Current Role of Endoscopic Endonasal Approach for Craniopharyngiomas: A 10-Year Systematic Review and Meta-Analysis Comparison with the Open Transcranial Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Data Extraction and Data Quality
Eligibility Criteria and Study Selection
2.3. Outcomes
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
2.6. Meta-Analysis
2.7. Quality and Bias Assessment
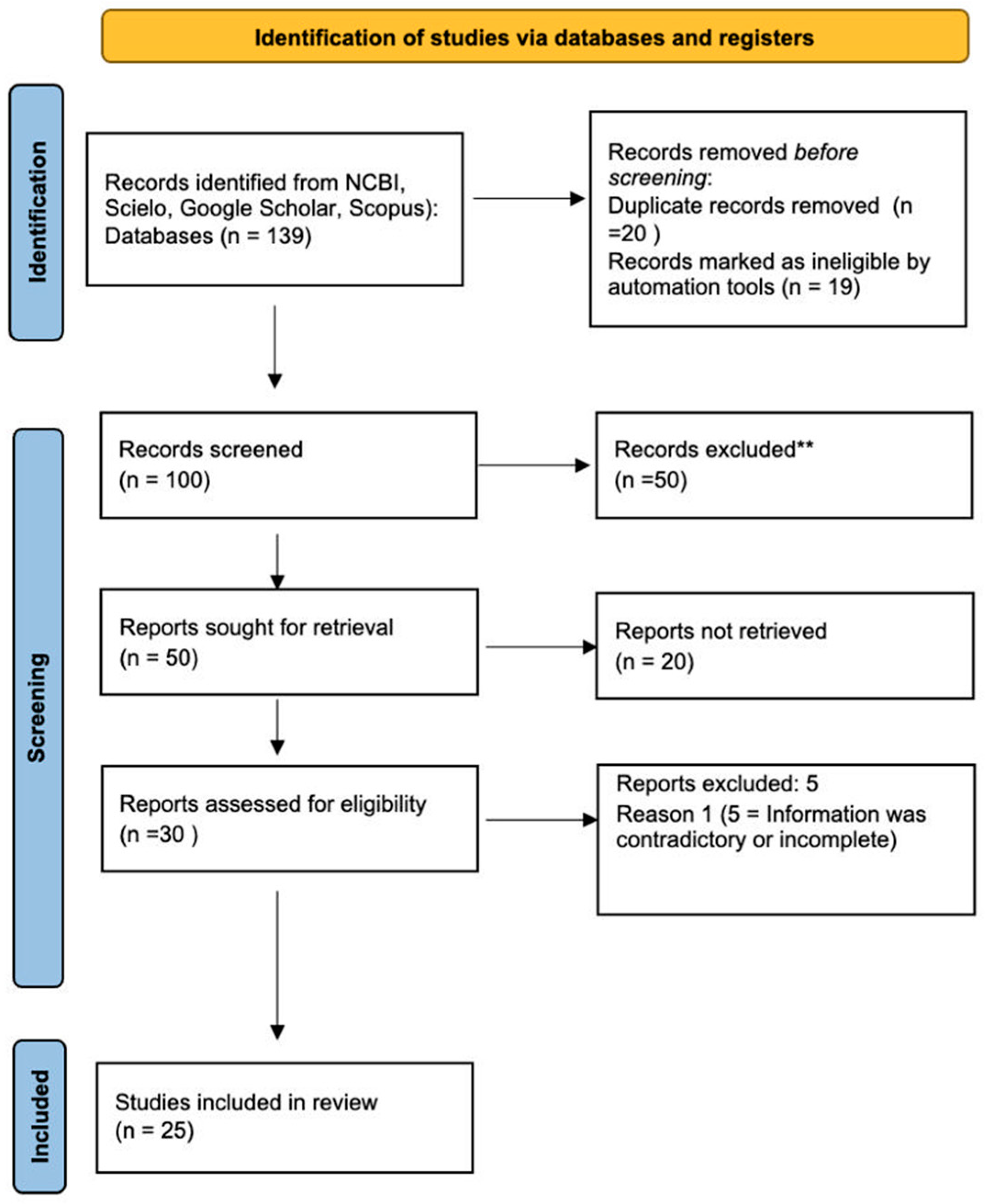
3. Results
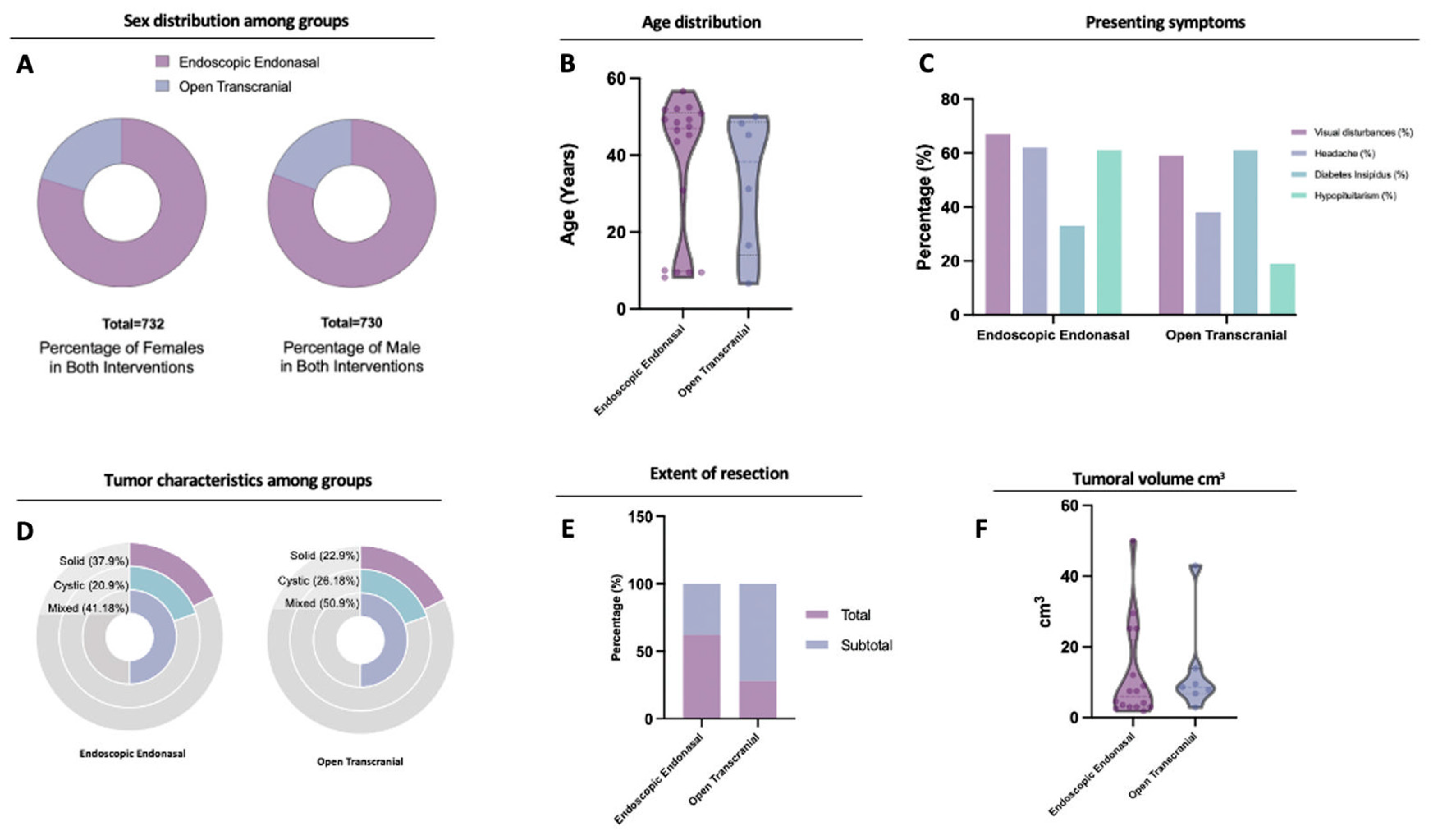
3.1. Included Studies and Patient Characteristics
3.2. Clinical Presentation and Extent of Resection
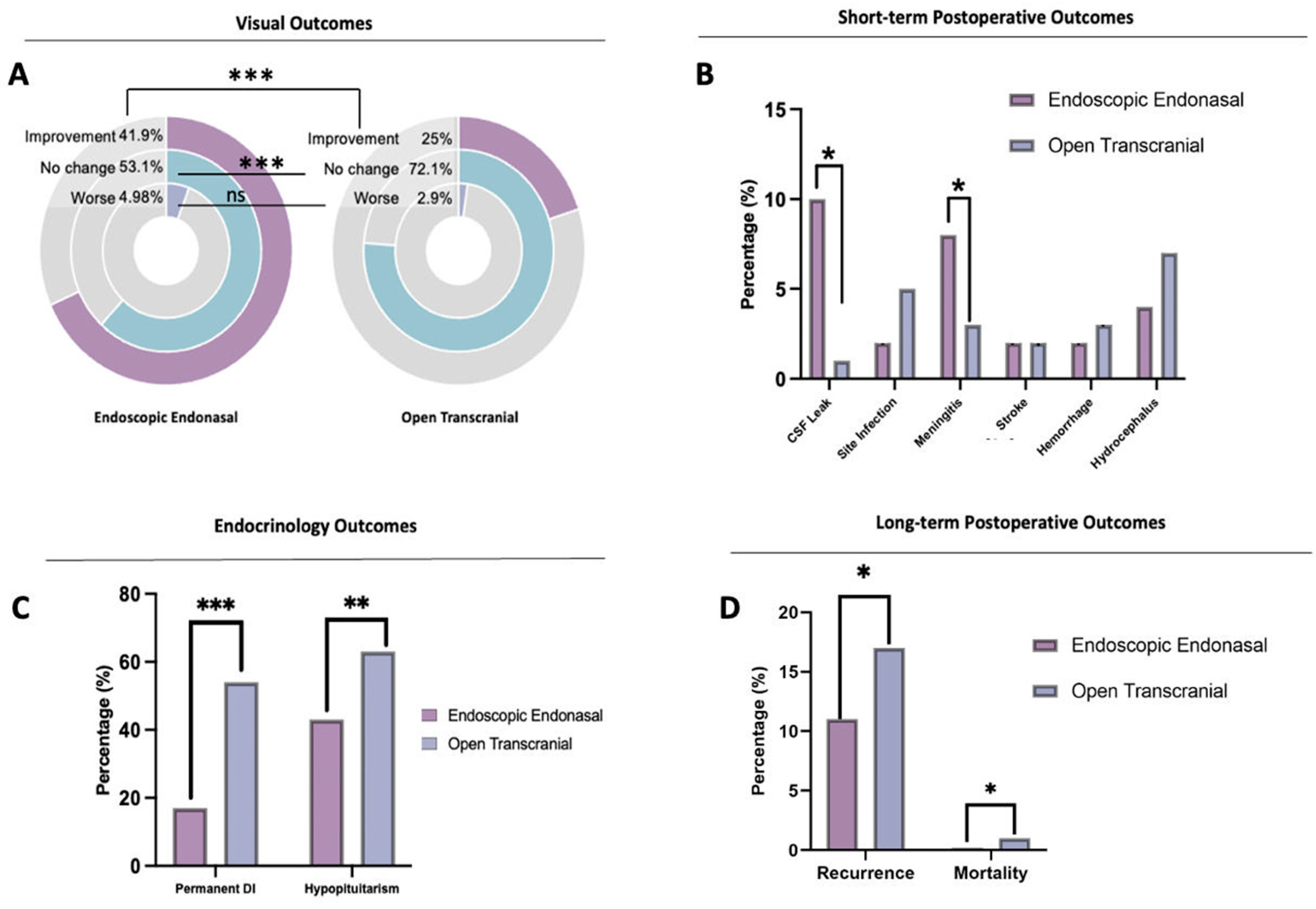
3.3. Surgical Outcomes
3.4. Outcomes: Meta-Analysis
4. Discussion
4.1. Presenting Symptoms
4.2. The Extent of Resection
4.3. CSF Leak, SITE Infection, and Meningitis Risk
4.4. Endocrinologic Outcomes
4.5. Stroke, Hemorrhage, and Hydrocephalus
4.6. Recurrence and Mortality
4.7. Hypothalamic Involvement
4.8. Perspective on Medical Treatments for Craniopharyngiomas: BRAF-Targeted Therapies
4.9. Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, B.; Xie, S.H.; Xiao, L.M.; Huang, G.L.; Wang, Z.G.; Yang, L.; Yang, X.Y.; Xu, S.; Chen, Y.Y.; Ji, Y.Q.; et al. A Novel Endoscopic Classification for Craniopharyngioma Based on Its Origin. Sci. Rep. 2018, 8, 10215. [Google Scholar] [CrossRef] [PubMed]
- Komotar, R.J.; Starke, R.M.; Raper, D.M.S.; Anand, V.K.; Schwartz, T.H. Endoscopic Skull Base Surgery: A Comprehensive Comparison with Open Transcranial Approaches. Br. J. Neurosurg. 2012, 26, 637–648. [Google Scholar] [CrossRef]
- Cavallo, L.M.; Somma, T.; Solari, D.; Iannuzzo, G.; Frio, F.; Baiano, C.; Cappabianca, P. Endoscopic Endonasal Transsphenoidal Surgery: History and Evolution. World Neurosurg. 2019, 127, 686–694. [Google Scholar] [CrossRef]
- Na, M.K.; Jang, B.; Choi, K.-S.; Lim, T.H.; Kim, W.; Cho, Y.; Shin, H.-G.; Ahn, C.; Kim, J.G.; Lee, J.; et al. Craniopharyngioma Resection by Endoscopic Endonasal Approach versus Transcranial Approach: A Systematic Review and Meta-Analysis of Comparative Studies. Front. Oncol. 2022, 12, 1058329. [Google Scholar] [CrossRef] [PubMed]
- Komotar, R.J.; Starke, R.M.; Raper, D.M.S.; Anand, V.K.; Schwartz, T.H. Endoscopic Endonasal Compared with Microscopic Transsphenoidal and Open Transcranial Resection of Craniopharyngiomas. World Neurosurg. 2012, 77, 329–341. [Google Scholar] [CrossRef]
