Abstract
An understanding of the neurocognitive profile underlying the use of social networking sites (SNSs) can help inform decisions about the classification of problematic SNS use as an addictive disorder and elucidate how/when ‘SNS addiction’ might develop. The present review aimed to synthesize structural and functional MRI research investigating problematic/compulsive forms of SNS use or regular (non-addicted) SNS use behaviours. We conducted a systematic search for research articles published in English using the Web of Science, PubMed, and Scopus databases up to October 2022. Studies meeting our inclusion criteria were assessed for quality and a narrative synthesis of the results was conducted. Twenty-eight relevant articles were identified comprising structural MRI (n = 9), resting-state fMRI (n = 6) and task-based fMRI studies (n = 13). Current evidence suggests that problematic SNS use might be characterised by (1) reduced volume of the ventral striatum, amygdala, subgenual anterior cingulate cortex, orbitofrontal cortex and posterior insula; (2) increased ventral striatum and precuneus activity in response to SNS cues; (3) abnormal functional connectivity involving the dorsal attention network; (4) inter-hemispheric communication deficits. Regular SNS use behaviours appear to recruit regions involved in the mentalising network, the self-referential cognition network, the salience network, the reward network and the default mode network. Such findings are at least partially consistent with observations from the substance addiction literature and provide some provisional support for the addictive potential of SNSs. Nonetheless, the present review is limited by the small number of eligible studies and large heterogeneity in the methods employed, and so our conclusions should remain tentative. Moreover, there is a lack of longitudinal evidence suggesting SNSs cause neuroadaptations and thus conclusions that problematic SNS use represents a disease process akin to substance use addictions are premature. More well-powered longitudinal research is needed to establish the neural consequences of excessive and problematic SNS use.
1. Introduction
The use of social networking sites (SNSs) is a ubiquitously popular activity that has seen a surge over the last decade. In 2012, it was estimated that there were approximately 1.5 billion SNS users [1]. In 2022, the number of SNS users had reached 4.6 billion, which represents a global penetration rate of 58.4% and an increase of more than 200% over the last 10 years [2] (it should be noted that SNS users might not represent unique individuals and thus these data may not be a true reflection of the current number of global users). Not only are more people using SNSs, but users are also spending longer on these sites each day. Worldwide, the daily average time spent using SNSs has risen from 90 min in 2012 to 145 min in 2020 [3]. Of concern is that SNS use has been linked to reduced well-being and an increase in mental health disorders, particularly in younger populations [4,5,6], although such findings are the subject of debate [7,8]. Additionally, a recent meta-analysis suggests that between 5 and 25% of the global population report symptoms resulting from their SNS use that resemble criteria traditionally used to diagnose substance use disorders and other behavioural addictions [9]. Yet, other studies employing experimental methods offer a more sceptical view of the addictive potential of modern technologies [10,11,12,13], and these findings are mirrored by fears that we could be over-pathologising normal everyday activities [14,15,16].
Importantly, addictions are known to result in structural and functional adaptations in the brain that render the user more susceptible to the drug (or behaviour) and associated cues [17,18]. Neuroimaging studies have been instrumental in improving our understanding of the neural basis of substance use addictions [19] and have also provided key insights into the neural similarities and differences between the use of addictive substances and pathological behaviours (e.g., gambling disorder; [20]). For example, a study by Limbrick-Oldfield et al. [21] found that regions in the brain’s reward circuit (including the bilateral insula and ventral striatum) were activated in problem gamblers in response to cravings elicited by gambling-related images, which is similar to observations in substance use disorders [22,23]. Nonetheless, a recent meta-analysis comparing the activation likelihood estimation from fMRI studies of gambling disorder and alcohol use disorder during tasks of executive function revealed that the two conditions were associated with distinct changes in neural activity [24]. While gambling disorder was associated with activation in regions of the fronto-striatal reward network, alcohol use disorder was associated with both activations and deactivations of different nodes of this network. Additionally, research comparing brain structure between individuals with alcohol use disorder and problem gamblers has shown dissimilar brain morphology [25]. While alcohol use disorder was associated with reduced volume of brain regions involved in cognitive control and reward processing, no structural abnormalities were identified in problem gamblers. Such differences might reflect that behavioural addictions do not expose the brain to toxic chemical substances that may be responsible for the neuroadaptations associated with addictions to drugs. Neuroimaging techniques have thus provided a vital tool for improving our understanding of the neural correlates of different addictive disorders, indicating that similar as well as distinct neural abnormalities might underlie behavioural and substance-related addictions.
