Meningioangiomatosis Combined with Calcifying Pseudoneoplasms of Neuraxis
Abstract
1. Introduction
2. Materials and Methods
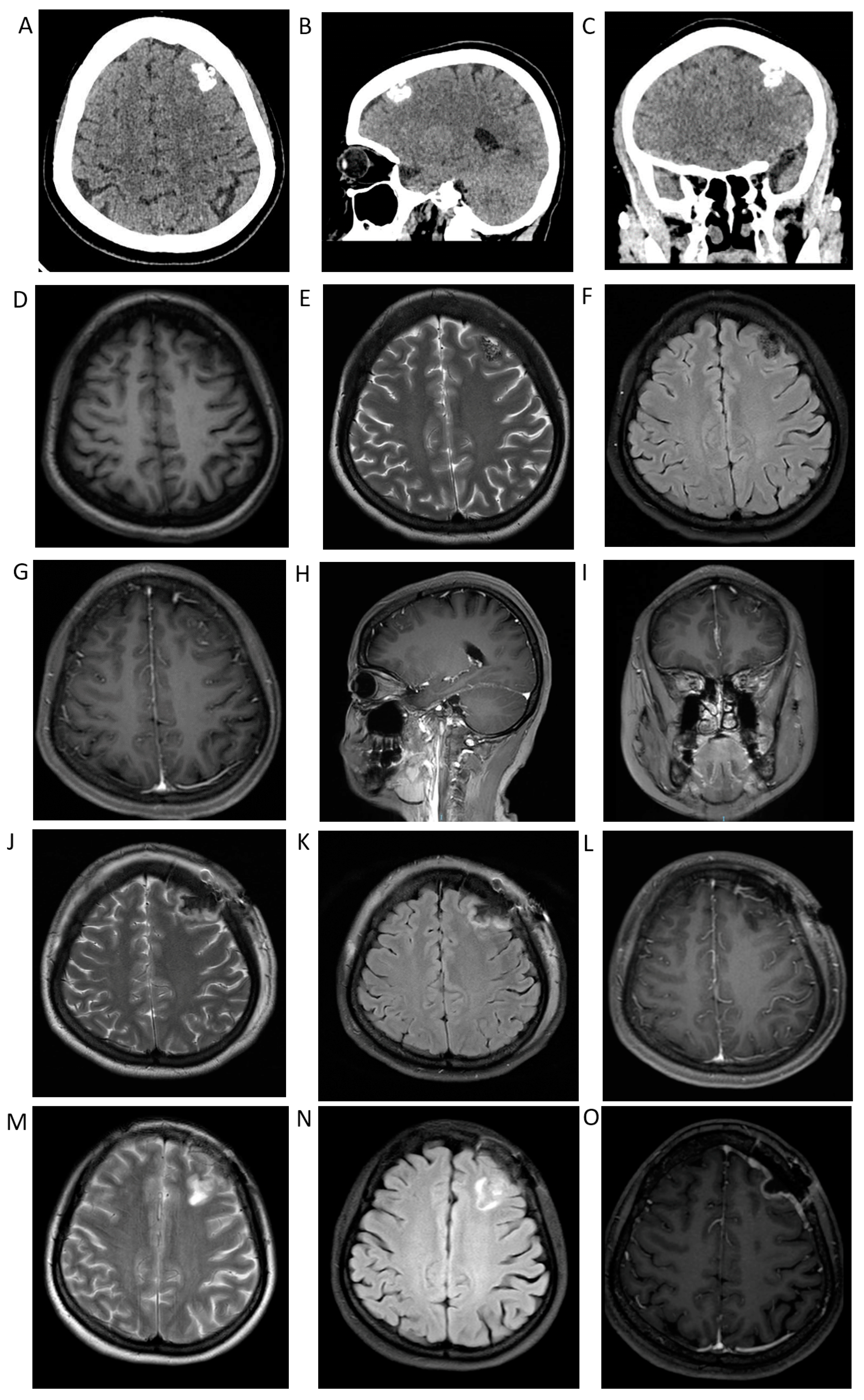
3. Case Presentation
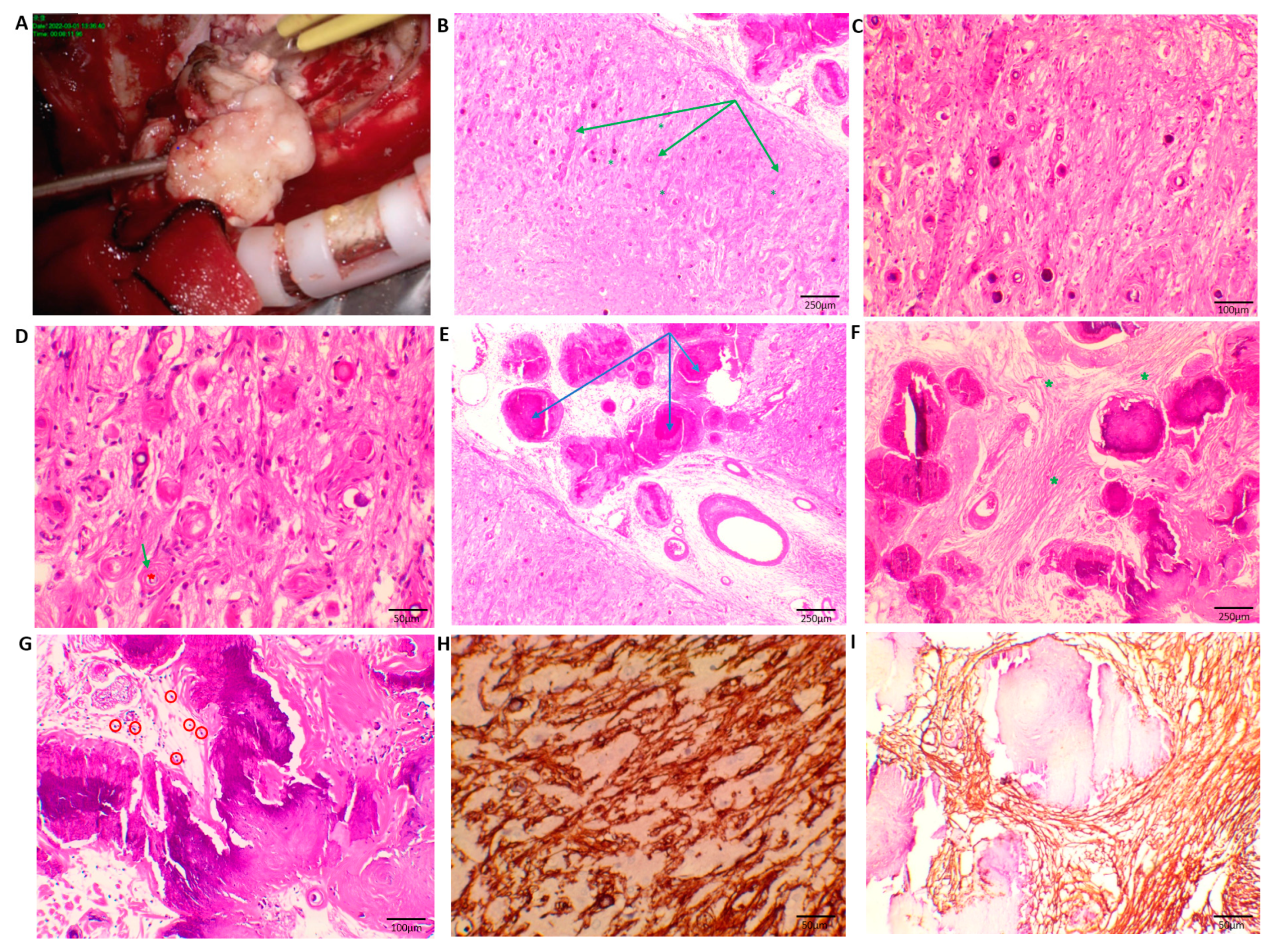
4. Histopathology
5. Results
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Free, S.; Berg, A.; Asfora, W.; Freeman, J. Sporadic Meningioangiomatosis: Bystander or Curious Culprit. South Dakota Med. 2018, 71, 120–124. [Google Scholar]
- Bassoe, P.; Nuzum, F. Report of a Case of Central and Peripheral Neurofibromatosis. J. Nerv. Ment. Dis. 1915, 42, 785–796. [Google Scholar] [CrossRef]
- Worster-Drought, C.; Dickson, W.E.C.; Mcmenemey, W.H. Multiple Meningeal and Perineural Tumours with Analogous Changes in the Glia and Ependyma (Neurofibroblastomatosis): With Report of Two Cases. Brain 1937, 60, 85–117. [Google Scholar] [CrossRef]
- Lu, J.-Q.; Popovic, S.; Provias, J.; Cenic, A. Collision Lesions of Calcifying Pseudoneoplasm of the Neuraxis and Rheumatoid Nodules: A Case Report with New Pathogenic Insights. Int. J. Surg. Pathol. 2021, 29, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Q.; Wang, S.-H.; Zhang, Z.-W.; Chen, J.; Li, Y.-M.; Lv, Z.-C.; Cao, H.-T.; Ma, X.-M.; Liu, H.-M.; Zhu, Z. Calcifying pseudoneoplasms of the neuraxis (CAPNON). A case report. Neuropathology 2021, 41, 371–375. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, R.H.; Davis, R.L. An unusual fibro-osseous component in intracranial lesions. Hum. Pathol. 1978, 9, 309–319. [Google Scholar] [CrossRef]
- Stienen, M.N.; Abdulazim, A.; Gautschi, O.P.; Schneiderhan, T.M.; Hildebrandt, G.; Lücke, S. Calcifying pseudoneoplasms of the neuraxis (CAPNON): Clinical features and therapeutic options. Acta Neurochir. 2013, 155, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, A.; Metrock, K.; Li, R.; Singh, S. Cystic meningioangiomatosis and cerebellar ependymoma in a child with neurofibromatosis type 2. Radiol. Case Rep. 2022, 17, 1082–1087. [Google Scholar] [CrossRef] [PubMed]
- Hassan, O.; Ghanchi, H.; Reyes, K.D.; Raghavan, R.; Minasian, T. Pediatric Temporal Lobe Meningioma with Meningioangiomatosis Mimicking Invasive Meningioma. Cureus 2021, 13, e18819. [Google Scholar] [CrossRef]
- Galloway, L.; Zilani, G.; Lammie, A.; Leach, P. Meningioma with rhabdoid features combined with meningioangiomatosis in infancy: A novel combination. Child’s Nerv. Syst. 2020, 36, 1311–1314. [Google Scholar] [CrossRef]
- Iorgulescu, J.B.; Ferris, S.; Agarwal, A.; Zambrano, S.C.; Hill, D.A.; Schmidt, R.; Perry, A. Non-meningothelial meningeal tumours with meningioangiomatosis-like pattern of spread. Neuropathol. Appl. Neurobiol. 2018, 44, 743–746. [Google Scholar] [CrossRef] [PubMed]
