Review of Plant Extracts and Active Components: Mechanisms of Action for the Treatment of Obesity-Induced Cognitive Impairment
Abstract
1. Introduction
2. Methods
3. Obesity-Induced Cognitive Impairment: Role of Hippocampus and Prefrontal Cortex
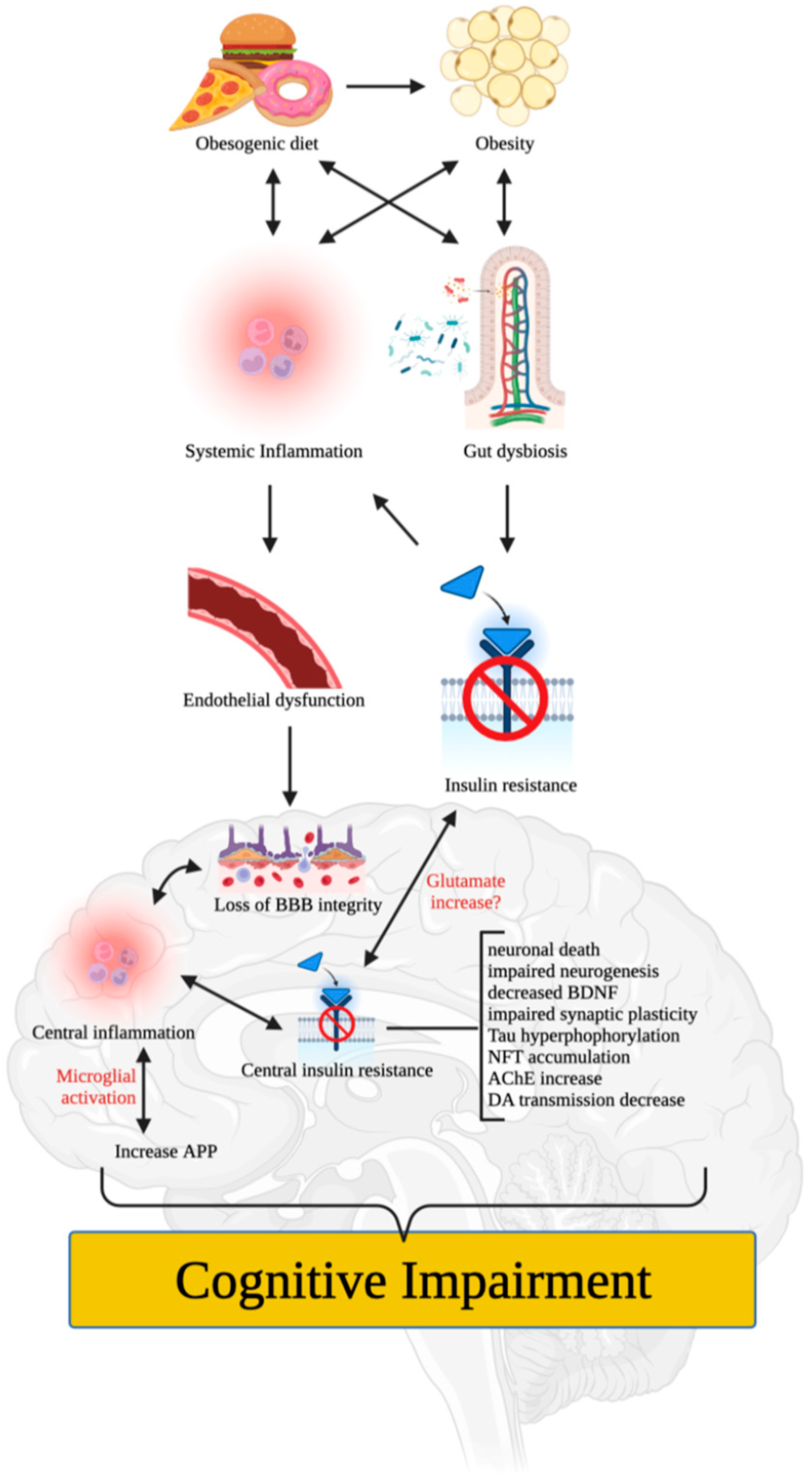
4. Putative Mechanisms of Obesity-Induced Cognitive Impairment
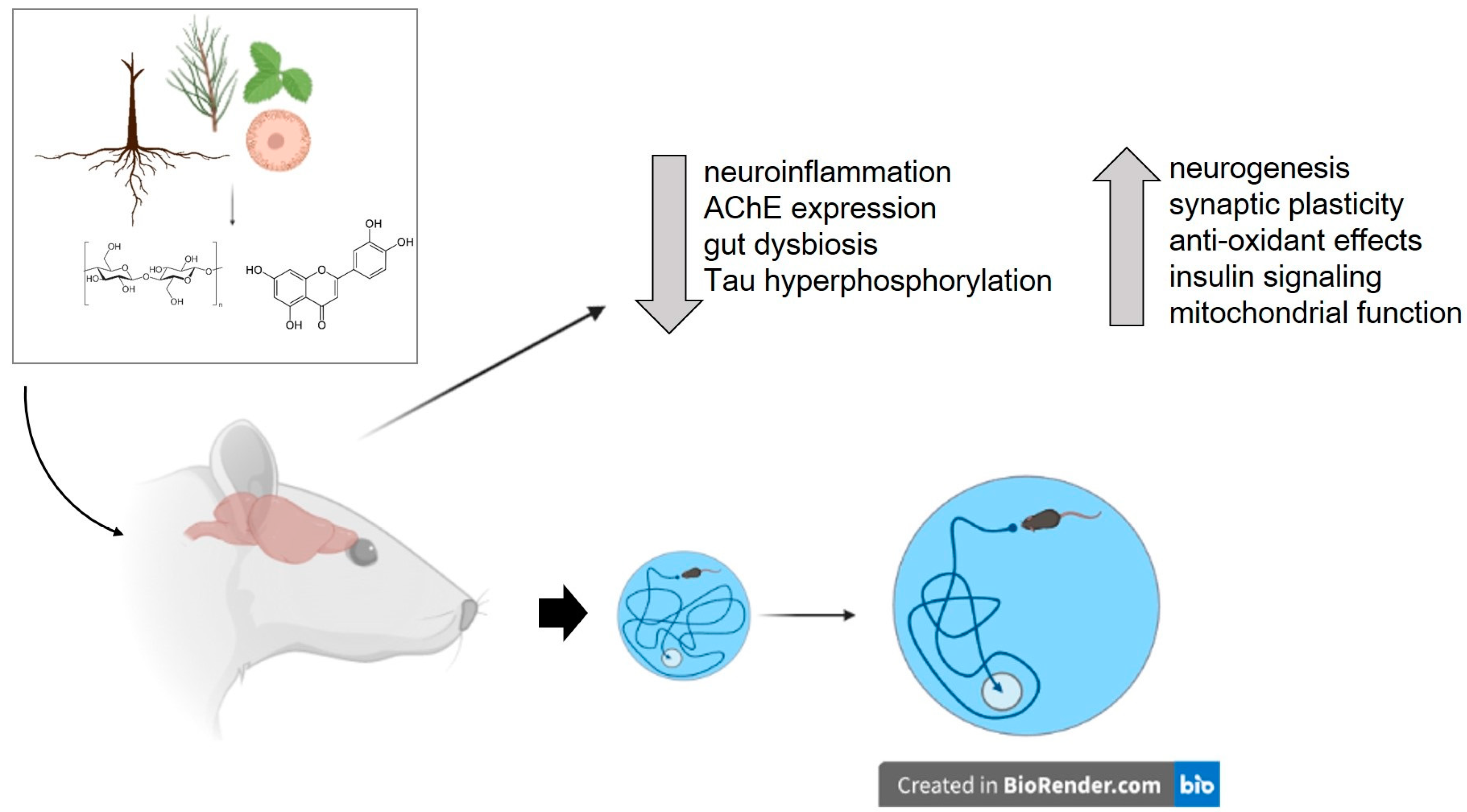
5. Plants Investigated for Their Potential to Ameliorate Obesity-Induced Cognitive Impairment
5.1. Ashwagandha (Withania somnifera)
5.2. Adzuki Bean (Vigna angularis)
5.3. Dwarf Goat’s Beard (Aruncus dioicus var. kamtschaticus)
5.4. Hardy Kiwi (Actinidia arguta)
5.5. Japanese Aster [Aster yomena (Kitam.) Honda]
5.6. Mango Ginger (Curcuma amada)
5.7. Mulberry Root-Bark (Mori radices cortex)
5.8. Olive (Olea europaea)
5.9. Pineapple (Ananas comosus)
6. Plant-Derived Substances Tested to Ameliorate Obesity-Induced Cognitive Impairment
6.1. β-Glucan
6.2. Caffeine
6.3. Chlorogenic Acid
6.4. Curcumin
6.5. Formononetin
6.6. Huperzine A
6.7. Iso-α-Acids
6.8. Isorhamnetin
6.9. Luteolin
6.10. Naringin
6.11. Panax japonicus Saponins
6.12. Purple Sweet Potato Color Anthocyanins
6.13. Rhein
6.14. Roxburgh’s Jewel Orchid Polysaccharides
6.15. Sea-Buckthorn Flavonoids
6.16. Silymarin
6.17. Tea Saponins
6.18. Xanthohumol Derivatives
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Lancet Gastroenterology & Hepatology. Obesity: Another ongoing pandemic. Lancet Gastroenterol. Hepatol. 2021, 6, 411. [Google Scholar] [CrossRef]
- Luppino, F.S.; de Wit, L.M.; Bouvy, P.F.; Stijnen, T.; Cuijpers, P.; Penninx, B.W.J.H.; Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Meta-analysis of Longitudinal Studies. Arch. Gen. Psychiatry 2010, 67, 220–229. [Google Scholar] [CrossRef]
- Bocarsly, M.E.; Fasolino, M.; Kane, G.A.; LaMarca, E.A.; Kirschen, G.W.; Karatsoreos, I.N.; McEwen, B.S.; Gould, E. Obesity diminishes synaptic markers, alters microglial morphology, and impairs cognitive function. Proc. Natl. Acad. Sci. USA 2015, 112, 15731–15736. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, J.C.D.; Killcross, A.S.; Jenkins, T.A. Obesity and cognitive decline: Role of inflammation and vascular changes. Front. Neurosci. 2014, 8, 375. [Google Scholar] [CrossRef]
- O’Brien, P.D.; Hinder, L.M.; Callaghan, B.C.; Feldman, E.L. Neurological consequences of obesity. Lancet Neurol. 2017, 16, 465–477. [Google Scholar] [CrossRef]
- Salas-Venegas, V.; Flores-Torres, R.P.; Rodríguez-Cortés, Y.M.; Rodríguez-Retana, D.; Ramírez-Carreto, R.J.; Concepción-Carrillo, L.E.; Pérez-Flores, L.J.; Alarcón-Aguilar, A.; López-Díazguerrero, N.E.; Gómez-González, B.; et al. The Obese Brain: Mechanisms of Systemic and Local Inflammation, and Interventions to Reverse the Cognitive Deficit. Front. Integr. Neurosci. 2022, 16, 798995. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhao, W.; Lu, M.; Zhang, X.; Zhang, P.; Xin, Z.; Sun, R.; Tian, W.; Cardoso, M.A.; Yang, J.; et al. Relationship between Central Obesity and the incidence of Cognitive Impairment and Dementia from Cohort Studies Involving 5,060,687 Participants. Neurosci. Biobehav. Rev. 2021, 130, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Hu, H.Y.; Ou, Y.N.; Shen, X.N.; Xu, W.; Wang, Z.T.; Dong, Q.; Tan, L.; Yu, J.T. Association of body mass index with risk of cognitive impairment and dementia: A systematic review and meta-analysis of prospective studies. Neurosci. Biobehav. Rev. 2020, 115, 189–198. [Google Scholar] [CrossRef]
- Dye, L.; Boyle, N.B.; Champ, C.; Lawton, C. The relationship between obesity and cognitive health and decline. Proc. Nutr. Soc. 2017, 76, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Lowe, C.J.; Reichelt, A.C.; Hall, P.A. The Prefrontal Cortex and Obesity: A Health Neuroscience Perspective. Trends Cogn. Sci. 2019, 23, 349–361. [Google Scholar] [CrossRef]
- dela Peña, I.C.; Figueroa, J.D.; Shi, W.-X. Hypothesis: Amelioration of obesity-induced cognitive dysfunction via a lorcaserin–betahistine combination treatment. Pharmacol. Res. Perspect. 2022, 10, e00947. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Anand, U.; Nandy, S.; Oleksak, P.; Qusti, S.; Alshammari, E.M.; El-Saber Batiha, G.; Koshy, E.P.; Dey, A. Herbal drugs and natural bioactive products as potential therapeutics: A review on pro-cognitives and brain boosters perspectives. Saudi Pharm. J. 2021, 29, 879–907. [Google Scholar] [CrossRef] [PubMed]
- Bolzenius, J.D.; Laidlaw, D.H.; Cabeen, R.P.; Conturo, T.E.; McMichael, A.R.; Lane, E.M.; Heaps, J.M.; Salminen, L.E.; Baker, L.M.; Scott, S.E.; et al. Brain structure and cognitive correlates of body mass index in healthy older adults. Behav. Brain Res. 2015, 278, 342–347. [Google Scholar] [CrossRef]
- Raji, C.A.; Ho, A.J.; Parikshak, N.N.; Becker, J.T.; Lopez, O.L.; Kuller, L.H.; Hua, X.; Leow, A.D.; Toga, A.W.; Thompson, P.M. Brain structure and obesity. Hum. Brain Mapp. 2010, 31, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Carlsson, C.M.; Trivedi, M.A.; Sager, M.A.; Johnson, S.C. The effect of body mass index on global brain volume in middle-aged adults: A cross sectional study. BMC Neurol. 2005, 5, 23. [Google Scholar] [CrossRef]
- Nota, M.H.C.; Vreeken, D.; Wiesmann, M.; Aarts, E.O.; Hazebroek, E.J.; Kiliaan, A.J. Obesity affects brain structure and function-rescue by bariatric surgery? Neurosci. Biobehav. Rev 2020, 108, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E. Cognitive and neuronal systems underlying obesity. Physiol. Behav. 2012, 106, 337–344. [Google Scholar] [CrossRef]
