Tensor Decomposition Analysis of Longitudinal EEG Signals Reveals Differential Oscillatory Dynamics in Eyes-Closed and Eyes-Open Motor Imagery BCI: A Case Report
Abstract
1. Introduction
2. Material and Methods
2.1. Participant
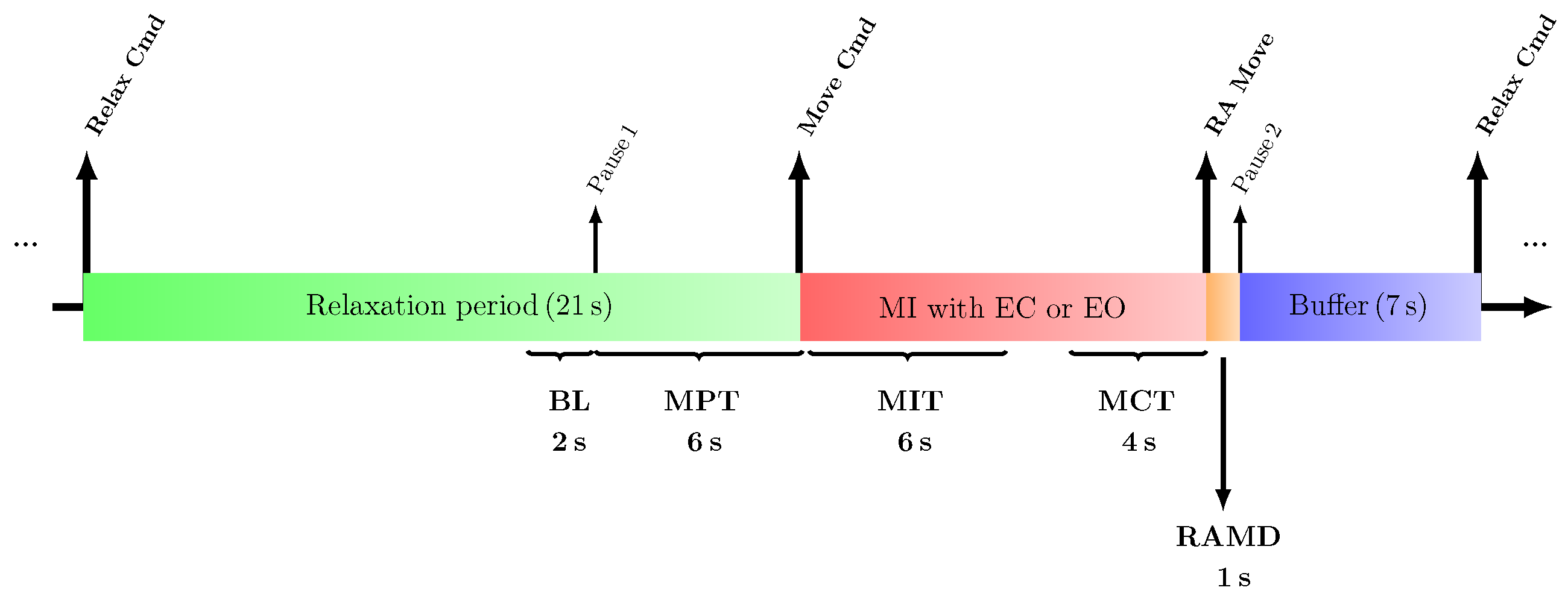
2.2. Motor Imagery Task
2.3. Data Acquisition
2.4. Data Pre-Processing
2.5. Tensor Analysis
2.5.1. Tensor Equation
2.5.2. Tensor Construction
2.5.3. Number of Factors
2.5.4. Generating Atom-Specific Time Scores
2.6. Statistical Analysis
3. Results
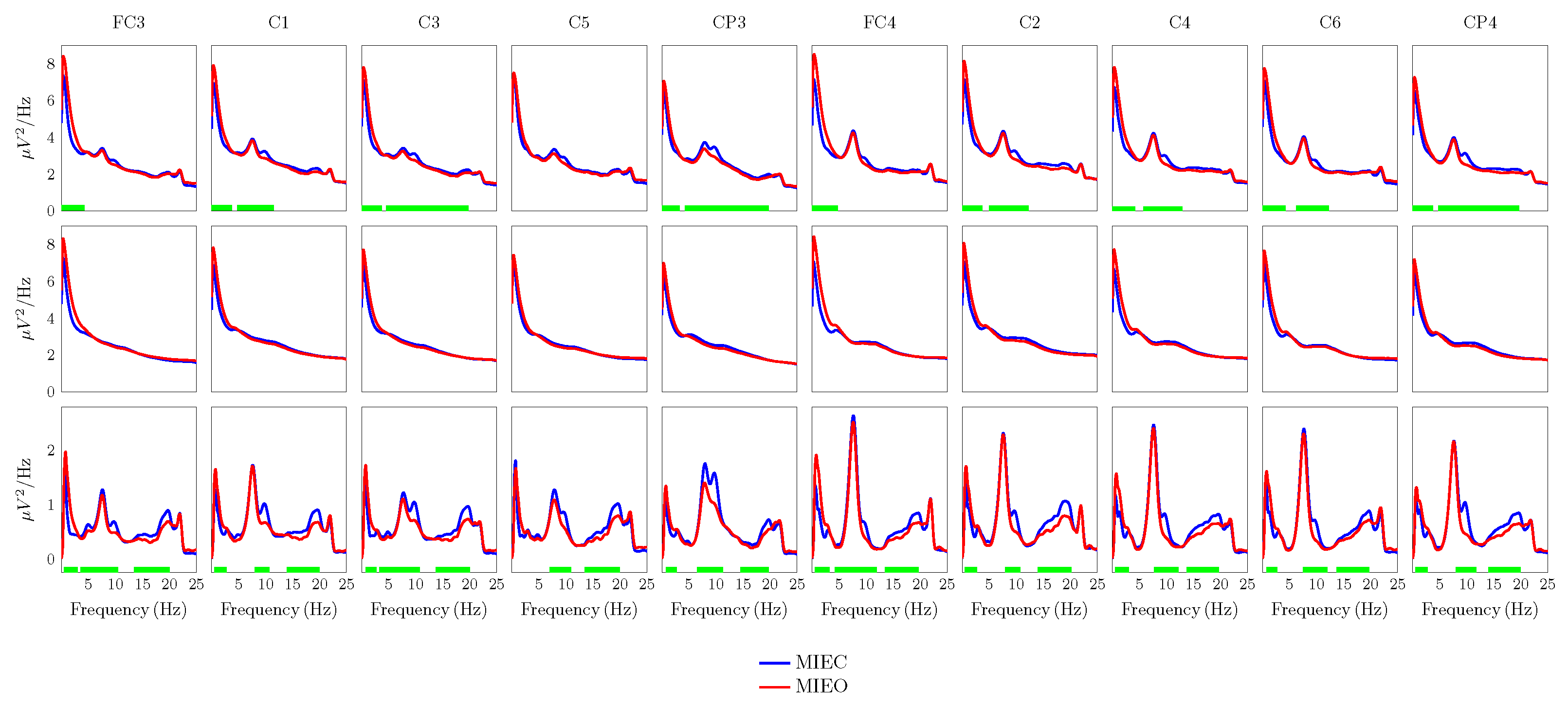
3.1. Eyes Condition-Specific Non-Oscillatory and Oscillatory Activities
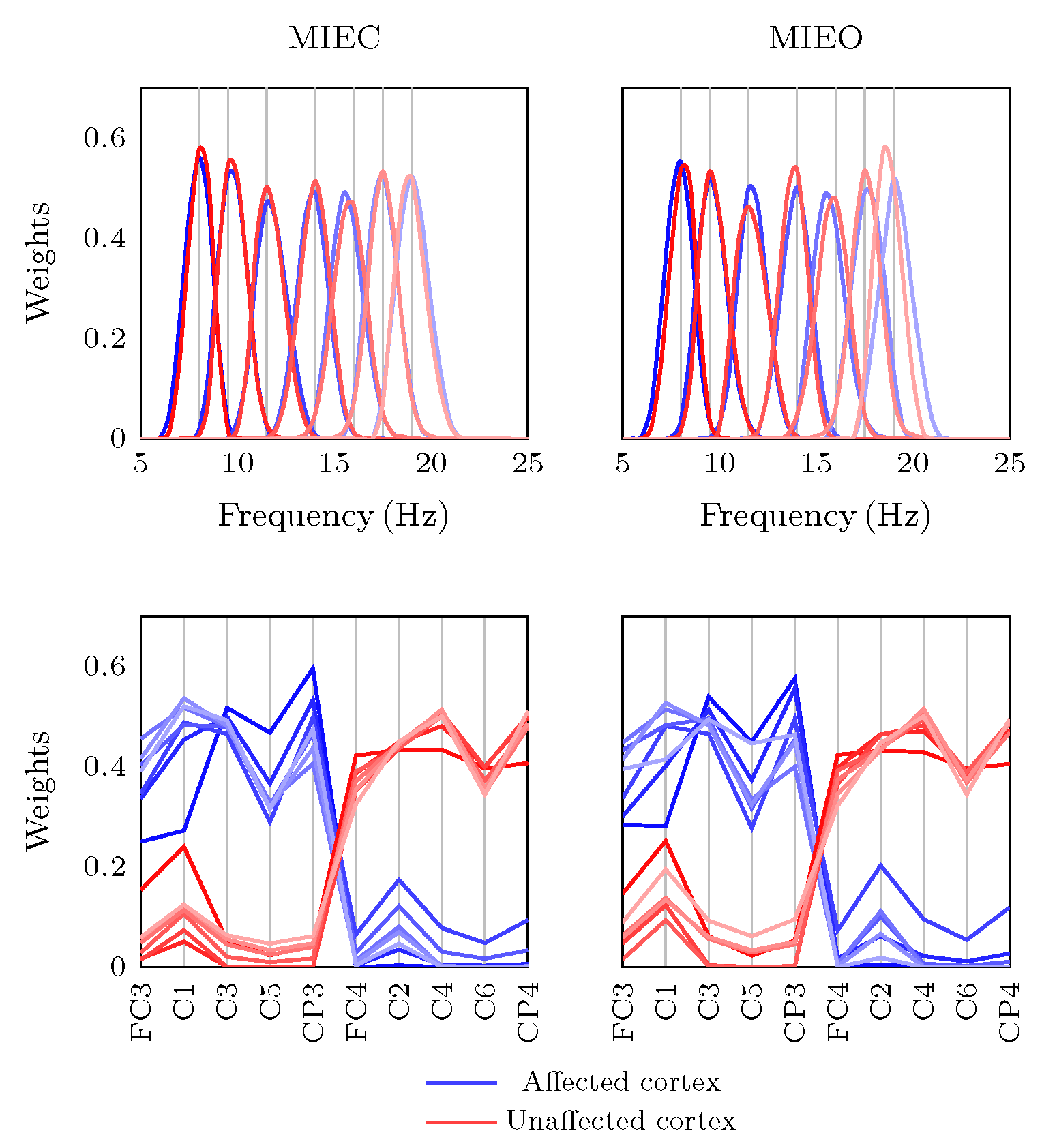
3.2. Eyes Condition-Specific Sensorimotor Oscillatory Activity
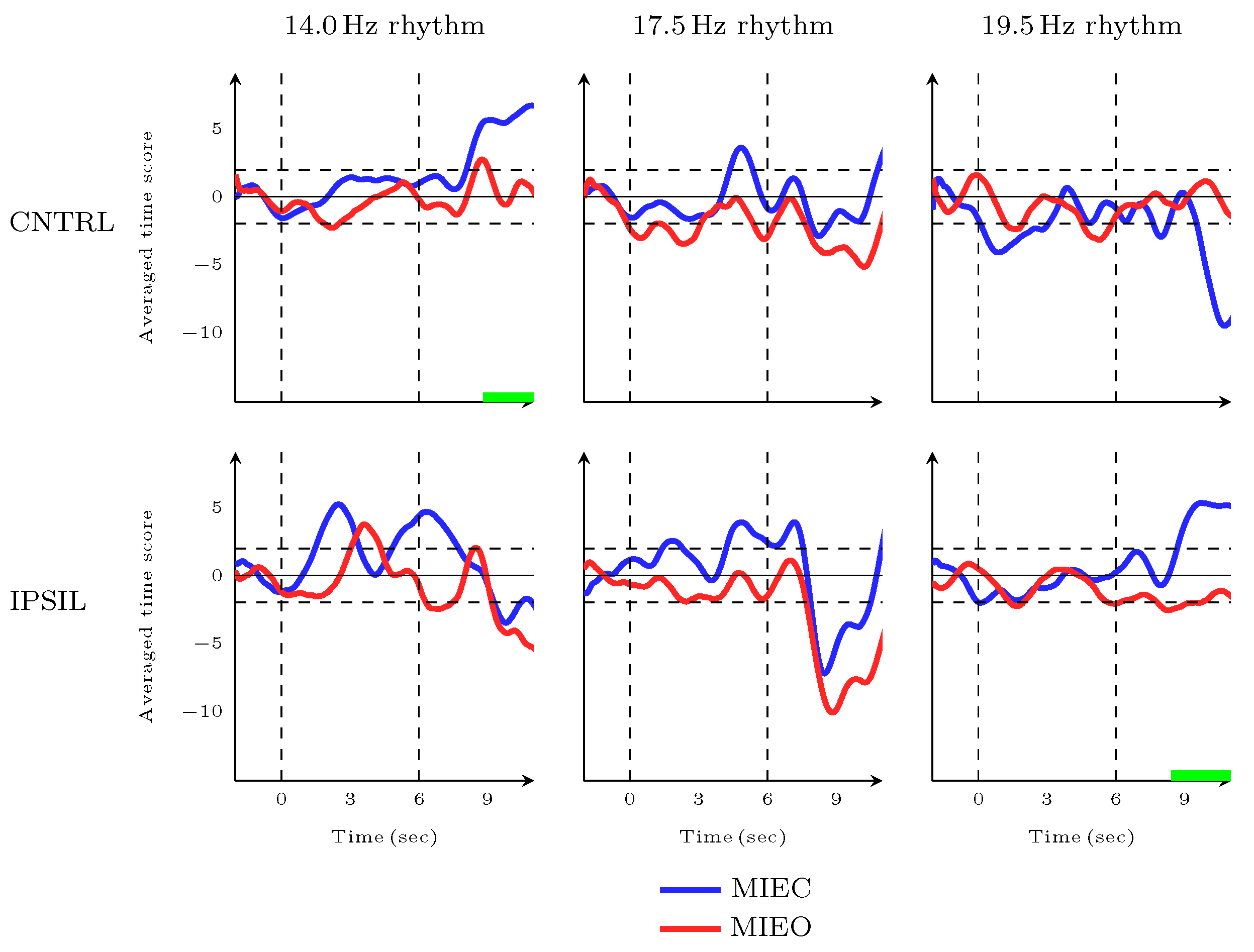
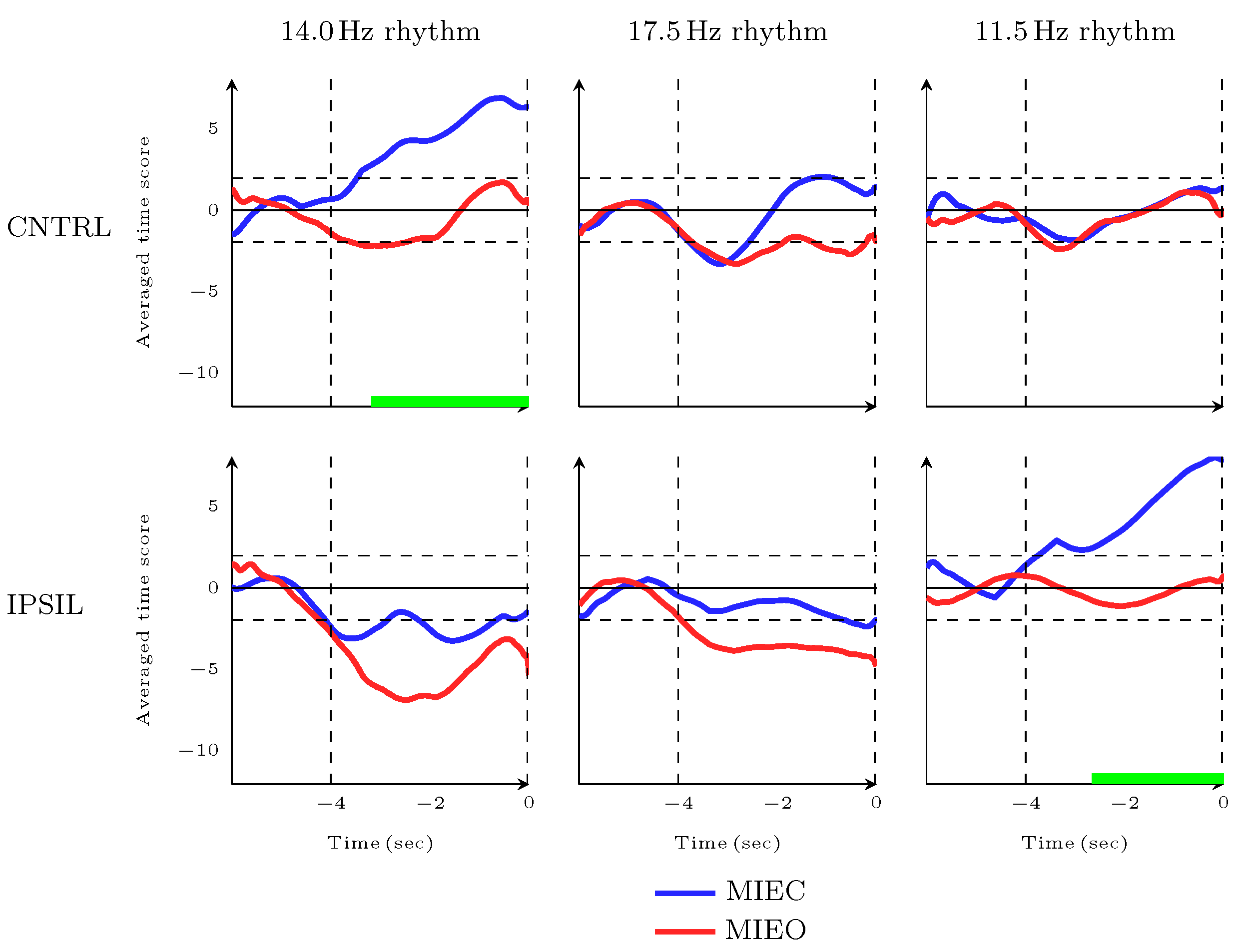
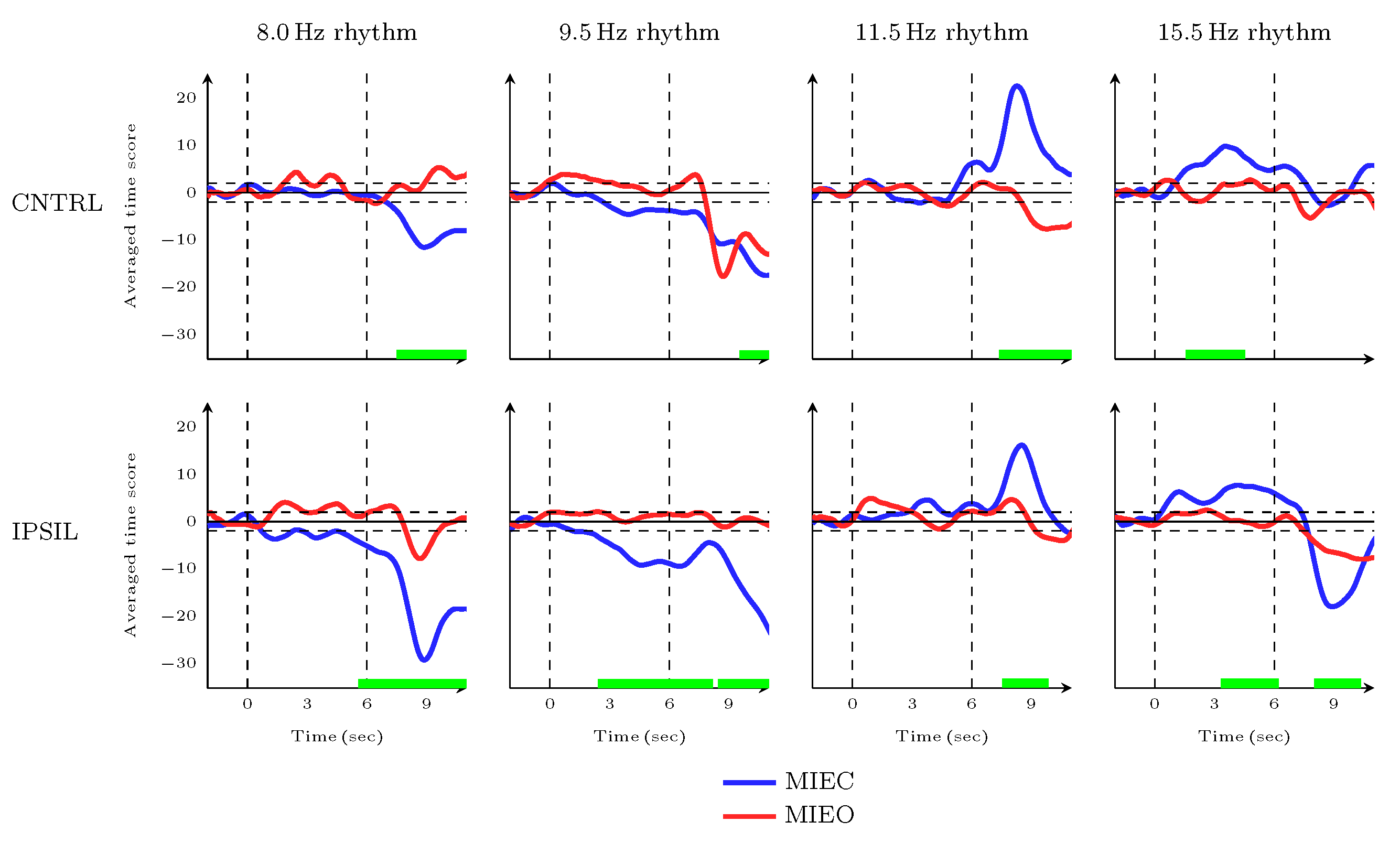
3.3. Dynamic Characteristics of MIEC Versus MIEO during Movement Preparation and Movement Initiation Time Frames
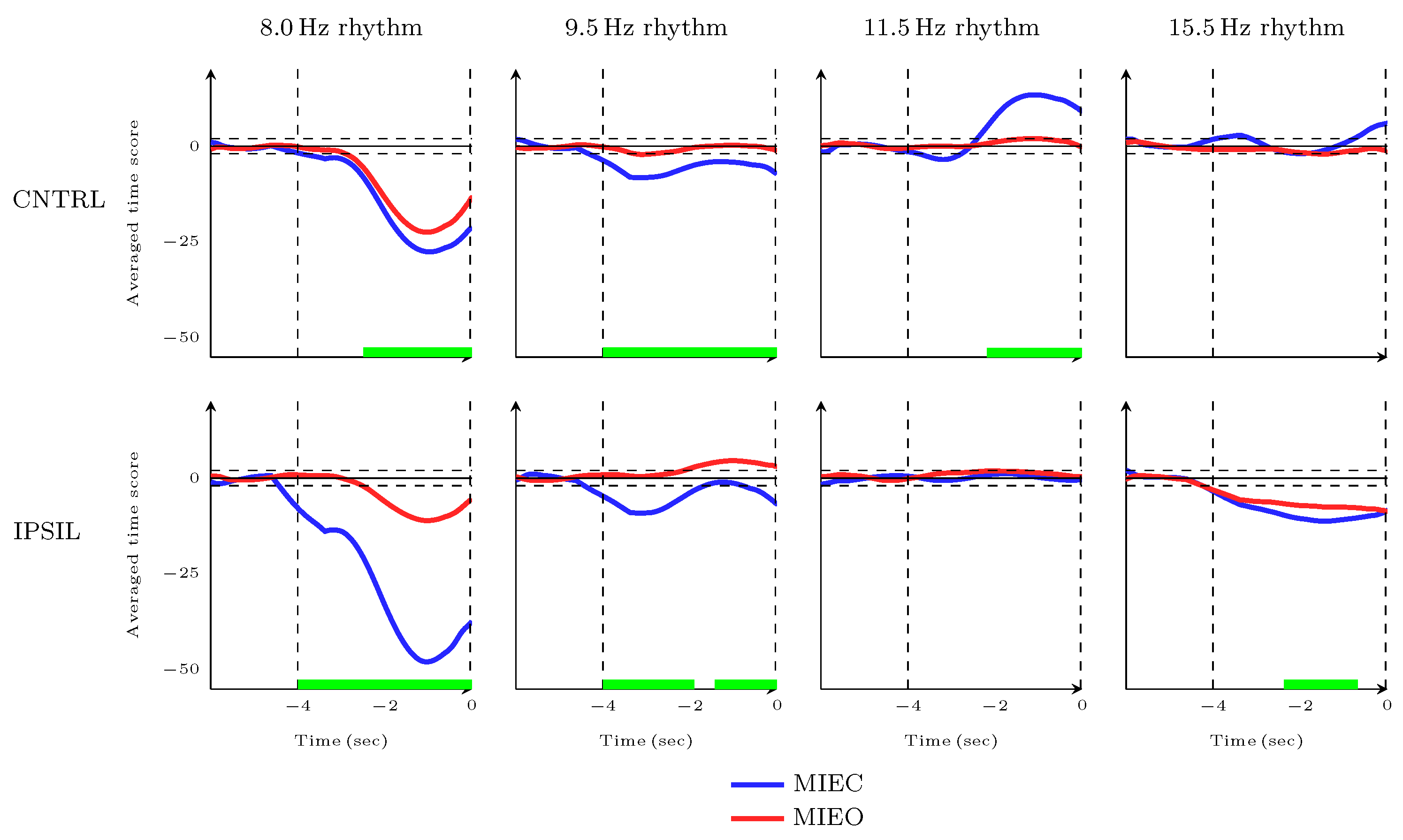
3.4. Dynamic Characteristics of MIEC Versus MIEO during Movement Completion Time Frame
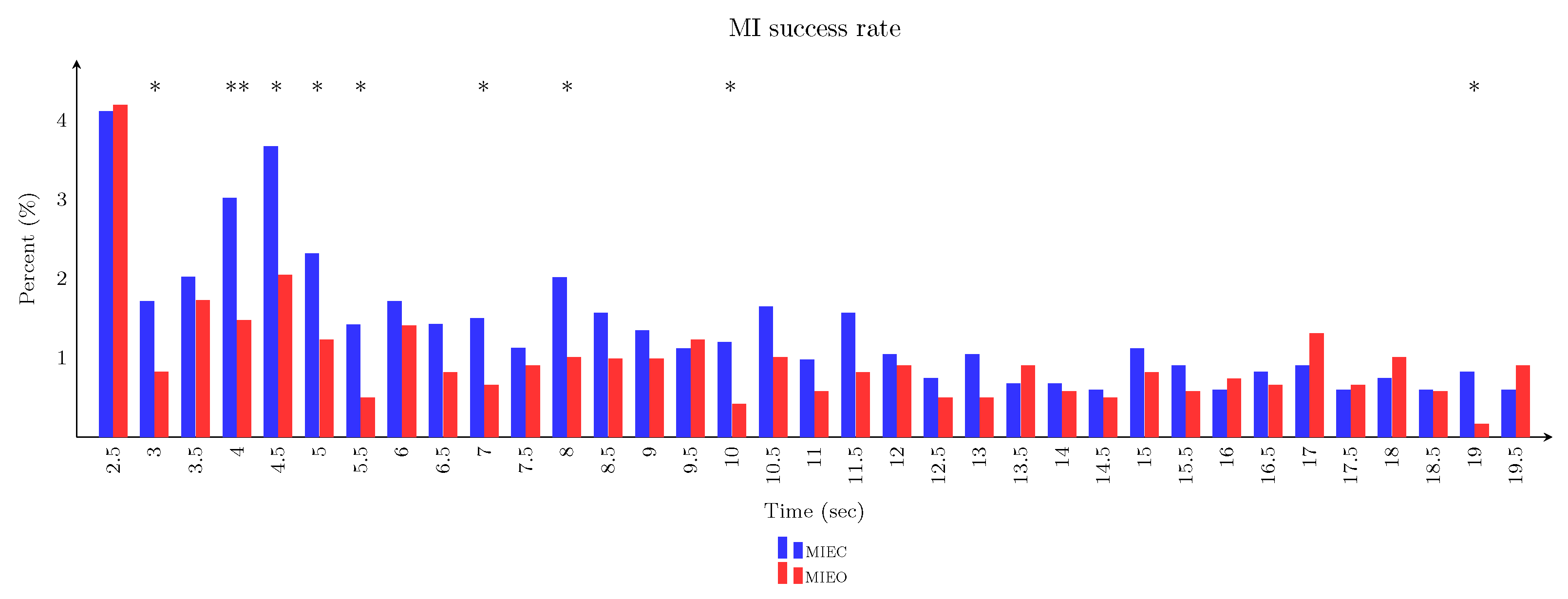
3.5. Imagery Task Performance in Eyes-Closed Versus Eyes-Open Conditions
4. Discussion
4.1. Summary
4.2. Main Findings
4.3. Physiological Interpretation of the Findings
4.4. Technical Considerations in Tensor Construction
4.5. Implications for BCI Domain
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- da Silva-Sauer, L.; Torre-Luque, A.d.l.; Silva, J.S.; Fernández-Calvo, B. New perspectives for cognitive rehabilitation: Could brain-computer interface systems benefit people with dementia? Psychol. Neurosci. 2019, 12, 25. [Google Scholar] [CrossRef]
- Hekmatmanesh, A.; Nardelli, P.H.; Handroos, H. Review of the state-of-the-art of brain-controlled vehicles. IEEE Access 2021, 9, 110173–110193. [Google Scholar] [CrossRef]
- Chaudhary, U.; Birbaumer, N.; Ramos-Murguialday, A. Brain–computer interfaces for communication and rehabilitation. Nat. Rev. Neurol. 2016, 12, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.J.; Wolpaw, J.R. Brain–computer interfaces in neurological rehabilitation. Lancet Neurol. 2008, 7, 1032–1043. [Google Scholar] [CrossRef]
- Hekmatmanesh, A.; Wu, H.; Li, M.; Handroos, H. A Combined Projection for Remote Control of a Vehicle Based on Movement Imagination: A Single Trial Brain Computer Interface Study. IEEE Access 2022, 10, 6165–6174. [Google Scholar] [CrossRef]
- Scherer, R.; Mohapp, A.; Grieshofer, P.; Pfurtscheller, G.; Neuper, C. Sensorimotor EEG patterns during motor imagery in hemiparetic stroke patients. Int. J. Bioelectromagn. 2007, 9, 155–162. [Google Scholar]