- Rossi, S.; Brenca, M.; Zanatta, L.; Trincia, E.; Guerriero, A.; Pizzato, C.; Fiorindi, A.; Viscardi, E.; Giangaspero, F.; Maestro, R.; et al. A Pediatric Intra-Axial Malignant SMARCB1-Deficient Desmoplastic Tumor Arising in Meningioangiomatosis. J. Neuropathol. Exp. Neurol. 2018, 77, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, Y.; Wang, X.; Zhang, J.-G.; Li, J.-J.; Hu, W.-H.; Zhang, K. Sporadic meningioangiomatosis with and without meningioma: Analysis of clinical differences and risk factors for poor seizure outcomes. Acta Neurochir. 2015, 157, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Jamil, O.; Ramkissoon, S.; Folkerth, R.; Smith, E. Multifocal meningioangiomatosis in a 3-year-old patient. J. Neurosurgery Pediatr. 2012, 10, 486–489. [Google Scholar] [CrossRef]
- Cui, H.; Shi, H.; Chen, X.; Wang, W.; Lai, R.; Han, A. Clinicopathological Features of Meningioangiomatosis Associated with Meningioma: A Case Report with Literature Review. Case Rep. Oncol. Med. 2012, 2012, 296286. [Google Scholar]
- Ho, M.; Eschbacher, K.L.; Paolini, M.A.; Raghunathan, A. New insights into calcifying pseudoneoplasm of the neuraxis (CAPNON): A 20-year radiological–pathological study of 37 cases. Histopathology 2020, 76, 1055–1069. [Google Scholar] [CrossRef]
- Paolini, M.A.; Ho, M.-L.; Monahan, H.R.; Raghunathan, A. Supratentorial CAPNON associated with WHO grade II meningioma: A case report. Neuropathology 2018, 38, 535–538. [Google Scholar] [CrossRef]
- Soukup, J.; Kohout, A.; Vosmikova, H.; Hacova, M.; Kaiser, M.; Klener, J.; Krejci, T.; Syrucek, M.; Wozniakova, M.; Gabalec, F.; et al. Calcifying pseudoneoplasm of neuroaxis (CAPNON): A comprehensive immunohistochemical and morphological characterization of five cases. Virchows Arch. 2022, 480, 415–423. [Google Scholar] [CrossRef]
- Inukai, M.; Shibahara, I.; Hotta, M.; Miyasaka, K.; Sato, S.; Hide, T.; Saegusa, M.; Kumabe, T. Case of Calcifying Pseudoneoplasms of the Neuraxis Coexisting with Interhemispheric Lipoma and Agenesis of the Corpus Callosum: Involvement of Infiltrating Macrophages. World Neurosurg. 2020, 134, 635–640.e1. [Google Scholar] [CrossRef]
- Watanabe, A.; Nakanishi, K.; Kataoka, K.; Wakasa, T.; Ohta, Y. Regrowth and progression of multiple calcifying pseudoneoplasms of the neuraxis: Case report. Surg. Neurol. Int. 2018, 9, 243. [Google Scholar] [CrossRef]
- Higa, N.; Yokoo, H.; Hirano, H.; Yonezawa, H.; Oyoshi, T.; Goto, Y.; Arita, K. Calcifying pseudoneoplasm of the neuraxis in direct continuity with a low-grade glioma: A case report and review of the literature. Neuropathology 2017, 37, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Makary, M.S.; Kobalka, P.; Giglio, P.; Slone, H.W. Meningioangiomatosis: Clinical, Imaging, and Histopathologic Characteristics. J. Clin. Imaging Sci. 2020, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Kashlan, O.N.; LaBorde, D.V.; Davison, L.; Saindane, A.M.; Brat, D.; Hudgins, P.A.; Gross, R.E. Meningioangiomatosis: A Case Report and Literature Review Emphasizing Diverse Appearance on Different Imaging Modalities. Case Rep. Neurol. Med. 2011, 2011, 361203. [Google Scholar] [CrossRef] [PubMed]