- Zhang, Z.; Coppin, G. To what extent memory could contribute to impaired food valuation and choices in obesity? Front. Psychol. 2018, 9, 2523. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Grill, H.J. Hippocampus contributions to food intake control: Mnemonic, neuroanatomical, and endocrine mechanisms. Biol. Psychiatry 2017, 81, 748–756. [Google Scholar] [CrossRef]
- Yang, Y.; Shields, G.S.; Guo, C.; Liu, Y. Executive function performance in obesity and overweight individuals: A meta-analysis and review. Neurosci. Biobehav. Rev. 2018, 84, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Gearhardt, A.N.; Yokum, S.; Stice, E.; Harris, J.L.; Brownell, K.D. Relation of obesity to neural activation in response to food commercials. Soc. Cogn. Affect. Neurosci. 2014, 9, 932–938. [Google Scholar] [CrossRef]
- Fernández-Andújar, M.; Morales-García, E.; García-Casares, N. Obesity and Gray Matter Volume Assessed by Neuroimaging: A Systematic Review. Brain Sci. 2021, 11, 999. [Google Scholar] [CrossRef] [PubMed]
- Proctor, C.; Thiennimitr, P.; Chattipakorn, N.; Chattipakorn, S.C. Diet, gut microbiota and cognition. Metab. Brain Dis. 2017, 32, 1–17. [Google Scholar] [CrossRef]
- Buie, J.J.; Watson, L.S.; Smith, C.J.; Sims-Robinson, C. Obesity-related cognitive impairment: The role of endothelial dysfunction. Neurobiol. Dis. 2019, 132, 104580. [Google Scholar] [CrossRef]
- Hargrave, S.L.; Jones, S.; Davidson, T.L. The Outward Spiral: A vicious cycle model of obesity and cognitive dysfunction. Curr. Opin. Behav. Sci. 2016, 9, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.-J.; Morris, M.J. Diet, inflammation and the gut microbiome: Mechanisms for obesity-associated cognitive impairment. BBA–Mol. Bas. Dis. 2020, 1866, 165767. [Google Scholar] [CrossRef] [PubMed]
- Solas, M.; Milagro, F.I.; Ramírez, M.J.; Martínez, J.A. Inflammation and gut-brain axis link obesity to cognitive dysfunction: Plausible pharmacological interventions. Curr. Opin. Pharmacol. 2017, 37, 87–92. [Google Scholar] [CrossRef]
- Xia, Y.; Prokop, S.; Giasson, B.I. “Don’t Phos Over Tau”: Recent developments in clinical biomarkers and therapies targeting tau phosphorylation in Alzheimer’s disease and other tauopathies. Mol. Neurodegener. 2021, 16, 37. [Google Scholar] [CrossRef]
- Wei, Z.; Koya, J.; Reznik, S.E. Insulin Resistance Exacerbates Alzheimer Disease via Multiple Mechanisms. Front. Neurosci. 2021, 15, 687157. [Google Scholar] [CrossRef]
- Gabbouj, S.; Ryhänen, S.; Marttinen, M.; Wittrahm, R.; Takalo, M.; Kemppainen, S.; Martiskainen, H.; Tanila, H.; Haapasalo, A.; Hiltunen, M.; et al. Altered Insulin Signaling in Alzheimer’s Disease Brain—Special Emphasis on PI3K-Akt Pathway. Front. Neurosci. 2019, 13, 629. [Google Scholar] [CrossRef]
- de Oliveira, J.; Kucharska, E.; Garcez, M.L.; Rodrigues, M.S.; Quevedo, J.; Moreno-Gonzalez, I.; Budni, J. Inflammatory Cascade in Alzheimer’s Disease Pathogenesis: A Review of Experimental Findings. Cells. 2021, 10, 2581. [Google Scholar] [CrossRef]
- Cohen, A.C.; Tong, M.; Wands, J.R.; De La Monte, S.M. Insulin and insulin-like growth factor resistance with neurodegeneration in an adult chronic ethanol exposure model. Alcohol. Clin. Exp. Res. 2007, 31, 1558–1573. [Google Scholar] [CrossRef]
- Geiger, B.M.; Behr, G.G.; Frank, L.E.; Caldera-Siu, A.D.; Beinfeld, M.C.; Kokkotou, E.G.; Pothos, E.N. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008, 22, 2740–2746. [Google Scholar] [CrossRef]
- Morris, J.K.; Bomhoff, G.L.; Gorres, B.K.; Davis, V.A.; Kim, J.; Lee, P.P.; Brooks, W.M.; Gerhardt, G.A.; Geiger, P.C.; Stanford, J.A. Insulin resistance impairs nigrostriatal dopamine function. Exp. Neurol. 2011, 231, 171–180. [Google Scholar] [CrossRef]
- Wallace, C.; Fordahl, S. Obesity and dietary fat influence dopamine neurotransmission: Exploring the convergence of metabolic state, physiological stress, and inflammation on dopaminergic control of food intake. Nutr. Res. Rev. 2022, 5, 236–251. [Google Scholar] [CrossRef] [PubMed]
- Labban, R.S.M.; Alfawaz, H.; Almnaizel, A.T.; Hassan, W.M.; Bhat, R.S.; Moubayed, N.M.; Bjørklund, G.; El-Ansary, A. High-fat diet-induced obesity and impairment of brain neurotransmitter pool. Transl. Neurosci. 2020, 11, 147–160. [Google Scholar] [CrossRef]
- Fritz, B.M.; Muñoz, B.; Yin, F.; Bauchle, C.; Atwood, B.K. A High-fat, High-sugar ‘Western’ Diet Alters Dorsal Striatal Glutamate, Opioid, and Dopamine Transmission in Mice. Neuroscience 2018, 372, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, S.; Kaur, G. Withania somnifera leaf alleviates cognitive dysfunction by enhancing hippocampal plasticity in high fat diet induced obesity model. BMC Complement. Altern. Med. 2017, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.M.; Lee, S.I.; Cho, E.J. Effect of Vigna angularis on High-Fat Diet-Induced Memory and Cognitive Impairments. J. Med. Food 2020, 23, 1155–1162. [Google Scholar] [CrossRef]