- Carrillo-de-la Peña, M.T.; Galdo-Alvarez, S.; Lastra-Barreira, C. Equivalent is not equal: Primary motor cortex (MI) activation during motor imagery and execution of sequential movements. Brain Res. 2008, 1226, 134–143. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Da Silva, F.L. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Alimardani, M.; Gherman, D.E. Individual Differences in Motor Imagery BCIs: A Study of Gender, Mental States and Mu Suppression. In Proceedings of the 2022 10th International Winter Conference on Brain-Computer Interface (BCI), IEEE, Gangwon, Republic of Korea, 21–23 February 2022; pp. 1–7. [Google Scholar]
- Jeunet, C.; N’Kaoua, B.; Lotte, F. Advances in user-training for mental-imagery-based BCI control: Psychological and cognitive factors and their neural correlates. Prog. Brain Res. 2016, 228, 3–35. [Google Scholar]
- Hekmatmanesh, A.; Azni, H.M.; Wu, H.; Afsharchi, M.; Li, M.; Handroos, H. Imaginary control of a mobile vehicle using deep learning algorithm: A brain computer interface study. IEEE Access 2021, 10, 20043–20052. [Google Scholar] [CrossRef]
- Hekmatmanesh, A.; Wu, H.; Handroos, H. Largest Lyapunov Exponent Optimization for Control of a Bionic-Hand: A Brain Computer Interface Study. Front. Rehabil. Sci. 2022, 2, 802070. [Google Scholar] [CrossRef] [PubMed]
- Hekmatmanesh, A.; Asl, R.M.; Wu, H.; Handroos, H. EEG control of a bionic hand with imagination based on chaotic approximation of largest Lyapunov exponent: A single trial BCI application study. IEEE Access 2019, 7, 105041–105053. [Google Scholar] [CrossRef]
- Hekmatmanesh, A.; Wu, H.; Li, M.; Nasrabadi, A.M.; Handroos, H. Optimized mother wavelet in a combination of wavelet packet with detrended fluctuation analysis for controlling a remote vehicle with imagery movement: A brain computer interface study. In New Trends in Medical and Service Robotics: Advances in Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2018; pp. 186–195. [Google Scholar]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Magee, C.A.; Rushby, J.A. EEG differences between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2007, 118, 2765–2773. [Google Scholar] [CrossRef] [PubMed]
- Barry, R.J.; Clarke, A.R.; Johnstone, S.J.; Brown, C.R. EEG differences in children between eyes-closed and eyes-open resting conditions. Clin. Neurophysiol. 2009, 120, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Berger, H. Über das elektroenkephalogramm des menschen. Archiv für Psychiatr. Und Nervenkrankh. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Li, L. The differences among eyes-closed, eyes-open and attention states: An EEG study. In Proceedings of the 2010 6th international conference on wireless communications networking and mobile computing (WiCOM). IEEE, Chengdu, China, 23–25 September 2010; pp. 1–4. [Google Scholar]