- Puget, S.; Garnett, M.; Wray, A.; Grill, J.; Habrand, J.-L.; Bodaert, N.; Zerah, M.; Bezerra, M.; Renier, D.; Pierre-Kahn, A.; et al. Pediatric Craniopharyngiomas: Classification and Treatment According to the Degree of Hypothalamic Involvement. J. Neurosurg: Pediatr. 2007, 106, 3–12. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological Index for Non-Randomized Studies (MINORS): Development and Validation of a New Instrument: Methodological Index for Non-Randomized Studies. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Leng, L.Z.; Greenfield, J.P.; Souweidane, M.M.; Anand, V.K.; Schwartz, T.H. Endoscopic, Endonasal Resection of Craniopharyngiomas: Analysis of Outcome Including Extent of Resection, Cerebrospinal Fluid Leak, Return to Preoperative Productivity, and Body Mass Index. Neurosurgery 2012, 70, 110–124. [Google Scholar] [CrossRef]
- Ali, Z.S.; Lang, S.-S.; Kamat, A.R.; Adappa, N.D.; Palmer, J.N.; Storm, P.B.; Lee, J.Y.K. Suprasellar Pediatric Craniopharyngioma Resection via Endonasal Endoscopic Approach. Childs Nerv. Syst. 2013, 29, 2065–2070. [Google Scholar] [CrossRef]
- Patel, K.S.; Raza, S.M.; McCoul, E.D.; Patrona, A.; Greenfield, J.P.; Souweidane, M.M.; Anand, V.K.; Schwartz, T.H. Long-Term Quality of Life after Endonasal Endoscopic Resection of Adult Craniopharyngiomas. J. Neurosurg. 2015, 123, 571–580. [Google Scholar] [CrossRef]
- Jeswani, S.; Nuño, M.; Wu, A.; Bonert, V.; Carmichael, J.D.; Black, K.L.; Chu, R.; King, W.; Mamelak, A.N. Comparative Analysis of Outcomes Following Craniotomy and Expanded Endoscopic Endonasal Transsphenoidal Resection of Craniopharyngioma and Related Tumors: A Single-Institution Study. J. Neurosurg. 2016, 124, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Moussazadeh, N.; Prabhu, V.; Bander, E.D.; Cusic, R.C.; Tsiouris, A.J.; Anand, V.K.; Schwartz, T.H. Endoscopic Endonasal versus Open Transcranial Resection of Craniopharyngiomas: A Case-Matched Single-Institution Analysis. Neurosurg. Focus 2016, 41, E7. [Google Scholar] [CrossRef] [PubMed]
- Bal, E.; Öge, K.; Berker, M. Endoscopic Endonasal Transsphenoidal Surgery, A Reliable Method for Treating Primary and Recurrent/Residual Craniopharyngiomas: Nine Years of Experience. World Neurosurg. 2016, 94, 375–385. [Google Scholar] [CrossRef]
- Fomichev, D.; Kalinin, P.; Kutin, M.; Sharipov, O. Extended Transsphenoidal Endoscopic Endonasal Surgery of Suprasellar Craniopharyngiomas. World Neurosurg. 2016, 94, 181–187. [Google Scholar] [CrossRef]
- Wannemuehler, T.J.; Rubel, K.E.; Hendricks, B.K.; Ting, J.Y.; Payner, T.D.; Shah, M.V.; Cohen-Gadol, A.A. Outcomes in Transcranial Microsurgery versus Extended Endoscopic Endonasal Approach for Primary Resection of Adult Craniopharyngiomas. Neurosurg. Focus 2016, 41, E6. [Google Scholar] [CrossRef]
- Pennacchietti, V.; Garzaro, M.; Grottoli, S.; Pacca, P.; Garbossa, D.; Ducati, A.; Zenga, F. Three-Dimensional Endoscopic Endonasal Approach and Outcomes in Sellar Lesions: A Single-Center Experience of 104 Cases. World Neurosurg. 2016, 89, 121–125. [Google Scholar] [CrossRef]
- Patel, V.S.; Thamboo, A.; Quon, J.; Nayak, J.V.; Hwang, P.H.; Edwards, M.; Patel, Z.M. Outcomes After Endoscopic Endonasal Resection of Craniopharyngiomas in the Pediatric Population. World Neurosurg 2017, 108, 6–14. [Google Scholar] [CrossRef]
