Google Scholar] [CrossRef]
- Linacre, J.M.; Heinemann, A.W.; Wright, B.D.; Granger, C.V.; Hamilton, B.B. The Structure and Stability of the Functional Independence Measure. Arch. Phys. Med. Rehabil. 1994, 75, 127–132. [Google Scholar] [CrossRef]
- Riberto, M.; Miyazaki, M.H.; Jucá, S.S.H.; Sakamoto, H.; Pinto, P.P.N.; Battistella, L.R. Validação Da Versão Brasileira Da Medida de Independência Funcional. Acta Fisiátrica 2004, 11, 72–76. [Google Scholar] [CrossRef]
- WHO. International Statistical Classification of Diseases and Related Health Problems; World Health Organization: Geneva, Switzerland, 2015.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-5, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 089042554. [Google Scholar]
- Da Silva, R.d.A.; Mograbi, D.C.; Landeira-Fernandez, J.; Cheniaux, E. Insight in Bipolar Disorder: A Systematic Review. J. Bras. Psiquiatr. 2014, 63, 242–254. [Google Scholar] [CrossRef]
- Shaltiel, G.; Shamir, A.; Nemanov, L.; Yaroslavsky, Y.; Nemets, B.; Ebstein, R.P.; Belmaker, R.H.; Agam, G. Inositol Monophosphatase Activity in Brain and Lymphocyte-Derived Cell Lines of Bipolar Patients. World J. Biol. Psychiatry 2001, 2, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Rangel, D.M.; Nóbrega, P.R.; Saraiva-Pereira, M.L.; Jardim, L.B.; Braga-Neto, P. A Case Series of Hereditary Cerebellar Ataxias in a Highly Consanguineous Population from Northeast Brazil. Park. Relat. Disord. 2019, 61, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, T.; Melo, U.S.; Pessoa, A.L.S.; Nobrega, P.R.; Kitajima, J.P.; Correa, I.; Zatz, M.; Kok, F.; Santos, S. Homozygous Missense Mutation in MED25 Segregates with Syndromic Intellectual Disability in a Large Consanguineous Family. J. Med. Genet. 2015, 52, 123–127. [Google Scholar] [CrossRef]
- Santos, S.; Kok, F.; Weller, M.; de Paiva, F.R.L.; Otto, P.A. Inbreeding Levels in Northeast Brazil: Strategies for the Prospecting of New Genetic Disorders. Genet. Mol. Biol. 2010, 33, 220–223. [Google Scholar] [CrossRef]
- Berry, G.T.; Wu, S.; Buccafusca, R.; Ren, J.; Gonzales, L.W.; Ballard, P.L.; Golden, J.A.; Stevens, M.J.; Greer, J.J. Loss of Murine Na+/Myo-Inositol Cotransporter Leads to Brain Myo-Inositol Depletion and Central Apnea. J. Biol. Chem. 2003, 278, 18297–18302. [Google Scholar] [CrossRef]
- Ohnishi, T.; Murata, T.; Watanabe, A.; Hida, A.; Ohba, H.; Iwayama, Y.; Mishima, K.; Gondo, Y.; Yoshikawa, T. Defective Craniofacial Development and Brain Function in a Mouse Model for Depletion of Intracellular Inositol Synthesis. J. Biol. Chem. 2014, 289, 10785. [Google Scholar] [CrossRef]
- Andreassi, C.; Zimmermann, C.; Mitter, R.; Fusco, S.; Devita, S.; Saiardi, A.; Riccio, A. An NGF-Responsive Element Targets Myo-Inositol Monophosphatase-1 MRNA to Sympathetic Neuron Axons. Nat. Neurosci. 2010, 13, 291–301. [Google Scholar] [CrossRef]
- Varela-Nallar, L.; Inestrosa, N.C. Wnt Signaling in the Regulation of Adult Hippocampal Neurogenesis. Front. Cell. Neurosci. 2013, 7, 51718. [Google Scholar] [CrossRef]
- Van Doze, A.; Perez, D.M. G-Protein-Coupled Receptors in Adult Neurogenesis. Pharmacol. Rev. 2012, 64, 645. [Google Scholar] [CrossRef]
- Ohtsuka, T.; Sakamoto, M.; Guillemot, F.; Kageyama, R. Roles of the Basic Helix-Loop-Helix Genes Hes1 and Hes5 in Expansion of Neural Stem Cells of the Developing Brain. J. Biol. Chem. 2001, 276, 30467–30474. [Google Scholar] [CrossRef] [PubMed]