- Legewie, H.; Simonova, O.; Creutzfeldt, O. EEG changes during performance of various tasks under open-and closed-eyed conditions. Electroencephalogr. Clin. Neurophysiol. 1969, 27, 470–479. [Google Scholar] [CrossRef]
- Marx, E.; Deutschländer, A.; Stephan, T.; Dieterich, M.; Wiesmann, M.; Brandt, T. Eyes open and eyes closed as rest conditions: Impact on brain activation patterns. Neuroimage 2004, 21, 1818–1824. [Google Scholar] [CrossRef]
- Yang, H.; Long, X.Y.; Yang, Y.; Yan, H.; Zhu, C.Z.; Zhou, X.P.; Zang, Y.F.; Gong, Q.Y. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage 2007, 36, 144–152. [Google Scholar] [CrossRef]
- McAvoy, M.; Larson-Prior, L.; Nolan, T.S.; Vaishnavi, S.N.; Raichle, M.E.; d’Avossa, G. Resting states affect spontaneous BOLD oscillations in sensory and paralimbic cortex. J. Neurophysiol. 2008, 100, 922–931. [Google Scholar] [CrossRef]
- Wei, J.; Chen, T.; Li, C.; Liu, G.; Qiu, J.; Wei, D. Eyes-open and eyes-closed resting states with opposite brain activity in sensorimotor and occipital regions: Multidimensional evidences from machine learning perspective. Front. Hum. Neurosci. 2018, 12, 422. [Google Scholar] [CrossRef]
- Weng, Y.; Liu, X.; Hu, H.; Huang, H.; Zheng, S.; Chen, Q.; Song, J.; Cao, B.; Wang, J.; Wang, S.; et al. Open eyes and closed eyes elicit different temporal properties of brain functional networks. NeuroImage 2020, 222, 117230. [Google Scholar] [CrossRef]
- Liang, B.; Zhang, D.; Wen, X.; Xu, P.; Peng, X.; Huang, X.; Liu, M.; Huang, R. Brain spontaneous fluctuations in sensorimotor regions were directly related to eyes open and eyes closed: Evidences from a machine learning approach. Front. Hum. Neurosci. 2014, 8, 645. [Google Scholar] [CrossRef]
- McFarland, D.J.; Miner, L.A.; Vaughan, T.M.; Wolpaw, J.R. Mu and beta rhythm topographies during motor imagery and actual movements. Brain Topogr. 2000, 12, 177–186. [Google Scholar] [CrossRef]
- Alegre, M.; Labarga, A.; Gurtubay, I.; Iriarte, J.; Malanda, A.; Artieda, J. Beta electroencephalograph changes during passive movements: Sensory afferences contribute to beta event-related desynchronization in humans. Neurosci. Lett. 2002, 331, 29–32. [Google Scholar] [CrossRef]
- Westphal, K.; Grözinger, B.; Diekmann, V.; Kornhuber, H. EEG-blocking before and during voluntary movements: Differences between the eyes-closed and the eyes-open condition. Arch. Ital. Biol. 1993, 131, 25–35. [Google Scholar] [PubMed]
- Rimbert, S.; Al-Chwa, R.; Zaepffel, M.; Bougrain, L. Electroencephalographic modulations during an open-or closed-eyes motor task. PeerJ 2018, 6, e4492. [Google Scholar] [CrossRef] [PubMed]