- Nagata, Y.; Watanabe, T.; Nagatani, T.; Takeuchi, K.; Chu, J.; Wakabayashi, T. Fully Endoscopic Combined Transsphenoidal and Supraorbital Keyhole Approach for Parasellar Lesions. J. Neurosurg. 2018, 128, 685–694. [Google Scholar] [CrossRef]
- Ishikawa, T.; Takeuchi, K.; Nagatani, T.; Aimi, Y.; Tanemura, E.; Tambara, M.; Nagata, Y.; Choo, J.; Wakabayashi, T. Quality of Life Changes Before and After Transsphenoidal Surgery for Sellar and Parasellar Lesions. World Neurosurg. 2019, 122, e1202–e1210. [Google Scholar] [CrossRef]
- Forbes, J.A.; Ordóñez-Rubiano, E.G.; Tomasiewicz, H.C.; Banu, M.A.; Younus, I.; Dobri, G.A.; Phillips, C.D.; Kacker, A.; Cisse, B.; Anand, V.K.; et al. Endonasal Endoscopic Transsphenoidal Resection of Intrinsic Third Ventricular Craniopharyngioma: Surgical Results. J. Neurosurg. 2019, 131, 1152–1162. [Google Scholar] [CrossRef]
- Yamada, S.; Fukuhara, N.; Yamaguchi-Okada, M.; Nishioka, H.; Takeshita, A.; Takeuchi, Y.; Inoshita, N.; Ito, J. Therapeutic Outcomes of Transsphenoidal Surgery in Pediatric Patients with Craniopharyngiomas: A Single-Center Study. J. Neurosurg. Pediatr. 2018, 21, 549–562. [Google Scholar] [CrossRef] [PubMed]
- Schelini, J.C.; Cavalheiro, S.; Dastoli, P.A.; Hirai, É.R.; Atallah, C.; Costa, M.; Nicacio, J.; Capellano, A.M.; Silva, N.; Zymberg, S.; et al. Endoscopic Endonasal Transsphenoidal Approach for Pediatric Craniopharyngiomas: A Case Series. Int. J. Pediatr. Otorhinolaryngol. 2020, 130, 109786. [Google Scholar] [CrossRef] [PubMed]
- Massa, D.; Glerean, M.; Rasmussen, J.; Altszul, M.; Fainstein-Day, P.; Ajler, P. Craneofaringiomas: Experiencia y resultados. Neurocirugía 2021, 32, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.P.-Y.; Tung, J.Y.-L.; Ku, D.T.-L.; Luk, C.-W.; Ling, A.S.-C.; Kwong, D.L.-W.; Cheng, K.K.-F.; Ho, W.W.-S.; Shing, M.M.-K.; Chan, G.C.-F. Outcome of Chinese Children with Craniopharyngioma: A 20-Year Population-Based Study by the Hong Kong Pediatric Hematology/Oncology Study Group. Childs Nerv. Syst. 2020, 36, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Marx, S.; Tsavdaridou, I.; Paul, S.; Steveling, A.; Schirmer, C.; Eördögh, M.; Nowak, S.; Matthes, M.; El Refaee, E.; Fleck, S.K.; et al. Quality of Life and Olfactory Function after Suprasellar Craniopharyngioma Surgery-a Single-Center Experience Comparing Transcranial and Endoscopic Endonasal Approaches. Neurosurg. Rev. 2021, 44, 1569–1582. [Google Scholar] [CrossRef]
- Javadpour, M.; Amoo, M.; Crimmins, D.; Caird, J.; Daly, P.; Pears, J.; Owens, C.; Capra, M.; Cody, D. Endoscopic Extended Transsphenoidal Surgery for Newly Diagnosed Paediatric Craniopharyngiomas. Childs Nerv. Syst. 2021, 37, 1547–1561. [Google Scholar] [CrossRef]