More recently, studies have begun to probe the neural underpinnings of SNS use behaviours. Based on neuroimaging research into offline social behaviours, the use of SNSs has been proposed to involve three key neural systems [26]: the mentalising network (i.e., dorsomedial prefrontal cortex, temporoparietal junction, anterior temporal lobe, inferior frontal gyrus, and the posterior cingulate cortex/precuneus) required for interpreting the emotions and mental states of others, the self-referential cognition network (i.e., medial prefrontal cortex and posterior cingulate cortex/precuneus) which enables self-reflections and social comparisons, and the reward network (i.e., ventromedial prefrontal cortex, ventral striatum, and ventral tegmental area) which is activated in response to social interactions. Given the pervasiveness of SNS use in society and the knowledge that a significant number of individuals report addiction-like symptoms, it seems also increasingly important to understand whether and how problematic SNS use might be reflected by structural and functional alterations in the brain. Recently we have argued for an incentive-sensitisation perspective to assessing the addictive potential of SNSs [27]. This theory of addiction posits that repeated drug use ‘hijacks’ the reward systems of the brain by sensitising them to the incentive properties of the drug, resulting in heightened drug ‘wanting’ without producing an increase in drug ‘liking’ [18,28]. In line with this, recent evidence has indicated that explicit cravings (i.e., ‘wanting’) are correlated with more problematic SNS use, independent of self-reported SNS liking [13,27]. Crucially, such an account would also predict adaptations of certain neural substrates in addicted SNS users. Thus, identifying neural similarities and differences between problematic SNS use and established addictive disorders would provide a valuable contribution in helping to determine whether some forms of SNS use might represent a behavioural addiction.
One way of investigating the neural correlates of SNS use is through the use of magnetic resonance imaging (MRI). Early neuroimaging studies have tended to investigate ‘internet addiction’ more broadly [29,30,31], and while the term ‘internet addiction’ is still commonly used to describe research investigating a range of problematic online activities, more recently, research has shifted away from this generalised construct to investigating specific online activities (e.g., online gaming, cybersex, social networking, online gambling, and online shopping; [32]). It therefore seems most appropriate to review the MRI literature for each of these behaviours in isolation when evaluating the addictive potential of a given activity. To that end, the present review will focus on discussing findings from studies that have specifically investigated SNS use behaviours, rather than internet or modern technology (e.g., smartphones) use more generally. Given that neuroimaging of ‘SNS addiction’ is still an emerging field, our review did not focus selectively on problematic (addictive) behaviours—as is typical in similar neuroscientific reviews of the drug addiction literature—but encompassed research that used MRI methods to study both problematic and nonproblematic (“healthy”) SNS use behaviours.
2. Method
2.1. Search Strategy
A literature search was conducted using the databases Web of Science, PubMed, and Scopus. The systematic review was conducted in accordance with the PRISMA statement (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). The search was restricted to peer-reviewed research articles published in English between 2010 and 2022. The following search algorithm and terms were used: (“social media” OR “social network*” OR Facebook OR Twitter OR Instagram OR YouTube OR Snapchat OR TikTok) AND (MRI OR fMRI OR “magnetic resonance imaging” OR neuroimaging OR “BOLD signal” OR “BOLD response” OR “gray matter” OR “grey matter” OR “white matter”). The most recent search was conducted on the 25 October 2022 and returned 774 unique results. A systematic review protocol was not registered.