- Hekmatmanesh, A.; Zhidchenko, V.; Kauranen, K.; Siitonen, K.; Handroos, H.; Soutukorva, S.; Kilpeläinen, A. Biosignals in human factors research for heavy equipment operators: A review of available methods and their feasibility in laboratory and ambulatory studies. IEEE Access 2021, 9, 97466–97482. [Google Scholar] [CrossRef]
- Murphy, T.H.; Corbett, D. Plasticity during stroke recovery: From synapse to behaviour. Nat. Rev. Neurosci. 2009, 10, 861–872. [Google Scholar] [CrossRef]
- Ang, K.K.; Guan, C.; Chua, K.S.G.; Ang, B.T.; Kuah, C.W.K.; Wang, C.; Phua, K.S.; Chin, Z.Y.; Zhang, H. A large clinical study on the ability of stroke patients to use an EEG-based motor imagery brain-computer interface. Clin. EEG Neurosci. 2011, 42, 253–258. [Google Scholar] [CrossRef]
- Monge-Pereira, E.; Ibañez-Pereda, J.; Alguacil-Diego, I.M.; Serrano, J.I.; Spottorno-Rubio, M.P.; Molina-Rueda, F. Use of electroencephalography brain-computer interface systems as a rehabilitative approach for upper limb function after a stroke: A systematic review. PM&R 2017, 9, 918–932. [Google Scholar]
- Toga, A.W.; Thompson, P.M. Mapping brain asymmetry. Nat. Rev. Neurosci. 2003, 4, 37–48. [Google Scholar] [CrossRef]
- Kapreli, E.; Athanasopoulos, S.; Papathanasiou, M.; Van Hecke, P.; Strimpakos, N.; Gouliamos, A.; Peeters, R.; Sunaert, S. Lateralization of brain activity during lower limb joints movement. An fMRI study. Neuroimage 2006, 32, 1709–1721. [Google Scholar] [CrossRef] [PubMed]
- Watrous, A.J.; Buchanan, R.J. The Oscillatory ReConstruction Algorithm adaptively identifies frequency bands to improve spectral decomposition in human and rodent neural recordings. J. Neurophysiol. 2020, 124, 1914–1922. [Google Scholar] [CrossRef] [PubMed]
- Donoghue, T.; Schaworonkow, N.; Voytek, B. Methodological considerations for studying neural oscillations. Eur. J. Neurosci. 2021, 55, 3502–3527. [Google Scholar] [CrossRef] [PubMed]
- Cichocki, A.; Washizawa, Y.; Rutkowski, T.; Bakardjian, H.; Phan, A.H.; Choi, S.; Lee, H.; Zhao, Q.; Zhang, L.; Li, Y. Noninvasive BCIs: Multiway signal-processing array decompositions. Computer 2008, 41, 34–42. [Google Scholar] [CrossRef]
- Cichocki, A.; Mandic, D.; De Lathauwer, L.; Zhou, G.; Zhao, Q.; Caiafa, C.; Phan, H.A. Tensor decompositions for signal processing applications: From two-way to multiway component analysis. IEEE Signal Process. Mag. 2015, 32, 145–163. [Google Scholar] [CrossRef]
- Harshman, R. Foundations of the PARAFAC Procedure: Models and Conditions for an “Explanatory” Multimodal Factor Analysis; UCLA working papers in phonetics; University of California at Los Angeles: Los Angeles, CA, USA, 1970. [Google Scholar]
- Rosipal, R.; Porubcová, N.; Barančok, P.; Cimrová, B.; Farkaš, I.; Trejo, L.J. Effects of mirror-box therapy on modulation of sensorimotor EEG oscillatory rhythms: A single-case longitudinal study. J. Neurophysiol. 2019, 121, 620–633. [Google Scholar] [CrossRef]