- Park, S.B.; Kang, J.Y.; Kim, J.M.; Park, S.K.; Yoo, S.K.; Lee, U.; Kim, D.-O.; Heo, H.J. Effect of Aruncus dioicus var. kamtschaticus Extract on Neurodegeneration Improvement: Ameliorating Role in Cognitive Disorder Caused by High-Fat Diet Induced Obesity. Nutrients 2019, 11, 1319. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.S.; Kang, J.Y.; Kang, J.E.; Park, S.K.; Kim, J.M.; Kim, C.W.; Oh, S.I.; Lee, U.; Kim, D.O.; Heo, H.J. Pentacyclic triterpenoid-rich fraction of the Hardy kiwi (Actinidia arguta) improves brain dysfunction in high fat diet-induced obese mice. Sci. Rep. 2020, 10, 5788. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Lee, S.; Cho, E.J.; Kim, H.Y. Protective effects of Aster yomena (Kitam.) Honda from cognitive dysfunction induced by high-fat diet. J. Food Biochem. 2022, 46, e14138. [Google Scholar] [CrossRef]
- Rao, L.S.N.; Kilari, E.K.; Kola, P.K. Protective effect of Curcuma amada acetone extract against high-fat and high-sugar diet-induced obesity and memory impairment. Nutr. Neurosci. 2021, 24, 212–225. [Google Scholar] [CrossRef]
- You, S.; Jang, M.; Kim, G.H. Mori Cortex Radicis Attenuates High Fat Diet-Induced Cognitive Impairment via an IRS/Akt Signaling Pathway. Nutrients 2020, 12, 1851. [Google Scholar] [CrossRef]
- Mikami, T.; Kim, J.; Park, J.; Lee, H.; Yaicharoen, P.; Suidasari, S.; Yokozawa, M.; Yamauchi, K. Olive leaf extract prevents obesity, cognitive decline, and depression and improves exercise capacity in mice. Sci. Rep. 2021, 11, 12495. [Google Scholar] [CrossRef]
- Ajayi, A.M.; John, K.A.; Emmanuel, I.B.; Chidebe, E.O.; Adedapo, A.D.A. High-fat diet-induced memory impairment and anxiety-like behavior in rats attenuated by peel extract of Ananas comosus fruit via atheroprotective, antioxidant and anti-inflammatory actions. Metab. Open 2021, 9, 100077. [Google Scholar] [CrossRef]
- Mary, N.; Babu, B.; Padikkala, J. Antiatherogenic effect of Caps HT2, a herbal Ayurvedic medicine formulation. Phytomedicine 2003, 10, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, N. Role of medicinal plants (brahmi and ashwagandha) in the treatment of Alzheimer’s. Int. J. Life Sci. Sci. Res. 2016, 2, 15–17. [Google Scholar]
- Soman, S.; Korah, P.; Jayanarayanan, S.; Mathew, J.; Paulose, C. Oxidative stress induced NMDA receptor alteration leads to spatial memory deficits in temporal lobe epilepsy: Ameliorative effects of Withania somnifera and Withanolide A. Neurochem. Res. 2012, 37, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Pearson, M.; Kebejian, L.; Golden, E.; Keselman, A.; Bender, M.; Carlson, O.; Egan, J.; Ladenheim, B.; Cadet, J.-L. Sex-dependent metabolic, neuroendocrine, and cognitive responses to dietary energy restriction and excess. Endocrinology 2007, 148, 4318–4333. [Google Scholar] [CrossRef]
- Kitano-Okada, T.; Ito, A.; Koide, A.; Nakamura, Y.; Han, K.H.; Shimada, K.; Sasaki, K.; Ohba, K.; Sibayama, S.; Fukushima, M. Anti-obesity role of adzuki bean extract containing polyphenols: In vivo and in vitro effects. J. Sci. Food Agric. 2012, 92, 2644–2651. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Min, J.W.; King, W.L.; He, X.H.; Li, J.X.; Peng, B.W. A review on the pharmacological effects of vitexin and isovitexin. Fitoterapia. 2016, 115, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, E.; Nassiri-Asl, M.; Shafeei, M.; Sheikhi, M. Neuroprotective effects of vitexin, a flavonoid, on pentylenetetrazole-induced seizure in rats. Chem. Biol. Drug Des. 2012, 80, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, E.J.; Kim, Y.M.; Jung, H.A. Effects of C-glycosylation on anti-diabetic, anti-Alzheimer’s disease and anti-inflammatory potential of apigenin. Food Chem. Toxicol. 2014, 64, 27–33. [Google Scholar] [CrossRef]
- Liu, R.; Zheng, Y.; Cai, Z.; Xu, B. Saponins and Flavonoids from Adzuki Bean (Vigna angularis L.) Ameliorate High-Fat Diet-Induced Obesity in ICR Mice. Front. Pharmacol. 2017, 8, 687. [Google Scholar] [CrossRef] [PubMed]
- Park, S.B.; Kang, J.Y.; Kim, J.M.; Park, S.K.; Park, S.H.; Kang, J.E.; Lee, C.J.; Kwon, B.S.; Yoo, S.K.; Lee, U. Aruncus dioicus var. kamtschaticus extract suppresses mitochondrial apoptosis induced-neurodegeneration in trimethyltin-injected ICR mice. J. Food Biochem. 2018, 42, e12667. [Google Scholar] [CrossRef]
- Latocha, P. The nutritional and health benefits of kiwiberry (Actinidia arguta)—A review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- Kurakane, S.; Yamada, N.; Sato, H.; Igarashi, K. Anti-diabetic effects of Actinidia arguta polyphenols on rats and KK-Ay mice. Food Sci. Technol. Res. 2011, 17, 93–102. [Google Scholar] [CrossRef]
- Bae, J.-S.; Kim, T.-H. Acetylcholinesterase inhibitory and antioxidant properties of Aster yomena extract. Korea J. Herbol. 2009, 24, 121–126. [Google Scholar]
- Kim, Y.J.; Cho, E.J.; Lee, A.Y.; Seo, W.T. Apigenin Ameliorates Oxidative Stress-induced Neuronal Apoptosis in SH-SY5Y Cells. Microbol. Biotechnol. Lett. 2021, 49, 138–147. [Google Scholar] [CrossRef]
- Xu, B.; Zhu, L.; Chu, J.; Ma, Z.; Fu, Q.; Wei, W.; Deng, X.; Ma, S. Esculetin improves cognitive impairments induced by transient cerebral ischaemia and reperfusion in mice via regulation of mitochondrial fragmentation and mitophagy. Behav. Brain Res. 2019, 372, 112007. [Google Scholar] [CrossRef] [PubMed]
- You, S.H.; Jang, M.; Kim, G.-H. Antioxidant activity and neuroprotective effect of root bark of Morus alba L. extract against hydrogen peroxide-induced cytotoxicity in PC12 Cells. J. Korean Soc. Food Sci. Nutr. 2018, 47, 519–527. [Google Scholar] [CrossRef]
- Winzell, M.S.; Ahrén, B. The high-fat diet-fed mouse: A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 2004, 53, S215–S219. [Google Scholar] [CrossRef]
- You, S.; Kim, G.-H. Protective effect of Mori Cortex radicis extract against high glucose-induced oxidative stress in PC12 cells. Biosci. Biotechnol. Biochem. 2019, 83, 1893–1900. [Google Scholar] [CrossRef] [PubMed]