- Bulut, E.; Mut, M.; Soylemezoglu, F.; Oguz, K.K. Meningioangiomatosis of the cerebellum: Radiopathologic characteristics of a case. Acta Neurochir. 2015, 157, 1371–1372. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, Y.; Zee, C.; Feng, X.; Sun, H. Computed Tomography and Magnetic Resonance Appearance of Sporadic Meningioangiomatosis Correlated with Pathological Findings. J. Comput. Assist. Tomogr. 2009, 33, 799–804. [Google Scholar] [CrossRef]
- Dallimore, C.A.; Quelle, M.; Désir, L.L.; Sham, S.; Harshan, M.; Wahl, S.J.; Zlochower, A.; Goodman, R.R.; Langer, D.J.; D’Amico, R.S. Calcifying Pseudoneoplasm of the Neuraxis in the Posterior Fossa: A Case Report and Literature Review. Cureus 2022, 14, e21562. [Google Scholar] [CrossRef]
- Domecq-Laplace, L.; Ruella, M.; Caffaratti, G.; Villamil, F.; Monsalve, M.; Alcorta, S.C.; Cervio, A. Posterior Fossa Calcifying Pseudoneoplasm of the Neuraxis (CAPNON). Presentation of three surgical cases. World Neurosurg. 2022, 167, e423–e431. [Google Scholar] [CrossRef]
- Anand, R.; Garling, R.J.; Poulik, J.; Sabolich, M.; Goodrich, D.J.; Sood, S.; Harris, C.A.; Haridas, A. Sporadic Meningioangiomatosis: A Series of Three Pediatric Cases. Cureus 2017, 9, e1640. [Google Scholar] [CrossRef]
- Yust-Katz, S.; Fuller, G.; Fichman-Horn, S.; Michaeli, N.; Inbar, E.; Lukman, J.; Limon, D.; Steiner, I.; Siegal, T. Progressive diffuse meningioangiomatosis: Response to bevacizumab treatment. Neurology 2016, 86, 1643–1644. [Google Scholar]
- Pisano, A.; Boschi, A.; Romoli, S. Calcifying pseudoneoplasms of the neuraxis: Not only surgical treatment. Asian J. Neurosurg. 2020, 15, 796–797. [Google Scholar]
- Kwan, M.K.; Abdelhai, A.M.; Saw, L.B.; Chan, C.Y.W. Symptomatic calcifying pseudotumor of the thoracic spine that resolved with the indomethacin treatment: A case report. Spine 2012, 37, E1676–E1679. [Google Scholar] [CrossRef] [PubMed]
| Authors | Case | Age (yr)/Sex | Site | Clinical Presentation | Combine with | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| Austin Wheeler | 1 | 2/male | Multiple intracranial lesions; light posterior fossa; bilateral basal ganglia; left temporal lobe; right frontal lobe, etc. | Unsteady gait | Cerebellar ependymoma | Surgery | No recurrence/alive |
| Omron Hassan | 1 | 11/male | Left temporal lobe | Diplopia; headache | Meningioma(WHO II) | Surgery | No recurrence/alive |
| Mina S. Makary | 1 | 17/female | Left temporal lobe | seizures; headache; dizziness | - | Surgery | No recurrence/alive; seizure-free |
| Brian Y.L. Chan | 1 | 2/male | Right temporal lobe; right frontal lobe | Squint; ptosis | Arachnoid cyst | Surgery | No recurrence/alive |
| Kunle Oyedokun | 1 | 3/male | Left parietal region | Seizures | - | Surgery | No recurrence/alive; seizure-free |
| Luke Galloway | 1 | 18-month old/male | Right temporal lobe | Seizures | Meningioma (WHO II) | Surgery | No recurrence/alive; seizure-free |
| Salvatore Stilo | 1 | 55/male | Left parietal region; head of the caudate nucleus, putamen, and thalamus | Seizures; memory and verbal impairment | B-cell central nervous system lymphoma | Surgery | NA |