- Tangwiriyasakul, C.; Premoli, I.; Spyrou, L.; Chin, R.F.; Escudero, J.; Richardson, M.P. Tensor decomposition of TMS-induced EEG oscillations reveals data-driven profiles of antiepileptic drug effects. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Miwakeichi, F.; Martınez-Montes, E.; Valdés-Sosa, P.A.; Nishiyama, N.; Mizuhara, H.; Yamaguchi, Y. Decomposing EEG data into space–time–frequency components using parallel factor analysis. NeuroImage 2004, 22, 1035–1045. [Google Scholar] [CrossRef]
- Rosipal, R.; Trejo, L.J.; Nunez, P.L. Application of multi-way EEG decomposition for cognitive workload monitoring. In Proceedings of the Proceedings of the 6th International Conference on Partial Least Squares and Related Methods, Beijing, China, 4–7 September 2009; pp. 145–149. [Google Scholar]
- Ji, H.; Li, J.; Lu, R.; Gu, R.; Cao, L.; Gong, X. EEG Classification for Hybrid Brain-Computer Interface Using a Tensor Based Multiclass Multimodal Analysis Scheme. Comput. Intell. Neurosci. 2016, 2016, 1732836. [Google Scholar] [CrossRef]
- Rošt’áková, Z.; Rosipal, R.; Seifpour, S.; Trejo, L.J. A comparison of non-negative Tucker decomposition and Parallel Factor Analysis for identification and measurement of human EEG rhythms. Meas. Sci. Rev. 2020, 20, 126–138. [Google Scholar] [CrossRef]
- Rošťáková, Z.; Rosipal, R.; Seifpour, S. Tucker Tensor Decomposition of Multi-session EEG Data. In Proceedings of the Artificial Neural Networks and Machine Learning—ICANN 2020, Bratislava, Slovakia, 15–18 September 2020; Farkaš, I., Masulli, P., Wermter, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 115–126. [Google Scholar]
- Fugl-Meyer, A.R.; Jääskö, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Duncan, P.W.; Wallace, D.; Lai, S.M.; Johnson, D.; Embretson, S.; Laster, L.J. The stroke impact scale version 2.0: Evaluation of reliability, validity, and sensitivity to change. Stroke 1999, 30, 2131–2140. [Google Scholar] [CrossRef]
- Charalambous, C.P. Interrater Reliability of a Modified Ashworth Scale of Muscle Spasticity. In Classic Papers in Orthopaedics; Banaszkiewicz, P.A., Kader, D.F., Eds.; Springer London: London, UK, 2014; pp. 415–417. [Google Scholar]
- Renard, Y.; Lotte, F.; Gibert, G.; Congedo, M.; Maby, E.; Delannoy, V.; Bertrand, O.; Lécuyer, A. Openvibe: An open-source software platform to design, test, and use brain–computer interfaces in real and virtual environments. Presence 2010, 19, 35–53. [Google Scholar] [CrossRef]
- Oostenveld, R.; Praamstra, P. The five percent electrode system for high-resolution EEG and ERP measurements. Clin. Neurophysiol. 2001, 112, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Liu, Z. Separating fractal and oscillatory components in the power spectrum of neurophysiological signal. Brain Topogr. 2016, 29, 13–26. [Google Scholar] [CrossRef]