2.2. Study Selection
One author, MW, conducted the screening process and uncertainties regarding the inclusion of studies were resolved through consultation with NI. After removing duplicate studies, titles and abstracts were read and screened for relevance. Articles were excluded if they: (1) did not investigate the functional or anatomical brain correlates of SNS use using MRI methods; (2) only assessed online network size as an alternative index for real-life (offline) social networks and therefore did not directly investigate SNS use as a primary topic; (3) investigated social media marketing. Initial screening reduced the results to 39 potentially relevant articles. Upon closer inspection, a further 11 papers were removed after reading the full-length articles. These studies did not meet the above criteria as SNS use was not the primary topic of investigation or online social network size was used as a proxy for offline social networks. One study employing a ‘Tweet Task’ was considered for inclusion but deemed ineligible as the aim of the study focused on assessing how individuals from historically marginalised groups were affected by viewing discriminatory content shared by Donald Trump [33]. Both authors agreed that since the study did not report whether participants were SNS users themselves, and because neither regular nor problematic SNS use was assessed, the study was not suitable. Additionally, one study investigated virtual social rejection using a paradigm that simulated an online chatroom [34], but the authors agreed that this study did not specifically assess SNS use and thus was not included. Two studies (with the same sample) investigating problematic smartphone users [35,36] were included because participant recruitment focused on individuals who use smartphones for internet communication/social networking, and those who primarily used smartphones for other purposes (e.g., gaming) were excluded. Similarly, one study investigating the neural correlates of ‘mobile technology engagement’ [37] was included since the scale employed specifically assesses phone-based social media use and frequency of public status updating. Furthermore, a study investigating ‘specific internet addiction’ [38] was deemed eligible for inclusion since the sample comprised subgroups of individuals with internet gaming and social network addiction.
The reference lists of included articles and previous systematic reviews of neuroimaging studies investigating internet addiction more broadly [39,40,41] were also hand-searched for any further relevant studies. However, no additional articles were deemed eligible for inclusion. Figure 1 illustrates our applied literature search using a PRISMA flow chart.
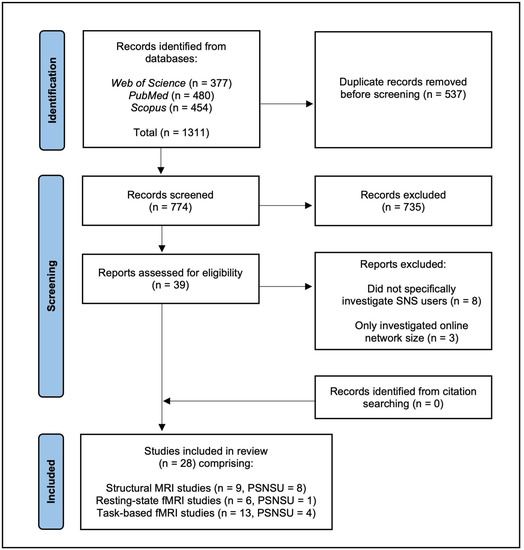
Figure 1.
PRISMA flow diagram showing the process of the systematic literature search; PSNSU (problematic social networking site use) refers to the number of studies that investigated the neural correlates of problematic/compulsive SNS use.
2.3. Data Extraction
Relevant data were extracted from each of the articles and summarised into tables according to the MRI method employed. The variables for which data were extracted included author names, year of publication, study design, number of participants, mean age, gender, type of SNS use, assessment tool for SNS use, MRI methodology and key findings (i.e., implicated brain regions). The data extraction process was conducted independently by MW.