| Alexandre Roux | 1 | 11/male | Left frontal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free |
| Laura Lavalle | 1 | 13/male | Right frontal | Stumble upon | - | Surgery | No recurrence/alive |
| Sara Free | 2 | 45/male | Left occipital region | Visual disturbances; Headache | - | Topiramate | Improvement/alive |
| 19/male | Left occipital lobe | Epilepsy history; headache; blurring of vision | - | Observation | Improvement/alive | ||
| J. Bryan Iorgulescu | 3 | 31/male | Frontal lobe | headache; nausea; vomiting; personality changes | Solitary fibrous tumour/hemangiopericytoma | Surgery | NA |
| 4/female | Parietal lobe | Seizures | Atypical teratoid/rhabdoid tumour | Surgery | No recurrence/alive; seizure-free | ||
| 2/male | Parietal lobe | Seizures | Rhabdomyosarcoma | Surgery and chemoradiotherapy | No recurrence/alive; seizure-free | ||
| Sabrina Rossi | 1 | 6/female | Right occipital lobe; right temporo-parietal region | Seizures; intellectual disability; dysfunctional behavior | Atypical teratoid/rhabdoid tumor | Surgery | Recurrence 2 months after operation; died 14 months from first diagnosis |
| Raja Anand | 3 | 6/female | Left frontal lobe | Blank stares; whole-body stiffening and rolling of the eyes | - | Surgery | One revision seven months after initial resection |
| 16/female | Right frontal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free | ||
| 2/male | Right parietal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free | ||
| Fábio A Nascimento | 1 | 25/male | Left parietal lobe | Seizures | - | Surgery | NA |
| Shlomit Yust-Katz | 1 | 67/male | Bilateral occipital lobes; right temporal lobe | Visual impairment | - | bevacizumab | Blind and clinically stable |
| Dorna Motevalli | 1 | 13/male | Frontal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free |
| Daniel Joseph Donovan | 1 | 16/female | Right temporal lobe | Seizures | - | Limited resection | No further growth/alive; seizure-free |
| Elif Bulut | 1 | 55/female | Left cerebellum | Vertigo | - | Surgery | No recurrence/alive |
| Zhihua Sun | 3 | 73/female | Left temporal lobe | Binocular diplopia; limited abduction of the left eye | - | Surgery | NA |
| 23/male | Light temporal lobe | Left hemianesthesia | - | Surgery | NA | ||
| 9/female | Left parietal lobe | Seizures | - | Surgery | NA | ||
| Y. Fu | 1 | 23/male | Right insular lobe | Seizures | - | Surgery | No recurrence/alive |
| Chao Zhang | 14 | 30/male | Right frontal lobe | Headache | Meningioma | Surgery | No recurrence/alive; seizure-free |
| 32/male | Right temporal lobe | Seizures | Meningioma | Surgery | No recurrence/alive; seizure-free | ||
| 3/male | Corpus callosum | Seizures | Meningioma | Surgery | No recurrence/alive; seizure-free | ||
| 12/male | Left parietal lobe | Seizures | Meningioma | Surgery | No recurrence/alive; seizure-free | ||
| 23/male | Right parietal lobe | Seizures | Meningioma | Surgery | No recurrence/alive; seizure-free | ||