- Kiers, H.A.L. Recent Developments in Three-Mode Factor Analysis: Constrained Three-Mode Factor Analysis and Core Rotations. In Proceedings of the Data Science, Classification, and Related Methods; Hayashi, C., Yajima, K., Bock, H.H., Ohsumi, N., Tanaka, Y., Baba, Y., Eds.; Springer: Tokyo, Japan, 1998; pp. 563–574. [Google Scholar]
- Pacific Development and Technology. APECSgui. Installation Guide and User Manual, Version 1.0. 2012. Available online: https://www.pacdel.com/upload/files/pdt-apecsgui-software-manual.pdf (accessed on 21 January 2022).
- Andersson, C.A.; Bro, R. The N-way toolbox for MATLAB. Chemom. Intell. Lab. Syst. 2000, 52, 1–4. [Google Scholar] [CrossRef]
- Rošt’áková, Z.; Rosipal, R. Determination of the number of components in the PARAFAC model with a nonnegative tensor structure: A simulated EEG data study. Neural Comput. Appl. 2022, 34, 14793–14805. [Google Scholar] [CrossRef]
- Ester, M.; Kriegel, H.P.; Sander, J.; Xu, X. A density-based algorithm for discovering clusters in large spatial databases with noise. In Proceedings of the KDD, Portland, OR, USA, 2–4 August 1996; Volume 96, pp. 226–231. [Google Scholar]
- MATLAB Version 9.7.0.1737446 (R2019b) Update 9; The Mathworks, Inc.: Natick, MA, USA, 2019.
- Maris, E.; Oostenveld, R. Nonparametric statistical testing of EEG-and MEG-data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Meng, J.; He, B. Exploring training effect in 42 human subjects using a non-invasive sensorimotor rhythm based online BCI. Front. Hum. Neurosci. 2019, 13, 128. [Google Scholar] [CrossRef]
- Grandchamp, R.; Delorme, A. Single-trial normalization for event-related spectral decomposition reduces sensitivity to noisy trials. Front. Psychol. 2011, 2, 236. [Google Scholar] [CrossRef]
- Nunez, P.L.; Srinivasan, R. Electric Fields of the Brain: The Neurophysics of EEG; Oxford University Press: Cary, NC, USA, 2006. [Google Scholar]
- Shelton, F.D.N.; Reding, M.J. Effect of lesion location on upper limb motor recovery after stroke. Stroke 2001, 32, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Muthukumaraswamy, S.D.; Johnson, B.W.; McNair, N.A. Mu rhythm modulation during observation of an object-directed grasp. Cogn. Brain Res. 2004, 19, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Oberman, L.M.; McCleery, J.P.; Ramachandran, V.S.; Pineda, J.A. EEG evidence for mirror neuron activity during the observation of human and robot actions: Toward an analysis of the human qualities of interactive robots. Neurocomputing 2007, 70, 2194–2203. [Google Scholar] [CrossRef]