- Mazzatenta, D.; Zoli, M.; Guaraldi, F.; Ambrosi, F.; Faustini Fustini, M.; Pasquini, E.; Asioli, S.; Zucchelli, M. Outcome of Endoscopic Endonasal Surgery in Pediatric Craniopharyngiomas. World Neurosurg. 2020, 134, e277–e288. [Google Scholar] [CrossRef]
- Cavallo, L.M.; Frank, G.; Cappabianca, P.; Solari, D.; Mazzatenta, D.; Villa, A.; Zoli, M.; D’Enza, A.I.; Esposito, F.; Pasquini, E. The Endoscopic Endonasal Approach for the Management of Craniopharyngiomas: A Series of 103 Patients: Clinical Article. J. Neurosurg. 2014, 121, 100–113. [Google Scholar] [CrossRef]
- Nie, C.; Ye, Y.; Wu, J.; Zhao, H.; Jiang, X.; Wang, H. Clinical Outcomes of Transcranial and Endoscopic Endonasal Surgery for Craniopharyngiomas: A Single-Institution Experience. Front. Oncol. 2022, 12, 755342. [Google Scholar] [CrossRef]
- Li, X.; Wu, W.; Miao, Q.; He, M.; Zhang, S.; Zhang, Z.; Lu, B.; Yang, Y.; Shou, X.; Li, Y.; et al. Endocrine and Metabolic Outcomes After Transcranial and Endoscopic Endonasal Approaches for Primary Resection of Craniopharyngiomas. World Neurosurg. 2019, 121, e8–e14. [Google Scholar] [CrossRef]
- Radovanovic, I.; Dehdashti, A.R.; Turel, M.K.; Almeida, J.P.; Godoy, B.L.; Doglietto, F.; Vescan, A.D.; Zadeh, G.; Gentili, F. Expanded Endonasal Endoscopic Surgery in Suprasellar Craniopharyngiomas: A Retrospective Analysis of 43 Surgeries Including Recurrent Cases. Operative Surg. 2019, 17, 132–142. [Google Scholar] [CrossRef] [PubMed]
- Madsen, P.J.; Buch, V.P.; Douglas, J.E.; Parasher, A.K.; Lerner, D.K.; Alexander, E.; Workman, A.D.; Palmer, J.N.; Lang, S.-S.; Kennedy, B.C.; et al. Endoscopic Endonasal Resection versus Open Surgery for Pediatric Craniopharyngioma: Comparison of Outcomes and Complications. J Neurosurg. Pediatr. 2019, 24, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Qiu, B.; Mei, F.; Mao, J.; Zhou, L.; Liu, F.; Fan, J.; Liu, Y.; Wen, G.; Qi, S.; et al. Clinical Impact of Craniopharyngioma Classification Based on Location Origin: A Multicenter Retrospective Study. Ann. Transl. Med 2021, 9, 1164. [Google Scholar] [CrossRef] [PubMed]
- Kassam, A.B.; Gardner, P.A.; Snyderman, C.H.; Carrau, R.L.; Mintz, A.H.; Prevedello, D.M. Expanded Endonasal Approach, a Fully Endoscopic Transnasal Approach for the Resection of Midline Suprasellar Craniopharyngiomas: A New Classification Based on the Infundibulum. J. Neurosurg. 2008, 108, 715–728. [Google Scholar] [CrossRef]
- Lei, C.; Chuzhong, L.; Chunhui, L.; Peng, Z.; Jiwei, B.; Xinsheng, W.; Yazhuo, Z.; Songbai, G. Approach Selection and Outcomes of Craniopharyngioma Resection: A Single-Institute Study. Neurosurg. Rev. 2021, 44, 1737–1746. [Google Scholar] [CrossRef]