- Gaforio, J.J.; Visioli, F.; Alarcón-de-la-Lastra, C.; Castañer, O.; Delgado-Rodríguez, M.; Fitó, M.; Hernández, A.F.; Huertas, J.R.; Martínez-González, M.A.; Menendez, J.A.; et al. Virgin Olive Oil and Health: Summary of the III International Conference on Virgin Olive Oil and Health Consensus Report, JAEN (Spain) 2018. Nutrients 2019, 11, 2039. [Google Scholar] [CrossRef]
- Ahamad, J.; Toufeeq, I.; Khan, M.A.; Ameen, M.S.M.; Anwer, E.T.; Uthirapathy, S.; Mir, S.R.; Ahmad, J. Oleuropein: A natural antioxidant molecule in the treatment of metabolic syndrome. Phytother. Res. 2019, 33, 3112–3128. [Google Scholar] [CrossRef]
- Sato, H.; Genet, C.; Strehle, A.; Thomas, C.; Lobstein, A.; Wagner, A.; Mioskowski, C.; Auwerx, J.; Saladin, R. Anti-hyperglycemic activity of a TGR5 agonist isolated from Olea europaea. Biochem. Biophys. Res. Commun. 2007, 362, 793–798. [Google Scholar] [CrossRef]
- Takayanagi, S.; Suzuki, H.; Yokozawa, M.; Uchiyama, K.; Ishikawa, M. The effect of the intake of ethanol/water extract of olive leaves (EEO) on body fat percentage and lean body mass percentage: A randomized, double-blind, placebo-controlled, parallel-group study of healthy japanese people. Pharmacometrics 2016, 91, 115–121. [Google Scholar]
- El-Shazly, S.A.; Ahmed, M.M.; Al-Harbi, M.S.; Alkafafy, M.E.; El-Sawy, H.B.; Amer, S.A. Physiological and molecular study on the anti-obesity effects of pineapple (Ananas comosus) juice in male Wistar rat. Food Sci. Biotechnol. 2018, 27, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef]
- Caesar, L.K.; Cech, N.B. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Shi, H.; Yu, Y.; Lin, D.; Zheng, P.; Zhang, P.; Hu, M.; Wang, Q.; Pan, W.; Yang, X.; Hu, T.; et al. β-glucan attenuates cognitive impairment via the gut-brain axis in diet-induced obese mice. Microbiome 2020, 8, 143. [Google Scholar] [CrossRef] [PubMed]
- Moy, G.A.; McNay, E.C. Caffeine prevents weight gain and cognitive impairment caused by a high-fat diet while elevating hippocampal BDNF. Physiol. Behav. 2013, 109, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; Tian, D.; Hu, C.Y.; Meng, Y.H. Chlorogenic Acid Ameliorates High-Fat and High-Fructose Diet-Induced Cognitive Impairment via Mediating the Microbiota–Gut–Brain Axis. J. Agric. Food Chem. 2022, 70, 2600–2615. [Google Scholar] [CrossRef]
- Sarker, M.R.; Franks, S.; Sumien, N.; Thangthaeng, N.; Filipetto, F.; Forster, M. Curcumin Mimics the Neurocognitive and Anti-Inflammatory Effects of Caloric Restriction in a Mouse Model of Midlife Obesity. PLoS ONE 2015, 10, e0140431. [Google Scholar] [CrossRef]
- Fu, X.; Qin, T.; Yu, J.; Jiao, J.; Ma, Z.; Fu, Q.; Deng, X.; Ma, S. Formononetin Ameliorates Cognitive Disorder via PGC-1α Pathway in Neuroinflammation Conditions in High-Fat Diet-Induced Mice. CNS Neurosci. Disord. Drug Targets 2019, 18, 566–577. [Google Scholar] [CrossRef]
- Wang, H.-y.; Wu, M.; Diao, J.-l.; Li, J.-b.; Sun, Y.-x.; Xiao, X.-q. Huperzine A ameliorates obesity-related cognitive performance impairments involving neuronal insulin signaling pathway in mice. Acta Pharmacol. Sin. 2020, 41, 145–153. [Google Scholar] [CrossRef]
- Ayabe, T.; Ohya, R.; Kondo, K.; Ano, Y. Iso-α-acids, bitter components of beer, prevent obesity-induced cognitive decline. Sci. Rep. 2018, 8, 4760. [Google Scholar] [CrossRef]
- Mulati, A.; Zhang, X.; Zhao, T.; Ren, B.; Wang, L.; Liu, X.; Lan, Y.; Liu, X. Isorhamnetin attenuates high-fat and high-fructose diet induced cognitive impairments and neuroinflammation by mediating MAPK and NFκB signaling pathways. Food Funct. 2021, 12, 9261–9272. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, X.; Lan, N.; Li, S.; Zhang, J.; Wang, S.; Li, C.; Shang, Y.; Huang, T.; Zhang, L. Luteolin protects against high fat diet-induced cognitive deficits in obesity mice. Behav. Brain Res. 2014, 267, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yan, J.; Chen, J.; Wu, W.; Zhu, X.; Wang, Y. Naringin Improves Neuronal Insulin Signaling, Brain Mitochondrial Function, and Cognitive Function in High-Fat Diet-Induced Obese Mice. Cell. Mol. Neurobiol. 2015, 35, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, D.; Zhou, Z.; Zhang, X.; Zhang, C.; He, Y.; Liu, C.; Yuan, C.; Yuan, D.; Wang, T. Saponins from Panax japonicus alleviate HFD-induced impaired behaviors through inhibiting NLRP3 inflammasome to upregulate AMPA receptors. Neurochem. Int. 2021, 148, 105098. [Google Scholar] [CrossRef]
- Zhuang, J.; Lu, J.; Wang, X.; Wang, X.; Hu, W.; Hong, F.; Zhao, X.-x.; Zheng, Y.-l. Purple sweet potato color protects against high-fat diet-induced cognitive deficits through AMPK-mediated autophagy in mouse hippocampus. J. Nutr. Biochem. 2019, 65, 35–45. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.F.; Zhang, P.; Wang, H.; Zhang, Q.; Yu, S.; Yu, Y. Chronic rhein treatment improves recognition memory in high-fat diet-induced obese male mice. J. Nutr. Biochem. 2016, 36, 42–50. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, W.; Tian, D.; Tang, Y.; Ye, Y.; Wei, Q.; Zhang, C.; Qiu, W.; Qin, D.; Yang, X.; et al. Dietary Supplement of Anoectochilus roxburghii (Wall.) Lindl. Polysaccharides Ameliorates Cognitive Dysfunction Induced by High Fat Diet via “Gut-Brain” Axis. Drug Des. Devel. Ther. 2022, 16, 1931–1945. [Google Scholar] [CrossRef]