| 13/male | Third ventricle | Diabetes insipidus | Meningioma | Surgery | Dead | ||
| 23/male | Right temporal lobe | Seizures | Meningioma | Surgery | No recurrence/alive; seizure-free | ||
| 10/female | Right frontal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free | ||
| 25/female | Right temporal lobe | Seizures | - | Surgery | No recurrence/alive; seizure recurrence | ||
| 26/female | Right parietal lobe | Seizures | - | Surgery | No recurrence/alive; seizure improved | ||
| 5/female | Right temporal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free | ||
| 10/male | Right parietal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free | ||
| 3.5/male | Anterior cranial fossa | Seizures | - | Surgery | No recurrence/alive; seizure-free | ||
| 27/male | Right temporal lobe | Seizures | - | Surgery | No recurrence/alive; seizure improved | ||
| Peifeng Li | 1 | 21/female | Right temporal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free |
| Nobutaka Mukae | 2 | 17/male | Left frontal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free |
| 16/male | Right frontal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free | ||
| A. Abdulazim | 1 | 41/male | Right frontoparietal lobe | Monoparesis of the left leg | - | Surgery | No recurrence/alive |
| Ayush Batra | 1 | 23/male | Right frontal lobe | Seizures; migraine headaches | - | Surgery | No recurrence/alive; seizure-free |
| Rui Feng | 10 | 18/male | Right frontal lobe | Seizures | - | Surgery | No recurrence/alive; Engel I seizure-free |
| 18/male | Right frontal lobe | Seizures | - | Surgery | No recurrence/alive; Engel I seizure-free | ||
| 13/female | Left parietal lobe | Seizures | - | Surgery | No recurrence/alive; Engel I seizure-free | ||
| 39/female | Right temporal lobe | Seizures | - | Surgery | No recurrence/alive; Engel II seizure improved | ||
| 8/male | Right frontal lobe | Seizures | - | Surgery | No recurrence/alive; Engel I seizure-free | ||
| 21/male | Left parietal lobe | Seizures | - | Surgery | No recurrence/alive; Engel I seizure-free | ||
| 14/male | Left frontal lobe | Seizures | - | Surgery | No recurrence/alive; Engel III seizure improved | ||
| 17/female | Right temporal lobe | Seizures | - | Surgery | No recurrence/alive; Engel II seizure improved | ||
| 34/female | Left occipital lobe | Seizures | - | Surgery | No recurrence/alive; Engel I seizure-free | ||
| 13/male | Right parietal lobe | Seizures | - | Surgery | No recurrence/alive; Engel I seizure-free | ||
| Sara Marzi | 1 | 37/male | Right frontal lobe | Headache | - | Surgery | No recurrence/alive |
| Osama Jamil | 1 | 3/female | Left frontotemporal; left gyrus rectus | Seizures | Meningioma | Surgery | No recurrence/alive; seizure-free |
| Everton Barbosa-Silva | 1 | 32/male | Right frontal lobe; Right parietal lobe; Right occipital lobe | Seizures | - | Sedation; anti-epileptic drugs | Recurrent seizures/dead |
| T. C. Yasha | 1 | 19/male | Left temporal lobe | Headache; Seizures | - | Surgery | NA |