- Lee, I.H.; Kim, D.H.; Park, J.-S.; Jeun, S.-S.; Hong, Y.-K.; Kim, S.W. Cerebrospinal Fluid Leakage Repair of Various Grades Developing during Endoscopic Transnasal Transsphenoidal Surgery. PLoS ONE 2021, 16, e0248229. [Google Scholar] [CrossRef]
- Dho, Y.-S.; Kim, Y.H.; Se, Y.-B.; Han, D.H.; Kim, J.H.; Park, C.-K.; Wang, K.-C.; Kim, D.G. Endoscopic Endonasal Approach for Craniopharyngioma: The Importance of the Relationship between Pituitary Stalk and Tumor. J. Neurosurg. 2018, 129, 611–619. [Google Scholar] [CrossRef]
- Luginbuhl, A.J.; Campbell, P.G.; Evans, J.; Rosen, M. Endoscopic Repair of High-Flow Cranial Base Defects Using a Bilayer Button. Laryngoscope 2010, 120, 876–880. [Google Scholar] [CrossRef]
- Leng, L.Z.; Brown, S.; Anand, V.K.; Schwartz, T.H. “GASKET-SEAL” WATERTIGHT CLOSURE IN MINIMAL-ACCESS ENDOSCOPIC CRANIAL BASE SURGERY. Oper. Neurosurg. 2008, 62, ONSE342–ONSE343. [Google Scholar] [CrossRef]
- Akinduro, O.O.; Izzo, A.; Lu, V.M.; Ricciardi, L.; Trifiletti, D.; Peterson, J.L.; Bernet, V.; Donaldson, A.; Eggenberger, E.; Olomu, O.; et al. Endocrine and Visual Outcomes Following Gross Total Resection and Subtotal Resection of Adult Craniopharyngioma: Systematic Review and Meta-Analysis. World Neurosurg. 2019, 127, e656–e668. [Google Scholar] [CrossRef]
- Hong, C.S.; Omay, S.B. The Role of Surgical Approaches in the Multi-Modal Management of Adult Craniopharyngiomas. Curr. Oncol. 2022, 29, 1408–1421. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez-Rubiano, E.G.; Forbes, J.A.; Morgenstern, P.F.; Arko, L.; Dobri, G.A.; Greenfield, J.P.; Souweidane, M.M.; Tsiouris, A.J.; Anand, V.K.; Kacker, A.; et al. Preserve or Sacrifice the Stalk? Endocrinological Outcomes, Extent of Resection, and Recurrence Rates Following Endoscopic Endonasal Resection of Craniopharyngiomas. J. Neurosurg. 2019, 131, 1163–1171. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.P.; Kalyvas, A.; Mohan, N.; Oswari, S.; Takami, H.; Velasquez, C.; Asha, M.; Zadeh, G.; Gentili, F. Current Results of Surgical Treatment of Craniopharyngiomas: The Impact of Endoscopic Endonasal Approaches. World Neurosurgery 2020, 142, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.K.; Sevak, I.A.; Carmel, P.W.; Eloy, J.A. Microscopic versus Endoscopic Approaches for Craniopharyngiomas: Choosing the Optimal Surgical Corridor for Maximizing Extent of Resection and Complication Avoidance Using a Personalized, Tailored Approach. Neurosurg. Focus 2016, 41, E5. [Google Scholar] [CrossRef]
- Cagnazzo, F.; Zoli, M.; Mazzatenta, D.; Gompel, J. Endoscopic and Microscopic Transsphenoidal Surgery of Craniopharyngiomas: A Systematic Review of Surgical Outcomes Over Two Decades. J. Neurol. Surg. A Cent. Eur. Neurosurg. 2018, 79, 247–256. [Google Scholar] [CrossRef]