- Oberman, L.M.; Pineda, J.A.; Ramachandran, V.S. The human mirror neuron system: A link between action observation and social skills. Soc. Cogn. Affect. Neurosci. 2007, 2, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Hobson, H.M.; Bishop, D.V. The interpretation of mu suppression as an index of mirror neuron activity: Past, present and future. R. Soc. Open Sci. 2017, 4, 160662. [Google Scholar] [CrossRef] [PubMed]
- Garakh, Z.; Novototsky-Vlasov, V.; Larionova, E.; Zaytseva, Y. Mu rhythm separation from the mix with alpha rhythm: Principal component analyses and factor topography. J. Neurosci. Methods 2020, 346, 108892. [Google Scholar] [CrossRef]
- Kuhlman, W.N. Functional topography of the human mu rhythm. Electroencephalogr. Clin. Neurophysiol. 1978, 44, 83–93. [Google Scholar] [CrossRef]
- Pineda, J.A. The functional significance of mu rhythms: Translating “seeing” and “hearing” into “doing”. Brain Res. Rev. 2005, 50, 57–68. [Google Scholar] [CrossRef]
- Jensen, O.; Mazaheri, A. Shaping Functional Architecture by Oscillatory Alpha Activity: Gating by Inhibition. Front. Hum. Neurosci. 2010, 4, 186. [Google Scholar] [CrossRef]
- Haegens, S.; Osipova, D.; Oostenveld, R.; Jensen, O. Somatosensory working memory performance in humans depends on both engagement and disengagement of regions in a distributed network. Hum. Brain Mapp. 2010, 31, 26–35. [Google Scholar] [CrossRef]
- Andriot, T.; Ohnmacht, P.; Vuilleumier, P.; Thorens, G.; Khazaal, Y.; Ginovart, N.; Ros, T. Electrophysiological and behavioral correlates of cannabis use disorder. Cogn. Affect. Behav. Neurosci. 2022, 22, 1421–1431. [Google Scholar] [CrossRef] [PubMed]
- Del Percio, C.; Babiloni, C.; Bertollo, M.; Marzano, N.; Iacoboni, M.; Infarinato, F.; Lizio, R.; Stocchi, M.; Robazza, C.; Cibelli, G.; et al. Visuo-attentional and sensorimotor alpha rhythms are related to visuo-motor performance in athletes. Hum. Brain Mapp. 2009, 30, 3527–3540. [Google Scholar] [CrossRef] [PubMed]
- Zaepffel, M.; Trachel, R.; Kilavik, B.E.; Brochier, T. Modulations of EEG beta power during planning and execution of grasping movements. PLoS ONE 2013, 8, e60060. [Google Scholar] [CrossRef] [PubMed]
- Kilavik, B.E.; Ponce-Alvarez, A.; Trachel, R.; Confais, J.; Takerkart, S.; Riehle, A. Context-related frequency modulations of macaque motor cortical LFP beta oscillations. Cereb. Cortex 2012, 22, 2148–2159. [Google Scholar] [CrossRef]
- Saleh, M.; Reimer, J.; Penn, R.; Ojakangas, C.L.; Hatsopoulos, N.G. Fast and slow oscillations in human primary motor cortex predict oncoming behaviorally relevant cues. Neuron 2010, 65, 461–471. [Google Scholar] [CrossRef]
- Kilavik, B.E.; Zaepffel, M.; Brovelli, A.; MacKay, W.A.; Riehle, A. The ups and downs of beta oscillations in sensorimotor cortex. Exp. Neurol. 2013, 245, 15–26. [Google Scholar] [CrossRef]