| Huajuan Cui | 1 | 33/male | Left temporal lobe | Seizures | Meningioma | Surgery | No recurrence/alive; seizure-free |
| Katrien Jansen | 1 | 8-month-old/male | Right temporal lobe | Seizures | - | Surgery | No recurrence/alive; seizure-free |
| Authors | Case | Age/Sex | Site | Presentation | Treatment | Outcome | EMA | Vimentin | S-100 | GFAP |
|---|---|---|---|---|---|---|---|---|---|---|
| Jiri Soukup | 5 | 38/F | Intracranial; supratentorial; central sulcus | Seizures | Surgery | No recurrence/alive | + | + | - | - |
| 72/F | Intracranial; supratentorial; falx cerebri | Right-sided hemiparesis; Organic psychosyndrome | Surgery | Died | + | + | - | - | ||
| 68/F | Intracranial; supratentorial; right lateral ventricle | Headaches; fainting; hydrocephalus with organic psychosyndrom | Surgery | No recurrence/alive | + | + | - | - | ||
| 50/F | Intracranial; subtentorial; right cerebellar hemisphere; and vermis | - | Surgery | No recurrence/alive | + | + | - | - | ||
| 53/F | Intracranial; subtentorial; partially intraaxial; pons and pontocerebellar angle | Headache; facial nerve palsy; tinnitus; fainting | Surgery | No recurrence/alive | + | + | - | - | ||
| Colin A. Dallimore | 1 | 53/F | Intracranial; subtentorial; posterior fossa | Headache | Surgery | No recurrence/alive | - | NA | - | - |
| Wei-Qing Li | 1 | 56/F | Intracranial; supratentorial; right frontal lobe | Headache | Surgery | No recurrence/alive | + | + | - | - |
| Jian-Qiang Lu | 1 | 51/F | Spinal; paravertebral fascia in the midline at the levels of L3–4 vertebral bodies | Lower back pain; lower back mass | Surgery | NA | NA | NA | NA | NA |
| Lei Yan | 1 | 44/F | Intracranial; subtentorial; skull base | Headache | Surgery | NA | - | NA | - | NA |
| John C. Benson | 1 | 58/M | Intracranial; subtentorial; posterior fossa | Headache | Surgery | No recurrence/alive; Hydrocephalus | NA | NA | NA | NA |
| Yujian Li | 1 | 19/F | Intracranial; supratentorial; right temporal | Seizures | Surgery | No recurrence/alive; Seizure-free | - | + | NA | + |
| Andrea Boschi | 1 | 44/F | Spinal; right preforaminal extradural lesion | Back pain | Indomethacin | No recurrence/alive | NA | NA | NA | NA |
| Marian Preetham Suresh | 1 | 63/M | Intracranial; supratentorial; posterior third ventricle | Cognitive impairment; gait disturbance | V-P shunt | No progress/alive | + | NA | NA | NA |
| Kaiyun Yang | 2 | 57/M | Intracranial; subtentorial; extraaxial, right cerebellopontine angle (CPA) | Hoarseness; dysphagia; gait imbalance | Surgery | No recurrence/alive | + | NA | NA | NA |
| 70/M | Intracranial; supratentorial; right frontal lobe | Headache; gait difficulty, with falls; confusion and mood changes | Surgery | Headache improved | + | NA | NA | NA | ||
| Madoka Inukai | 1 | 64/F | Intracranial; supratentorial; corpus callosum | Weakness of the left leg persisting | Surgery | No recurrence/alive; weakness improvement | NA | NA | - | - |