- Alexandraki, K.I.; Kaltsas, G.A.; Karavitaki, N.; Grossman, A.B. The Medical Therapy of Craniopharyngiomas: The Way Ahead. J. Clin. Endocrinol. Metab. 2019, 104, 5751–5764. [Google Scholar] [CrossRef]
- Juratli, T.A.; Jones, P.S.; Wang, N.; Subramanian, M.; Aylwin, S.J.B.; Odia, Y.; Rostami, E.; Gudjonsson, O.; Shaw, B.L.; Cahill, D.P.; et al. Targeted Treatment of Papillary Craniopharyngiomas Harboring BRAF V600E Mutations. Cancer 2019, 125, 2910–2914. [Google Scholar] [CrossRef]
- Almeida, J.P.; Workewych, A.; Takami, H.; Velasquez, C.; Oswari, S.; Asha, M.; Bernardo, A.; Gentili, F. Surgical Anatomy Applied to the Resection of Craniopharyngiomas: Anatomic Compartments and Surgical Classifications. World Neurosurg. 2020, 142, 611–625. [Google Scholar] [CrossRef]
- Brastianos, P.K.; Twohy, E.; Geyer, S.M.; Gerstner, E.R.; Kaufmann, T.J.; Ruff, M.; Bota, D.A.; Reardon, D.A.; Cohen, A.L.; De La Fuente, M.I.; et al. Alliance A071601: Phase II Trial of BRAF/MEK Inhibition in Newly Diagnosed Papillary Craniopharyngiomas. J. Clin. Oncol. 2021, 39, 2000. [Google Scholar] [CrossRef]

| Author | Year of Publication | Type of Study | Total Number of Patients | Total Follow-Up (Months) | Number of Patients with Endoscopic Resection | Number of Patients of Open Transcranial Resection |
|---|---|---|---|---|---|---|
| Leng et al. [8] | 2011 | Case Series | 24 | 35 | 24 | 0 |
| Ali et al. [9] | 2013 | Retrospective cohort | 7 | 6.3 | 7 | 0 |
| Patel et al. [10] | 2015 | Single Institution Review | 31 | 40 | 31 | 0 |
| Jeswani et al. [11] | 2015 | Single Institution retrospective Review | 87 | 34.9 | 53 | 34 |
| Moussazadeh et al. [12] | 2016 | Single Institution Review | 26 | 30 | 21 | 5 |
| Bal et al. [13] | 2016 | Case Series | 25 | 56.4 | 25 | 0 |
| Fomichev et al. [14] | 2016 | Case Series | 136 | 10 | 136 | 0 |
| Wannemuehler et al. [15] | 2016 | Retrospective review | 21 | 7.2 | 9 | 12 |
| Pennacchietti et al. [16] | 2016 | Retrospective review | 104 | 18 | 104 | 0 |
| Patel et al. [17] | 2017 | Cross-sectional | 16 | 56.2 | 16 | 0 |
| Nagata et al. [18] | 2018 | Retrospective review | 12 | 41 | 0 | 0 |
| Ishikawa et al. [19] | 2018 | Case Series | 178 | 6 | 178 | 0 |
| Forbes et al. [20] | 2018 | Prospective Cohort | 10 | 46.8 | 10 | 0 |
| Yamada et al. [21] | 2018 | Clinical series | 65 | 93.6 | 65 | 0 |
| Schelini et al. [22] | 2019 | Case Series | 20 | 63.6 | 20 | 0 |
| Massa et al. [23] | 2020 | Case Series | 30 | 42.7 | 30 | 0 |
| Pak-Yin Liu et al. [24] | 2020 | Retrospective cohort | 28 | 60 | 0 | 28 |
| Marx et al. [25] | 2020 | Retrospective cohort | 30 | 136 | 17 | 13 |
| Javadpour et al. [26] | 2020 | Prospective database | 15 | 74 | 15 | 0 |
| Mazzatenta et al. [27] | 2020 | Cross-sectional | 25 | 72 | 25 | 0 |
| Fomichev et al. [14] | 2016 | Retrospective Cohort | 136 | 42 | 136 | 0 |
| Cavallo et al. [28] | 2014 | Case Series | 103 | 48 | 103 | 0 |