- Mulati, A.; Ma, S.; Zhang, H.; Ren, B.; Zhao, B.; Wang, L.; Liu, X.; Zhao, T.; Kamanova, S.; Sair, A.T.; et al. Sea-Buckthorn Flavonoids Alleviate High-Fat and High-Fructose Diet-Induced Cognitive Impairment by Inhibiting Insulin Resistance and Neuroinflammation. J. Agric. Food Chem. 2020, 68, 5835–5846. [Google Scholar] [CrossRef] [PubMed]
- Neha; Kumar, A.; Jaggi, A.S.; Sodhi, R.K.; Singh, N. Silymarin ameliorates memory deficits and neuropathological changes in mouse model of high-fat-diet-induced experimental dementia. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 777–787. [Google Scholar] [CrossRef]
- Wang, S.; Huang, X.-F.; Zhang, P.; Newell, K.A.; Wang, H.; Zheng, K.; Yu, Y. Dietary teasaponin ameliorates alteration of gut microbiota and cognitive decline in diet-induced obese mice. Sci. Rep. 2017, 7, 12203. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.L.; Elias, V.D.; Hay, J.J.; Choi, J.; Reed, R.L.; Stevens, J.F. Xanthohumol improves dysfunctional glucose and lipid metabolism in diet-induced obese C57BL/6J mice. Arch. Biochem. Biophys. 2016, 599, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Jiang, P.; Zhao, J.; Shi, H.; Zhang, P.; Yang, X.; Biazik, J.; Hu, M.; Hua, H.; Ge, X.; et al. β-Glucan from Lentinula edodes prevents cognitive impairments in high-fat diet-induced obese mice: Involvement of colon-brain axis. J. Transl. Med. 2021, 19, 54. [Google Scholar] [CrossRef]
- Magne, F.; Gotteland, M.; Gauthier, L.; Zazueta, A.; Pesoa, S.; Navarrete, P.; Balamurugan, R. The Firmicutes/Bacteroidetes Ratio: A Relevant Marker of Gut Dysbiosis in Obese Patients? Nutrients 2020, 12, 1474. [Google Scholar] [CrossRef]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic acid improves high fat diet-induced hepatic steatosis and insulin resistance in mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Yesil, A.; Yilmaz, Y. Review article: Coffee consumption, the metabolic syndrome and non-alcoholic fatty liver disease. Aliment. Pharmacol. Ther. 2013, 38, 1038–1044. [Google Scholar] [CrossRef]
- Nishitsuji, K.; Watanabe, S.; Xiao, J.; Nagatomo, R.; Ogawa, H.; Tsunematsu, T.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K. Effect of coffee or coffee components on gut microbiome and short-chain fatty acids in a mouse model of metabolic syndrome. Sci. Rep. 2018, 8, 16173. [Google Scholar] [CrossRef]
- Fernandes, M.Y.D.; Dobrachinski, F.; Silva, H.B.; Lopes, J.P.; Gonçalves, F.Q.; Soares, F.A.; Porciúncula, L.O.; Andrade, G.M.; Cunha, R.A.; Tomé, A.R. Neuromodulation and neuroprotective effects of chlorogenic acids in excitatory synapses of mouse hippocampal slices. Sci. Rep. 2021, 11, 10488. [Google Scholar] [CrossRef]
- Ishida, K.; Yamamoto, M.; Misawa, K.; Nishimura, H.; Misawa, K.; Ota, N.; Shimotoyodome, A. Coffee polyphenols prevent cognitive dysfunction and suppress amyloid β plaques in APP/PS2 transgenic mouse. Neurosci. Res. 2020, 154, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.; Ku, N.; Mortensen, R. Regulation of cytokine-induced human C-reactive protein production by transforming growth factor-beta. J. Immunol. 1990, 145, 2507–2513. [Google Scholar] [CrossRef]
- Yajima, H.; Ikeshima, E.; Shiraki, M.; Kanaya, T.; Fujiwara, D.; Odai, H.; Tsuboyama-Kasaoka, N.; Ezaki, O.; Oikawa, S.; Kondo, K. Isohumulones, bitter acids derived from hops, activate both peroxisome proliferator-activated receptor α and γ and reduce insulin resistance. J. Biol. Chem. 2004, 279, 33456–33462. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Hosono, M.; Oyamada, C.; Odai, H.; Oikawa, S.; Kondo, K. Dietary isohumulones, the bitter components of beer, raise plasma HDL-cholesterol levels and reduce liver cholesterol and triacylglycerol contents similar to PPARα activations in C57BL/6 mice. Br. J. Nutr. 2005, 93, 559–567. [Google Scholar] [CrossRef]
- Obara, K.; Mizutani, M.; Hitomi, Y.; Yajima, H.; Kondo, K. Isohumulones, the bitter component of beer, improve hyperglycemia and decrease body fat in Japanese subjects with prediabetes. Clin. Nutr. 2009, 28, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-α-acids, bitter components of beer, prevent inflammation and cognitive decline induced in a mouse model of Alzheimer’s disease. J. Biol. Chem. 2017, 292, 3720–3728. [Google Scholar] [CrossRef]
- Fuenzalida, K.; Quintanilla, R.; Ramos, P.; Piderit, D.; Fuentealba, R.A.; Martinez, G.; Inestrosa, N.C.; Bronfman, M. Peroxisome proliferator-activated receptor γ up-regulates the Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial stabilization and protection against oxidative stress and apoptosis. J. Biol. Chem. 2007, 282, 37006–37015. [Google Scholar] [CrossRef] [PubMed]
- Pathan, A.R.; Gaikwad, A.B.; Viswanad, B.; Ramarao, P. Rosiglitazone attenuates the cognitive deficits induced by high fat diet feeding in rats. Eur. J. Pharmacol. 2008, 589, 176–179. [Google Scholar] [CrossRef]
- Ho, P.C.; Saville, D.J.; Coville, P.F.; Wanwimolruk, S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products. Pharm. Acta Helv. 2000, 74, 379–385. [Google Scholar] [CrossRef]
- Kim, H.-J.; Song, J.Y.; Park, H.J.; Park, H.-K.; Yun, D.H.; Chung, J.-H. Naringin protects against rotenone-induced apoptosis in human neuroblastoma SH-SY5Y cells. Korean J. Physiol. Pharmacol. 2009, 13, 281–285. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, A. Protective effect of hesperidin and naringin against 3-nitropropionic acid induced Huntington’s like symptoms in rats: Possible role of nitric oxide. Behav. Brain Res. 2010, 206, 38–46. [Google Scholar] [CrossRef]