| Jiahua Huang | 1 | 39/M | Intracranial; subtentorial; skull base | Visual disturbance; headache | Surgery | No recurrence/alive | + | NA | - | NA |
| Prashanth Raghu | 1 | NA/M | Intracranial; supratentorial; right medial temporal lobe | Seizures | Anti-epileptic drugs | EEG found normal; symptomatically better | NA | NA | NA | NA |
| Frederic A Vallejo | 1 | 35/M | Intracranial; supratentorial; left-posterior temporal lobe | Seizures; headaches; vertigo | Surgery | No recurrence/alive; seizure-free | NA | + | NA | + |
| Pithon RFA | 1 | 17/M | Intracranial; supratentorial; left frontal lobe | Seizures | Surgery | No recurrence/alive; seizure-free | NA | NA | NA | NA |
| Yuta Tanoue | 1 | 52/M | Intracranial; supratentorial; left medial temporal lobe | Seizures | Surgery | No recurrence/alive; seizure-free | - | + | + | + |
| Zaman SKU | 1 | 10/M | Intracranial; supratentorial; right thalamic | Left-sided hemiparesis with mixed movement disorder with hemiballism, choreoathetosis, and dystonia | Medication; physiotherapy | No progress/alive | NA | NA | NA | NA |
| A J Gauden | 1 | 69/M | cranio-cervical junction | Neck pain | Surgery | No recurrence/alive | + | NA | NA | NA |
| Thakur B | 1 | 67/F | Intracranial; subtentorial; cerebellum | Difficulty walking | Surgery | No recurrence/alive | - | NA | NA | + |
| Eric S Nussbaum | 1 | 39/F | Intracranial–extradural; subtentorial | Right-sided deafness and tinnitus | Surgery | No recurrence/alive | NA | NA | NA | NA |
| Akira Watanabe | 1 | 40/F | Intracranial; supratentorial; right frontal lobe | Somnolence | Surgery | Recurrence (after 14 months) | NA | NA | NA | NA |
| Atin Saha | 1 | 67/M | Spinal; vertebral canal | Left lower extremity pain; weakness and gait instability | Surgery | No recurrence/alive; symptom improvement | NA | NA | NA | NA |
| Zerehpoosh FB | 1 | 25/M | Intracranial; supratentorial; left temporal lobe | Incidental finding | Surgery | No recurrence/alive | NA | NA | NA | NA |
| Sean M Barber | 1 | 31/F | Intracranial; supratentorial; right temporal lobe | Seizures | Surgery | No recurrence/alive; symptom improvement | + | + | - | - |
| Michael M Safaee | 1 | 8/M | Intracranial; supratentorial; right frontal lobe | Seizures | Surgery | No recurrence/alive; seizure-free | NA | NA | NA | NA |
| Timothy C Blood | 1 | 65/F | Intracranial; supratentorial; anterior cranial fossa | Deafness | Surgery | No recurrence/alive | + | NA | NA | NA |
| Michael A Paolini | 1 | 17/M | Intracranial; supratentorial; left occipitoparietal lobe | Seizures | Surgery | No recurrence/alive | + | NA | NA | NA |
| Brasiliense LB | 1 | 67/F | Intracranial; subtentorial; ventral midbrain and supratentorial; left frontal lobe | Seizures | Surgery | No recurrence/alive; seizure-free | NA | NA | - | - |
| Abdaljaleel M | 1 | 62/F | Intracranial; supratentorial; temporal, parietal, and occipital lobes | Seizures; headaches | Surgery | NA | NA | NA | - | NA |
| Nayuta Higa | 1 | 62/M | Intracranial; supratentorial; left cingulate gyrus | Headache | Surgery | Recurrence | NA | NA | + | + |