| Nie et al. [29] | 2022 | Case Series | 273 | 30.5 | 88 | 185 |
| Li et al. [30] | 2018 | Retrospective cohort | 43 | 9 | 17 | 26 |
| Radovanovic et al. [31] | 2019 | Case Series | 43 | 56.8 | 43 | 0 |
| Endoscopic Endonasal | Open Transcranial | p Value | |
|---|---|---|---|
| Patient Characteristics | |||
| Total Patients | 1144 | 305 | 0.1 |
| Mean Age (Range) | 37.4 | 37.1 | 0.96 |
| Female (%) | 50.54 | 51.38 | 0.84 |
| Male (%) | 49.46 | 48.62 | 0.85 |
| Mean follow-up (months) | 39.9 | 43.9 | 0.76 |
| Presenting Symptoms | |||
| Visual disturbances (%) * | 65.70 | 58.70 | 0.03 |
| Headache (%) * | 62.10 | 37.90 | <0.0001 |
| Diabetes Insipidus (%) * | 30.70 | 61.10 | 0.005 |
| Hypopituitarism (%) * | 60.60 | 18.70 | <0.0001 |
| Tumor characteristics | |||
| Volume (cm3) | 11.97 | 13.23 | 0.83 |
| Solid (%) | 37.91 | 22.91 | 0.71 |
| Cystic (%) | 20.92 | 26.18 | 0.61 |
| Mixed solid and cystic (%) | 41.18 | 50.90 | 0.83 |
| Extent of resection | |||
| Gross Total (%) | 62.00 | 28.17 | 0.62 |
| Subtotal (%) | 37.98 | 71.83 | 0.57 |
| Endoscopic Endonasal (%) | Open Transcranial (%) | p Value | OR | LCI | SCI | |
|---|---|---|---|---|---|---|
| Visual Outcomes | ||||||
| 41.96 | 25 | <0.0001 | 7.7 | 5.4 | 11.1 | |
| No change * | 53.06 | 72.09 | <0.001 | 0.4 | 0.28 | 0.55 |
| Worse | 4.98 | 2.91 | 0.14 | 0.13 | 0.83 | 4.9 |
| Endocrine Outcomes | ||||||
| New Onset Permanent DI * | 29.20 | 67.40 | <0.0001 | 0.2 | 0.14 | 0.27 |
| New Onset Hypopituitarism * | 46.80 | 66.32 | <0.0001 | 0.4 | 0.33 | 0.58 |
| Postoperative Outcomes | ||||||
| CSF Leak * | 9.94 | 0.70 | <0.0001 | 15.8 | 4.2 | 66.2 |
| Site Infection | 1.65 | 4.57 | 0.09 | 0.35 | 0.11 | 1.22 |
| Meningitis * | 8.12 | 2.55 | 0.009 | 3.38 | 1.59 | 7.62 |
| Stroke | 2.10 | 2.12 | 0.98 | 0.98 | 0.39 | 2.5 |
| Hemorrhage | 2.30 | 3.21 | 0.59 | 0.71 | 0.24 | 2.1 |
| Hydrocephalus | 3.50 | 6.50 | 0.4 | 0.5 | 0.15 | 1.77 |
| Follow-Up | ||||||
| Recurrence * | 15.50 | 21.20 | 0.04 | 0.7 | 0.47 | 0.97 |
| Mortality * | 0.77 | 2.22 | 0.05 | 0.9 | 0.75 | 12.82 |
| EEA (Cases/Control) | TCA (Cases/Control) | p-Value | RR | LCI | SCI | |
|---|---|---|---|---|---|---|
| Visual Outcomes | ||||||
| Improvement | 72/78 | 43/172 | 1.17 | 0.71 | 0.39 | 1.03 |
| Outcomes | ||||||
| Permanent DI | 86/79 | 182/88 | 0.04 | −0.22 | −0.44 | −0.01 |
| Hypopituitarism | 100/76 | 189/96 | 0.5 | −0.10 | −0.4 | 0.20 |
| CSF Leak | 31/181 | 2/288 | 0.01 | 1.39 | 0.29 | 2.50 |
| Meningitis | 6/163 | 7/268 | 0.9 | 0.06 | −0.97 | 1.09 |
| Recurrence | 20/175 | 56/208 | 0.002 | −0.73 | −1.20 | −0.25 |