- Prakash, A.; Shur, B.; Kumar, A. Naringin protects memory impairment and mitochondrial oxidative damage against aluminum-induced neurotoxicity in rats. Int. J. Neurosci. 2013, 123, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhang, C.; He, H.; Wang, T.; Liu, Z.; Liu, G.; Sun, Z.; Zhou, Z.; Bai, C.; Yuan, D. Protective effect of saponins extract from Panax japonicus on myocardial infarction: Involvement of NF-κB, Sirt1 and mitogen-activated protein kinase signalling pathways and inhibition of inflammation. J. Pharm. Pharmacol. 2014, 66, 1641–1651. [Google Scholar] [CrossRef]
- Yuan, D.; Xiang, T.; Huo, Y.; Liu, C.; Wang, T.; Zhou, Z.; Dun, Y.; Zhao, H.; Zhang, C. Preventive effects of total saponins of Panax japonicus on fatty liver fibrosis in mice. Arch. Med. Sci. 2018, 14, 396–406. [Google Scholar] [CrossRef]
- Kulkarni, A.; Chen, J.; Maday, S. Neuronal autophagy and intercellular regulation of homeostasis in the brain. Curr. Opin. Neurobiol. 2018, 51, 29–36. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, S.; Hu, N.; Gu, M.; Chu, C.; Li, Y.; Lu, X.; Huang, C. Rhein Reduces Fat Weight in db/db Mouse and Prevents Diet-Induced Obesity in C57Bl/6 Mouse through the Inhibition of PPARγ Signaling. PPAR Res. 2012, 2012, 374936. [Google Scholar] [CrossRef]
- Sheng, X.; Zhu, X.; Zhang, Y.; Cui, G.; Peng, L.; Lu, X.; Zang, Y.Q. Rhein protects against obesity and related metabolic disorders through liver X receptor-mediated uncoupling protein 1 upregulation in brown adipose tissue. Int. J. Biol. Sci. 2012, 8, 1375–1384. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.; Duan, X.; Ke, Y.; Zhang, L.; Shen, Y.; Hu, B.; Liu, A.; Chen, H.; Li, C.; Wu, W.; et al. Antidiabetic activities of polysaccharides from Anoectochilus roxburghii and Anoectochilus formosanus in STZ-induced diabetic mice. Int. J. Biol. Macromol. 2018, 112, 882–888. [Google Scholar] [CrossRef]
- Zeng, B.; Su, M.; Chen, Q.; Chang, Q.; Wang, W.; Li, H. Antioxidant and hepatoprotective activities of polysaccharides from Anoectochilus roxburghii. Carbohydr. Polym. 2016, 153, 391–398. [Google Scholar] [CrossRef]
- Liu, Z.L.; Zhang, J.G.; Liu, Q.; Yi, L.T.; Li, Y.M.; Li, Y. The vascular protective effects of Anoectochilus roxburghii polysaccharose under high glucose conditions. J. Ethnopharmacol. 2017, 202, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Q.; Zhuang, S.; Wen, Y.; Cheng, W.; Zeng, Z.; Jiang, T.; Tang, C. Effect of Anoectochilus roxburghii flavonoids extract on H2O2—Induced oxidative stress in LO2 cells and D-gal induced aging mice model. J. Ethnopharmacol. 2020, 254, 112670. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Sheng, S. Anti-aging effects and mechanisms of Anoectochilus roxburghii polysaccharose. J. Huaqiao Univ. (Nat. Sci.) 2020, 41, 77–83. [Google Scholar]
- Sagar, S.M. Future directions for research on Silybum marianum for cancer patients. Integr. Cancer Ther. 2007, 6, 166–173. [Google Scholar] [CrossRef]
- Yao, J.; Zhi, M.; Minhu, C. Effect of silybin on high-fat-induced fatty liver in rats. Braz. J. Med. Biol. Res. 2011, 44, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Wolfram, S.; Wang, Y.; Thielecke, F. Anti-obesity effects of green tea: From bedside to bench. Mol. Nutr. Food Res. 2006, 50, 176–187. [Google Scholar] [CrossRef]
- Li, Q.; Zhao, H.; Zhang, Z.; Liu, Z.; Pei, X.; Wang, J.; Li, Y. Long-term green tea catechin administration prevents spatial learning and memory impairment in senescence-accelerated mouse prone-8 mice by decreasing Aβ1-42 oligomers and upregulating synaptic plasticity–related proteins in the hippocampus. Neuroscience 2009, 163, 741–749. [Google Scholar] [CrossRef] [PubMed]
- van Duynhoven, J.; Vaughan, E.E.; van Dorsten, F.; Gomez-Roldan, V.; de Vos, R.; Vervoort, J.; van der Hooft, J.J.; Roger, L.; Draijer, R.; Jacobs, D.M. Interactions of black tea polyphenols with human gut microbiota: Implications for gut and cardiovascular health. Am. J. Clin. Nutr. 2013, 98, 1631S–1641S. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Legette, L.L.; Luna, A.Y.M.; Reed, R.L.; Miranda, C.L.; Bobe, G.; Proteau, R.R.; Stevens, J.F. Xanthohumol lowers body weight and fasting plasma glucose in obese male Zucker fa/fa rats. Phytochemistry 2013, 91, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Dorn, C.; Bataille, F.; Gaebele, E.; Heilmann, J.; Hellerbrand, C. Xanthohumol feeding does not impair organ function and homoeostasis in mice. Food Chem. Toxicol. 2010, 48, 1890–1897. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.; Kalita, J.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249. [Google Scholar] [CrossRef]
- Miranda, C.L.; Johnson, L.A.; de Montgolfier, O.; Elias, V.D.; Ullrich, L.S.; Hay, J.J.; Paraiso, I.L.; Choi, J.; Reed, R.L.; Revel, J.S.; et al. Non-estrogenic Xanthohumol Derivatives Mitigate Insulin Resistance and Cognitive Impairment in High-Fat Diet-induced Obese Mice. Sci. Rep. 2018, 8, 613. [Google Scholar] [CrossRef]
- Al Dahhan, N.Z.; De Felice, F.G.; Munoz, D.P. Potentials and pitfalls of cross-translational models of cognitive impairment. Front. Behav. Neurosci. 2019, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Lewis, A.R.; Singh, S.; Youssef, F.F. Cafeteria-diet induced obesity results in impaired cognitive functioning in a rodent model. Heliyon 2019, 5, e01412. [Google Scholar] [CrossRef] [PubMed]