| Hong Gang Wu | 1 | 39/F | Spine; sacral canal | Sacrococcygeal pain | Surgery | No recurrence/alive | NA | NA | NA | NA |
| Sara García Duque | 4 | 48/F | Intracranial; supratentorial; left occipital lobe | Headache | Surgery | No recurrence/alive | NA | NA | NA | NA |
| 51/F | Spinal cord; L2 | Lower back pain | Surgery | No recurrence/alive | NA | NA | NA | NA | ||
| 46/F | Spinal cord; C3 | Posterior neck pain | Surgery | No recurrence/alive | NA | NA | NA | NA | ||
| 73/M | Spinal cord; T2 | Progressive paraparesis | Surgery | No recurrence/alive | NA | NA | NA | NA | ||
| Joseph Ghaemi | 1 | 18/M | Intracranial; subtentorial; skull base | Headache; diplopia | Surgery | NA | + | NA | NA | NA |
| Arthur J M Lopes | 1 | 72/F | spinal cord; L2 | Low back pain | Surgery | No recurrence/alive | + | NA | + | + |
| Mohammed Alshareef | 1 | 59/F | cranio-cervical junction | Gait instability; balance difficulty | Surgery | No recurrence/alive | NA | NA | NA | NA |
| Molly Hubbard | 1 | 38/F | Intracranial; supratentorial; bilateral frontal lobe | Headache; left facial numbness | Surgery | No recurrence/alive; symptom improvement | NA | NA | NA | NA |
| Karol Wiśniewski | 1 | 29/M | Intracranial; subtentorial; foramen magnum | Headache | Surgery | No recurrence/alive | + | NA | - | NA |
| Kirill Lyapichev | 1 | 24/M | Intracranial; supratentorial; right temporo-occipital lobe | Headache; seizures; loss of vision | Surgery | No recurrence/alive | - | NA | NA | + |
| M N Stienen | 2 | 46/M | Intracranial; supratentorial; right parietal lobe | Seizures | Surgery | No recurrence/alive | NA | NA | NA | NA |
| 55/F | Intracranial; supratentorial; left frontoparietal lobe | Progressive hallucinosis; behavioral disorders | Surgery | No recurrence/alive; symptom improvement | NA | NA | NA | NA | ||
| Edward E Kerr | 1 | 56/M | Intracranial; subtentorial; posterior fossa | Headache | Surgery | No recurrence/alive; symptom improvement | + | NA | NA | - |
| Mun Keong Kwan | 1 | 48/M | Spinal, extradural mass located dorsal to the T9–T10 disc | Radicular pain | Indomethacin | No recurrence/alive | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Xu, C.; Cai, Y.; Pan, Z.; Li, Z. Meningioangiomatosis Combined with Calcifying Pseudoneoplasms of Neuraxis. Brain Sci. 2023, 13, 786. https://doi.org/10.3390/brainsci13050786
Sun X, Xu C, Cai Y, Pan Z, Li Z. Meningioangiomatosis Combined with Calcifying Pseudoneoplasms of Neuraxis. Brain Sciences. 2023; 13(5):786. https://doi.org/10.3390/brainsci13050786
Chicago/Turabian StyleSun, Xiangyu, Chengshi Xu, Yuxiang Cai, Zhiyong Pan, and Zhiqiang Li. 2023. "Meningioangiomatosis Combined with Calcifying Pseudoneoplasms of Neuraxis" Brain Sciences 13, no. 5: 786. https://doi.org/10.3390/brainsci13050786
APA StyleSun, X., Xu, C., Cai, Y., Pan, Z., & Li, Z. (2023). Meningioangiomatosis Combined with Calcifying Pseudoneoplasms of Neuraxis. Brain Sciences, 13(5), 786. https://doi.org/10.3390/brainsci13050786