2.4. Quality Assessment
Given that no standardised criteria exist for assessing the quality of neuroimaging studies, previous systematic reviews of MRI research have modified criteria from existing tools [42]. We opted to use a modified version of the Effective Public Health Practice Project (EPHPP) tool for quality assessment/risk of bias [43], similar to other systematic reviews of neuroimaging studies [44,45]. Our modified EPHPP comprised 10 quality criteria which assessed studies for selection bias, study design, neuroimaging methodology, and statistical analysis. Meeting a criterion was scored as 1 = ‘Yes’ or 0 = ‘No’ or ‘Unclear’, and ratings were summed to produce an overall quality score (range 0–10). A low (score = 0–4), moderate (score = 5–7) or high (score = 8–10) quality rating was then assigned to each study. The quality assessment was conducted independently by MW who consulted with NI to resolve any uncertainty.
3. Results
A total of 28 papers that met the eligibility criteria were identified and included in this review. The included studies were all published between 2013 and 2022 and comprised structural MRI studies (n = 9), resting-state fMRI studies (n = 6) and task-based fMRI studies (n = 13). Of the 28 studies only 13 investigated the neural correlates of compulsive or problematic SNS use (the majority of these being structural MRI studies, n = 8). All of the included studies were rated as moderate or high quality. Because of the heterogeneity of MRI methods, a meta-analysis was not conducted.
3.1. Structural MRI Studies
Of the nine structural MRI studies identified in the literature search, most employed a sample of Facebook users. The mean age of participants in the included studies ranged from 16 to 31.2. All but two studies used voxel-based morphometry (VBM) to assess grey matter volume (GMV), with one assessing changes in the surface area and cortical thickness of regions of interest (ROIs) using a longitudinal design and another study using diffusion tensor imaging (DTI) to assess the connectivity of white matter microstructure. Two studies were rated as moderate quality and seven studies as high quality. A summary of the included structural MRI studies is provided in Table 1.
Of the studies investigating GMV, the majority observed a negative correlation between excessive/problematic SNS use and GMV in regions that make up the brain’s reward systems. However, the implicated brain regions varied across studies and results are largely inconsistent. Three studies reported structural alterations of the nucleus accumbens (NAc; a component of the ventral striatum), which plays a key role in motivating reward-related behaviour. Montag et al. [46] found a negative correlation between bilateral NAc GMV and Facebook use intensity and reduced GMV in the right NAc was also associated with higher Facebook addiction scores. Correspondingly, He et al. [47] reported a negative correlation between GMV of the right ventral striatum and compulsive Facebook use. The only other study to report significant structural alterations of the NAc found that reduced GMV in this region was associated with higher use of the WeChat paying function [48]. However, in this instance, the specific association of smaller NAc GMV with the paying function and not with other functions (e.g., messaging and voice calling) or WeChat addiction scores could be argued to be more indicative of compulsive buying/shopping-related problems rather than as representing an SNS addiction. It has also been argued that the failure to observe reduced NAc structure for more problematic SNS users in other studies might indicate an important difference between SNS and substance use addictions [49]. Nonetheless, it is important to consider that the study by Montag et al. [46] employed a larger sample size (n = 62) and used the most objective index of Facebook use when compared to most other studies that have failed to observe this effect. In the study, participants installed a mobile app that tracked their use of Facebook across a 5-week period, whereas in all other structural MRI assays self-reports of usage/addiction severity were acquired. Thus, other studies may have been too underpowered to observe an effect or their reliance on self-report measures may have influenced the results.