- Brinkman, L.; Stolk, A.; Dijkerman, H.C.; de Lange, F.P.; Toni, I. Distinct roles for alpha-and beta-band oscillations during mental simulation of goal-directed actions. J. Neurosci. 2014, 34, 14783–14792. [Google Scholar] [CrossRef]
- Stolk, A.; Brinkman, L.; Vansteensel, M.J.; Aarnoutse, E.; Leijten, F.S.; Dijkerman, C.H.; Knight, R.T.; de Lange, F.P.; Toni, I. Electrocorticographic dissociation of alpha and beta rhythmic activity in the human sensorimotor system. eLife 2019, 8, e48065. [Google Scholar] [CrossRef] [PubMed]
- Bro, R. PARAFAC. Tutorial and applications. Chemom. Intell. Lab. Syst. 1997, 38, 149–171. [Google Scholar] [CrossRef]
- Bro, R.; Kiers, H.A. A new efficient method for determining the number of components in PARAFAC models. J. Chemom. J. Chemom. Soc. 2003, 17, 274–286. [Google Scholar] [CrossRef]
- Mørup, M.; Hansen, L.K. Automatic relevance determination for multi-way models. J. Chemom. J. Chemom. Soc. 2009, 23, 352–363. [Google Scholar] [CrossRef]
- Buzsáki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed]
- He, B.J.; Zempel, J.M.; Snyder, A.Z.; Raichle, M.E. The temporal structures and functional significance of scale-free brain activity. Neuron 2010, 66, 353–369. [Google Scholar] [CrossRef] [PubMed]
- He, B.J. Scale-free brain activity: Past, present, and future. Trends Cogn. Sci. 2014, 18, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Egeth, H.E.; Yantis, S. Visual attention: Control, representation, and time course. Annu. Rev. Psychol. 1997, 48, 269–297. [Google Scholar] [CrossRef]
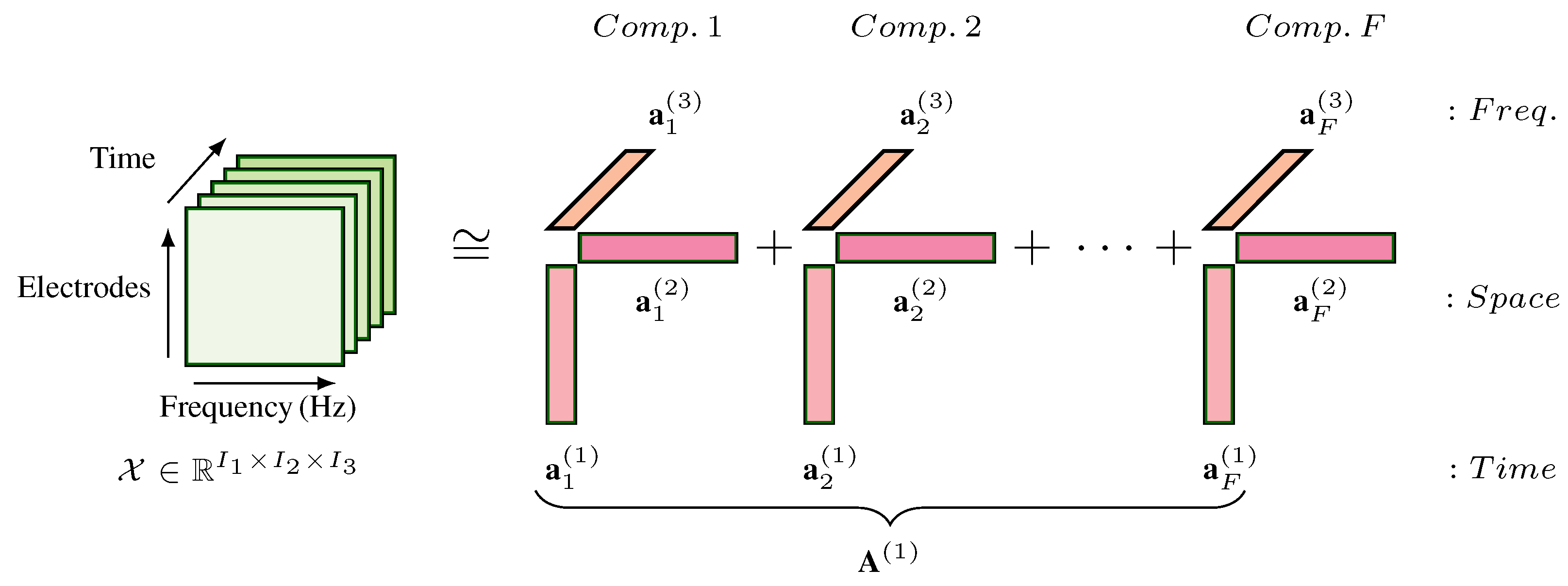
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seifpour, S.; Šatka, A. Tensor Decomposition Analysis of Longitudinal EEG Signals Reveals Differential Oscillatory Dynamics in Eyes-Closed and Eyes-Open Motor Imagery BCI: A Case Report. Brain Sci. 2023, 13, 1013. https://doi.org/10.3390/brainsci13071013
Seifpour S, Šatka A. Tensor Decomposition Analysis of Longitudinal EEG Signals Reveals Differential Oscillatory Dynamics in Eyes-Closed and Eyes-Open Motor Imagery BCI: A Case Report. Brain Sciences. 2023; 13(7):1013. https://doi.org/10.3390/brainsci13071013
Chicago/Turabian StyleSeifpour, Saman, and Alexander Šatka. 2023. "Tensor Decomposition Analysis of Longitudinal EEG Signals Reveals Differential Oscillatory Dynamics in Eyes-Closed and Eyes-Open Motor Imagery BCI: A Case Report" Brain Sciences 13, no. 7: 1013. https://doi.org/10.3390/brainsci13071013
APA StyleSeifpour, S., & Šatka, A. (2023). Tensor Decomposition Analysis of Longitudinal EEG Signals Reveals Differential Oscillatory Dynamics in Eyes-Closed and Eyes-Open Motor Imagery BCI: A Case Report. Brain Sciences, 13(7), 1013. https://doi.org/10.3390/brainsci13071013