- Hirata, H.; Ohtsuka, T.; Bessho, Y.; Kageyama, R. Generation of Structurally and Functionally Distinct Factors from the Basic Helix-Loop-Helix Gene Hes3 by Alternative First Exons. J. Biol. Chem. 2000, 275, 19083–19089. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Moriyoshi, K.; Sasai, Y.; Shiota, K.; Nakanishi, S.; Kageyama, R. Persistent Expression of Helix-Loop-Helix Factor HES-1 Prevents Mammalian Neural Differentiation in the Central Nervous System. EMBO J. 1994, 13, 1799–1805. [Google Scholar] [CrossRef]
- Youngs, R.M.; Chu, M.S.; Meloni, E.G.; Naydenov, A.; Carlezon, W.A.; Konradi, C. Lithium Administration to Preadolescent Rats Causes Long-Lasting Increases in Anxiety-Like Behavior and Has Molecular Consequences. J. Neurosci. 2006, 26, 6031. [Google Scholar] [CrossRef]
- Kimata, T.; Tanizawa, Y.; Can, Y.; Ikeda, S.; Kuhara, A.; Mori, I. Synaptic Polarity Depends on Phosphatidylinositol Signaling Regulated by Myo-Inositol Monophosphatase in Caenorhabditis Elegans. Genetics 2012, 191, 509. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, Y.; Kuhara, A.; Inada, H.; Kodama, E.; Mizuno, T.; Mori, I. Inositol Monophosphatase Regulates Localization of Synaptic Components and Behavior in the Mature Nervous System of C. Elegans. Genes Dev. 2006, 20, 3296. [Google Scholar] [CrossRef]
- Ohnishi, T.; Tanizawa, Y.; Watanabe, A.; Nakamura, T.; Ohba, H.; Hirata, H.; Kaneda, C.; Iwayama, Y.; Arimoto, T.; Watanabe, K.; et al. Human Myo-Inositol Monophosphatase 2 Rescues the Nematode Thermotaxis Mutant Ttx-7 More Efficiently than IMPA1: Functional and Evolutionary Considerations of the Two Mammalian Myo-Inositol Monophosphatase Genes. J. Neurochem. 2013, 124, 685–694. [Google Scholar] [CrossRef]
- Fisher, S.K.; Novak, J.E.; Agranoff, B.W. Inositol and Higher Inositol Phosphates in Neural Tissues: Homeostasis, Metabolism and Functional Significance. J. Neurochem. 2002, 82, 736–754. [Google Scholar] [CrossRef]
- Najmabadi, H.; Hu, H.; Garshasbi, M.; Zemojtel, T.; Abedini, S.S.; Chen, W.; Hosseini, M.; Behjati, F.; Haas, S.; Jamali, P.; et al. Deep Sequencing Reveals 50 Novel Genes for Recessive Cognitive Disorders. Nature 2011, 478, 57–63. [Google Scholar] [CrossRef]
- Arimura, N.; Kaibuchi, K. Neuronal Polarity: From Extracellular Signals to Intracellular Mechanisms. Nat. Rev. Neurosci. 2007, 8, 194–205. [Google Scholar] [CrossRef]
- Arimura, N.; Kaibuchi, K. Key Regulators in Neuronal Polarity. Neuron 2005, 48, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Wen, P.J.; Osborne, S.L.; Meunier, F.A. Phosphoinositides in Neuroexocytosis and Neuronal Diseases. Curr. Top. Microbiol. Immunol. 2012, 362, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Sade, Y.; Toker, L.; Kara, N.Z.; Einat, H.; Rapoport, S.; Moechars, D.; Berry, G.T.; Bersudsky, Y.; Agam, G. IP3 Accumulation and/or Inositol Depletion: Two Downstream Lithium’s Effects That May Mediate Its Behavioral and Cellular Changes. Transl. Psychiatry 2016, 6, e968. [Google Scholar] [CrossRef] [PubMed]
- Saiardi, A.; Mudge, A.W. Lithium and Fluoxetine Regulate the Rate of Phosphoinositide Synthesis in Neurons: A New View of Their Mechanisms of Action in Bipolar Disorder. Transl. Psychiatry 2018, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Ilhan Atagün, M.; Güntekin, B.; Tan, D.; Elif Tülay, E.; Başar, E. Lithium Excessively Enhances Event Related Beta Oscillations in Patients with Bipolar Disorder. J. Affect. Disord. 2015, 170, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Atagün, M.I. Brain Oscillations in Bipolar Disorder and Lithium-Induced Changes. Neuropsychiatr. Dis. Treat. 2016, 12, 589. [Google Scholar] [CrossRef]