| Plant | Treatment Dose; Duration | Central Effects | Other Effects | Obesity Model; Strain, Species; Gender | Reference |
|---|---|---|---|---|---|
| Ashwagandha (Withania somnifera) | Dry leaf powder (1 mg/g of BW) + HFD; 12 weeks | neuroprotection; enhancement of synaptic plasticity and cell survival | normalized corticosterone levels; improved locomotor coordination | HFD (30% fat by weight); Wistar albino rats; Female | [39] |
| Adzuki bean (Vigna angularis) | Ethanol extract (VA 100; 200 mg/kg of BW) + HFD; 4 weeks | not investigated | weight loss | HFD (60% fat); C57BL/6J mice; Male | [40] |
| Dwarf Goat’s Beard (Aruncus dioicus var. kamtschaticus) | Ethyl acetate fraction (EFAD 20; 40 mg/kg of BW); +HFD 4 weeks | reduction of oxidative stress, improvement of impaired cholinergic system and mitochondrial dysfunction, etc. | weight loss; improved impaired glucose tolerance | HFD (20 kcal%/g fat); C57BL/6 mice; Male | [41] |
| Hardy kiwi (Actinidia arguta) | Chloroform fraction (20 and 40 mg/kg); 4 weeks | improved impaired cholinergic, antioxidant system, and mitochondria functions; brain insulin signaling | improved glucose tolerance | HFD (20 kcal%/g, carbohydrate 20 kcal%/g and fat 60 kcal%/g, 5.24 kcal/g); C57BL/6 mice; Male | [42] |
| Japanese aster [Aster yomena (Kitam.) Honda] | Ethyl acetate fraction (EFAY 100 mg/kg; 200 mg/kg) + HFD; 4 weeks | decreased neuroinflammation; ameliorated insulin resistance | not investigated | HFD (60% fat); C57BL/6J mice; Male | [43] |
| Mango ginger (Curcuma amada) | Acetone extract (CAAE 100; 300 mg/kg of BW) + HFHS diet; 3 weeks | reduction in AchE and oxidative stress markers, improvement of impaired cholinergic system; increased dopamine and serotonin levels; neuroprotection | weight loss; decreased liver and serum lipid levels; increased HDLs | HFHSD (3 mL of ghee and 1 mL of coconut oil, and 25% of fructose); Wistar albino rats; Male | [44] |
| Mulberry root bark (Mori radicis cortex) | Dissolved extracts (100 or 200 mg/kg/day of BW); 6 weeks | decreased AChE expression; reduction of oxidative stress; inhibition of p-Tau expression; neuroprotection | weight loss; inhibited disruptions of lipid metabolic markers; lowered blood glucose spikes | HFD (60% kcal fat); C57BL/6 mice; Male | [45] |
| Olive (Olea europaea) | Ethanol/Water extract of olive leaves OLEAVITA (1 g olive leaf extract per 1000 g of HFD); 10 weeks | improvement of mitochondrial function and antioxidant capacity; increased BDNFexpression | weight loss; enhanced mitochondrial muscle mass and endurance exercise capacity; antidepressant-like effect | HFD (protein, 17.8 g; fat, 20.0 g; carbohydrate 49.0 g; calorie, 480.8 kcal) + 200 g butter) C57BL/6J mice; Male | [46] |
| Pineapple (Ananas comosus) | Methanol extract of peel (PEAC 200 mg/kg of BW); 3 weeks | increasing antioxidant capacity; decreased brain AChE levels; reduction of neuroinflammation | anxiolytic-like effect; improved lipid profile and decreased risk of atherogenicity | HFD (44% animal fat and 0.3% methionine); Wistar rats; Male | [47] |
| Isolated Compounds | Source(s) | Treatment Mode; Dose; Duration | Central Effects | Other Effects | Obesity Model; Strain; Species; Gender | Ref. |
|---|---|---|---|---|---|---|
| β-glucan | Oats | Oral β-glucan from oats (7 g/100 g of daily food intake) + HFD; 15 weeks and short-term for 1 week | decreased microglial activation and inflammation; promoted synaptogenesis; improved insulin signaling; inhibited Tau phosphorylation; improved synaptic plasticity | weight loss (long-term treatment); reversed gut barrier dysfunction; increased thickness of colonic mucus; increased level of tight junction proteins; ameliorated altered microbiota | HFFD (55% by energy; 5% fiber by weight); C57BL/6J mice; Male | [74] |
| Caffeine | Coffee, tea | Intraperitoneal caffeine; 20 mg/kg; once weekly; 11 weeks | reversed decrease in BDNF levels | weight loss; decreased plasma insulin levels | HFD (31.8% of energy as fat); SD rats; Male | [75] |
| Chlorogenic acid | Various plants | Oral chlorogenic acid; (150 mg/kg/day) + HFFD; 14 weeks | ameliorated hippocampal structural damage and synaptic dysfunction; decreased inflammation; improved cholinergic synapse and calcium signaling pathway | weight loss; reduced insulin resistance and lipid profile; increased gut microbiota diversity and bacteria producing SCFA; significant decrease in TG, TC, LDL, and significantly increased HDLs | HFHS (45% kcal from fat, 10% fructose in drinking water) C57BL/6J mice; Male | [76] |
| Curcumin | Turmeric (Curcuma longa) | Oral curcumin ad libitum; 1000 mg/kg diet; 12 weeks | anti-inflammatory or antioxidant actions | no weight loss effect | Ad libitum standard diet (4.1% energy as fat); C57BL/6 mice; Male | [77] |
| Formononetin | Red clover (Trifolium pratense) | Intragastric (20,40 mg/kg); 10 weeks | Decreased Tau hyperphosphorylation; reduced cytokines | weight loss | HFD (10% lard oil, 1% cholesterol, 0.2% cholate; 5% sucrose); ICR mice; Male | [78] |
| Huperzine A | Chinese club moss (Huperzia serrata) | Intragastric or oral Hup A (0.1 mg/kg/day and 0.3 mg/kg/day); 12 weeks | increased insulin and AKT activity; decreased beta-secretase expression | no weight loss effect | HFD (60% energy from fat, 20% from protein, and 20% from carbohydrates); C57 BL/6 mice; Male | [79] |