| Article | Number of Patients (No Previous Intervention) | Mean Age | Type of Intervention | Classification of Hypothalamic Involvement | Number of Patients with Hypothalamic Involvement | Pre-operatory Hypothalamic Dysfunction | Post-operatory New Hypothalamic Dysfunction | Extent of Resection in Cases with Hypothalamic Involvement | Postoperativs Radiation | Post-Operative Main Symptom |
|---|---|---|---|---|---|---|---|---|---|---|
| Bal, 2016 [13] | 15 | 31.2 (17–68) | EEA | Not Discussed | 4 | 50% (2/4) | 0% | GTR (50%) | Not Discussed | Not Discussed |
| STR (50%) | ||||||||||
| Yamada, 2018 [21] | 45 | 9.6 (0.8–17.9) | EEA | Grade 0 = 19 | 26 | 4.4% (2/45) | 4.4% (2/45) | GTR (98%) * | 1 | Weight Gain |
| Grade 1 = 8 | STR (2%) | |||||||||
| Grade 2 = 18 | ||||||||||
| Javadopour, 2021 [26] | 15 | 10 (5–18) | EEA | Grade 0 = 9 | 6 | 20% (3/15) | 6.6% (1/15) | STR (100%) | 6 | Weight Gain |
| Grade 1 = 0 | ||||||||||
| Grade 2 = 6 | ||||||||||
| Cavallo, 2014 [28] | 103 | 50.36 (18–83) | EEA | Not Discussed | 25 | 24.3% (25/103) | 17.5% (17/97) | GTR (30%) STR (70%) | 8 patients | Not Discussed |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueredo, L.F.; Martínez, A.L.; Suarez-Meade, P.; Marenco-Hillembrand, L.; Salazar, A.F.; Pabon, D.; Guzmán, J.; Murguiondo-Perez, R.; Hallak, H.; Godo, A.; et al. Current Role of Endoscopic Endonasal Approach for Craniopharyngiomas: A 10-Year Systematic Review and Meta-Analysis Comparison with the Open Transcranial Approach. Brain Sci. 2023, 13, 842. https://doi.org/10.3390/brainsci13060842
Figueredo LF, Martínez AL, Suarez-Meade P, Marenco-Hillembrand L, Salazar AF, Pabon D, Guzmán J, Murguiondo-Perez R, Hallak H, Godo A, et al. Current Role of Endoscopic Endonasal Approach for Craniopharyngiomas: A 10-Year Systematic Review and Meta-Analysis Comparison with the Open Transcranial Approach. Brain Sciences. 2023; 13(6):842. https://doi.org/10.3390/brainsci13060842
Chicago/Turabian StyleFigueredo, Luisa F., Andrea L. Martínez, Paola Suarez-Meade, Lina Marenco-Hillembrand, Andrés Felipe Salazar, Daniela Pabon, Juan Guzmán, Renata Murguiondo-Perez, Hana Hallak, Alex Godo, and et al. 2023. "Current Role of Endoscopic Endonasal Approach for Craniopharyngiomas: A 10-Year Systematic Review and Meta-Analysis Comparison with the Open Transcranial Approach" Brain Sciences 13, no. 6: 842. https://doi.org/10.3390/brainsci13060842
APA StyleFigueredo, L. F., Martínez, A. L., Suarez-Meade, P., Marenco-Hillembrand, L., Salazar, A. F., Pabon, D., Guzmán, J., Murguiondo-Perez, R., Hallak, H., Godo, A., Sandoval-Garcia, C., Ordoñez-Rubiano, E. G., Donaldson, A., Chaichana, K. L., Peris-Celda, M., Bendok, B. R., Samson, S. L., Quinones-Hinojosa, A., & Almeida, J. P. (2023). Current Role of Endoscopic Endonasal Approach for Craniopharyngiomas: A 10-Year Systematic Review and Meta-Analysis Comparison with the Open Transcranial Approach. Brain Sciences, 13(6), 842. https://doi.org/10.3390/brainsci13060842