- Debener, S.; Ullsperger, M.; Siegel, M.; Fiehler, K.; Von Cramon, D.Y.; Engel, A.K. Trial-by-Trial Coupling of Concurrent Electroencephalogram and Functional Magnetic Resonance Imaging Identifies the Dynamics of Performance Monitoring. J. Neurosci. 2005, 25, 11730. [Google Scholar] [CrossRef]
- Raghavachari, S.; Lisman, J.E.; Tully, M.; Madsen, J.R.; Bromfield, E.B.; Kahana, M.J. Theta Oscillations in Human Cortex during a Working-Memory Task: Evidence for Local Generators. J. Neurophysiol. 2006, 95, 1630–1638. [Google Scholar] [CrossRef]
- Onton, J.; Delorme, A.; Makeig, S. Frontal Midline EEG Dynamics during Working Memory. Neuroimage 2005, 27, 341–356. [Google Scholar] [CrossRef]
- Canolty, R.T.; Edwards, E.; Dalal, S.S.; Soltani, M.; Nagarajan, S.S.; Kirsch, H.E.; Berger, M.S.; Barbare, N.M.; Knight, R.T. High Gamma Power Is Phase-Locked to Theta Oscillations in Human Neocortex. Science 2006, 313, 1626. [Google Scholar] [CrossRef]
- Mitchell, D.J.; McNaughton, N.; Flanagan, D.; Kirk, I.J. Frontal-Midline Theta from the Perspective of Hippocampal “Theta”. Prog. Neurobiol. 2008, 86, 156–185. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | MRT59 (n = 9) | Carriers (n = 5) | Non-Carriers (n = 4) | p |
|---|---|---|---|---|
| Age mean (Range) | 53 (43–64) | 69 (54–89) | 55 (37–75) | 0.99 |
| Education (n) | 0.78 | |||
| No education or semi-literate | 9 | 2 | 2 | No education or semi-literate |
| Elementary school | 1 | 0 | ||
| High school | 0 | 1 | ||
| College | 2 | 1 |
| MRT59 (n = 9) | Carriers (n = 5) | Non-Carriers (n = 4) | |
|---|---|---|---|
| VIQ mean (SD) | 47.11 (2.80) | 77.00 (14.81) | 87.50 (19.05) |
| PIQ mean (SD) | 46.00 (1.58) | 85.40 (10.99) | 87.50 (15.99) |
| FISQ-4 mean (SD) | 41.33 (1.80) | 75.20 (12.85) | 86.25 (18.19) |
| FISQ-2 mean (SD) | 43.33 (5.07) | 79.20 (13.21) | 91.75 (12.55) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pessoa, A.L.S.; Quesada, A.A.; Nóbrega, P.R.; Viana, A.P.O.; de Oliveira, K.T.; Figueiredo, T.; Santos, S.; Kok, F. Neuropsychological Characterization of Autosomal Recessive Intellectual Developmental Disorder 59 Associated with IMPA1 (MRT59). Brain Sci. 2023, 13, 1048. https://doi.org/10.3390/brainsci13071048
Pessoa ALS, Quesada AA, Nóbrega PR, Viana APO, de Oliveira KT, Figueiredo T, Santos S, Kok F. Neuropsychological Characterization of Autosomal Recessive Intellectual Developmental Disorder 59 Associated with IMPA1 (MRT59). Brain Sciences. 2023; 13(7):1048. https://doi.org/10.3390/brainsci13071048
Chicago/Turabian StylePessoa, Andre Luiz Santos, Andrea Amaro Quesada, Paulo Ribeiro Nóbrega, Ana Priscila Oliveira Viana, Kécia Tavares de Oliveira, Thalita Figueiredo, Silvana Santos, and Fernando Kok. 2023. "Neuropsychological Characterization of Autosomal Recessive Intellectual Developmental Disorder 59 Associated with IMPA1 (MRT59)" Brain Sciences 13, no. 7: 1048. https://doi.org/10.3390/brainsci13071048
APA StylePessoa, A. L. S., Quesada, A. A., Nóbrega, P. R., Viana, A. P. O., de Oliveira, K. T., Figueiredo, T., Santos, S., & Kok, F. (2023). Neuropsychological Characterization of Autosomal Recessive Intellectual Developmental Disorder 59 Associated with IMPA1 (MRT59). Brain Sciences, 13(7), 1048. https://doi.org/10.3390/brainsci13071048