| Iso-α-acids | Hops (Humulus lupulus) | Oral iso-α-acids group; daily 0.05% (w/w) + HFD; 8 weeks | attenuated neuroinflammation and lipid peroxidation; prevented hippocampal atrophy | weight loss; decreased epididymal fat and plasma triglyceride levels | HFD (60 kcal% from fat); C57BL/6 J mice; Male | [80] |
| Isorhamnetin | Sea-buckthorn (Elaeagnaceae genus) | Oral daily isorhamnetin (0.03% w/w and 0.06% w/w) in HFFD; 14 weeks | inhibited microglial overactivation and neuroinflammation; increased activity of neurotrophic factors | weight loss; improved serum and liver lipids: TC, TG, LDL, HDL | HFHFD (45% kcal from fat, 10% kcal from fructose water); C57BL/6J; Male | [81] |
| Luteolin | Dyer’s rocket (Reseda luteola) | Oral daily; 10 mg/kg in HFD; 20 weeks | decreased neuroinflammation, oxidative stress, and neuronal insulin resistance; increased BDNF levels, synapsin U and PSD-95 | weight loss; restored blood adipokines to normal level; | HFD (Energy as 15% protein, 43% carbohydrate, and 42% fat); C57BL/6J mice; Male | [82] |
| Naringin | Grapefruit (Citrus × paradisi) | Daily oral naringin; 100 mg/kg + HFD; 20 weeks | ameliorated mitochondrial dysfunction, improved insulin signaling pathway, AMPK | weight loss; restored abnormal glucose, fatty acid, and cholesterol metabolism | HFD (unknown composition); C57BL/6J mice; Male | [83] |
| Panax japonicus saponins | Japanese ginseng (Panax japonicus) | Oral daily saponins; 15 mg/kg and 45 mg/kg; 16 weeks | decreased neurodegeneration and neuroinflammation; upregulated AMPA receptors signaling pathway | weight loss; antidepressant | HFD (60% total calories from fat); Balb/c mice; Male | [84] |
| Purple sweet potato color anthocyanins | Sweet potato (Ipomoea batatas) | Oral daily PSPC; 100 mg/kg + HFD; 20 weeks | enhanced autophagy, decreased levels of ROS; improved BDNF levels | weight loss; ameliorated peripheral insulin resistance | HFD (60% calories from fat); ICR mice; Male | [85] |
| Rhein | Rhubarb (Rheum rhabarbarum) | Daily oral rhein; 120 mg/kg + HFD; 6 weeks | improved BDNF levels; decreased neuroinflammation | weight loss; inhibited the increase in plasma LPS level and the proinflammatory macrophage accumulation in the colon and alteration of microbiota, improved glucose tolerance | HFD (60% fat by calories); C57BL/6J mice; Male | [86] |
| Roxburgh’s jewel orchid polysaccharides | Roxburgh’s Jewel Orchid [Anoectochilus roxburghii (Wall.) Lindl.] | Oral daily ARPs; (1 mg/g and 3 mg/g (w/w) + HFD; 14 weeks | decreased Tau phosphorylation; and neuroinflammation | weight loss; decreased plasma glucose, total cholesterol, inflammation; restored intestinal epithelial barrier; decreased abundance of Parabacteriodes | HFD (60% kcal from fat); C57BL/6J mice; Male | [87] |
| Sea-buckthorn flavonoids | Sea-buckthorn (Elaeagnaceae genus) | Oral daily Sea Buckthorn flavonoid; 0.06% and 0.31% w/w mixed in HFFD; 14 weeks | alleviated the synaptic damages, reduced neuroinflammation, normalized insulin signaling, increased neurotrophic growth factors levels | weight loss; reversed glucose intolerance increase and insulin sensitivity loss; significantly decreased TG, TC, LDL, significantly increased HDLs | HFHFD (45% kcal from fat, 10% fructose in drinking water); C57BL/6J mice; Male | [88] |
| Silymarin | Milk thistle (Silybum marianum) | Oral daily silymarin; 100 mg/kg and 200 mg/kg + HFD; 15 days | reversed increase in AChE activity; decreased oxidative stress; reduced nitrate/nitrite levels and myeloperoxidase activity; decreased brain neutrophil infiltration and Aβ burden | weight loss; lowered serum cholesterol level; | Cholesterol-rich diet; HFD (310 g/1000 g of total diet from fat); Swiss Albino mice; Male and Female | [89] |
| Tea saponins | Tea plant | Oral daily tea saponins; 0.5% mixed in HFD; 6 weeks | suppressed neuroinflammation; reduced microglia and astrocyte accumulation; raised BDNF levels | weight loss; reversed alteration of gut microbiota and systemic inflammation; reduced M1 macrophage accumulation in the colon; | HFD (60% energy from fat); C57BL/6J mice; Male | [90] |
| Xanthohumol Derivatives | Hops (Humulus lupulus) | Oral daily xanthohumol derivatives; 30 mg/kg; 13 weeks | not investigated | weight loss (TXN); improvement of impaired glucose tolerance; decreased HOMA-IR and plasma leptin | HFD (60% fat; 20% carbohydrate; 20% protein); C57BL/6J mice; Male | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Peña, I.; Afable, T.; Dahilig-Talan, V.R.; Cruz, P. Review of Plant Extracts and Active Components: Mechanisms of Action for the Treatment of Obesity-Induced Cognitive Impairment. Brain Sci. 2023, 13, 929. https://doi.org/10.3390/brainsci13060929
de la Peña I, Afable T, Dahilig-Talan VR, Cruz P. Review of Plant Extracts and Active Components: Mechanisms of Action for the Treatment of Obesity-Induced Cognitive Impairment. Brain Sciences. 2023; 13(6):929. https://doi.org/10.3390/brainsci13060929
Chicago/Turabian Stylede la Peña, Ike, Timothy Afable, Vina Rose Dahilig-Talan, and Philip Cruz. 2023. "Review of Plant Extracts and Active Components: Mechanisms of Action for the Treatment of Obesity-Induced Cognitive Impairment" Brain Sciences 13, no. 6: 929. https://doi.org/10.3390/brainsci13060929
APA Stylede la Peña, I., Afable, T., Dahilig-Talan, V. R., & Cruz, P. (2023). Review of Plant Extracts and Active Components: Mechanisms of Action for the Treatment of Obesity-Induced Cognitive Impairment. Brain Sciences, 13(6), 929. https://doi.org/10.3390/brainsci13060929