Two studies found correlations between problematic SNS use and GMV of the anterior cingulate cortex (ACC) but in different directions. In their study of WeChat users, Montag et al. [48] found that reduced GMV of the subgenual ACC was associated with higher addiction scores and this relationship remained stable after controlling for age, gender, anxiety and depression. This finding seems to square with the important role of the ACC in the cognitive control network [50] and thus reduced ACC volume would likely result in the inhibitory control deficits exhibited by individuals with addictive disorders [51]. However, no other structural MRI study investigating SNS use has corroborated this finding and notably one study reports the opposite association. He, Turel and Bechara [49] found that increased GMV in the ACC and midcingulate cortex (MCC) was associated with more addictive Facebook use. The authors postulated that the finding could be the result of an adaptation and compensation process in which the ACC/MCC increases in volume to improve the efficiency of the inhibitory system in response to reduced GMV in other regions implicated in more problematic SNS use (e.g., amygdala). However, there are also important differences between these two conflicting studies that could potentially account for their results. Compared to Montag et al. [48], the study by He, Turel and Bechara [49] employed a substantially smaller sample size (61 vs. 20) and both assessed different SNS platforms, using different scales to assess addiction severity. Since Montag et al. [48] was the only study to investigate WeChat users, the findings could potentially highlight a difference between SNSs that are primarily used as a communication tool (i.e., for instant messaging) and other content sharing/microblogging based platforms. Additionally, Montag et al. [48] used a more specific ROI analysis to investigate subregional differences in ACC GMV. Findings were specific to the subgenual ACC and no correlations with other subregions of the ACC were found, whereas the positive correlation reported by He, Turel and Bechara [49] related to more dorsal ACC. Thus, the results might indicate important subregional specificity within the ACC, in which reduced subgenual ACC volume is related to more addictive SNS use.
Two studies by the same group reported reduced amygdala GMV in more excessive/problematic users [47,49]. The finding is therefore consistent with substance use addictions, in which addicts present reduced GMV of the amygdala [52]. However, unlike results typically observed in established addictions only one study observed reduced GMV in the prefrontal region, namely in the right orbitofrontal cortex (OFC) in problematic SNS users vs. healthy controls, matched for age, sex and IQ [35]. Furthermore, reduced GMV of the right OFC was the only significant difference between the two groups and results remained significant when controlling for potential comorbid conditions (i.e., depression, anxiety and alcohol addiction). While being the only structural MRI study to implicate this region in problematic SNS users the study did employ a comparatively large sample size (n = 88) and the results fit with findings from other addictive disorders that suggest an important role for the OFC in reward and decision-making processes.
In one of the few studies to find positive correlations between GMV and SNS use, Turel et al. [53] reported larger bilateral posterior superior temporal gyrus/middle temporal gyrus, and left posterior fusiform gyrus in more frequent Facebook users. It is suggested that increased GMV of these regions is associated with an improved ability to deal with social-semantic demands involved in Facebook use (e.g., recognising faces and interpreting the mental states of others). While no negative associations between GMV and SNS behaviours were observed, the study did not correlate GMV with a measure of SNS addiction, unlike most other structural MRI studies. Contrastingly, in another study by the same group but employing a measure of Facebook addiction, only negative correlations were observed [54]. In this study, participants completed a delay-discounting task before undergoing structural MRI. The results revealed a negative correlation between GMV in bilateral posterior insula (PI) and addiction scores, and this relationship was mediated by delay discounting, showing a stronger relationship in those participants with a preference for immediate rewards. It is suggested that the PI plays an important role in interoceptive awareness and thus promotes subjective feelings of urges and cravings in addiction [55]. Consequently, abnormal morphology of this region may result in deficits in decision making and inhibition, which is also corroborated by the negative correlation between PI GMV and delay discounting [54]. However, no other negative associations, such as those reported in other structural MRI studies and described above, were identified.
While the above studies are useful in demonstrating associations between SNS behaviours and brain morphology, they all share an important limitation in that they are unable to establish whether such structural abnormalities are directly caused by more frequent/addictive SNS use. Nonetheless, in the most recent structural MRI study to investigate SNS use, a longitudinal design was employed in which 189 adolescents were followed across three annual assessments [56]. The researchers used latent class growth curve analysis to assess how the development of cortical thickness and surface area of ROIs differed between high vs. low social media users. The results showed that the high social media use group had higher baseline cortical thickness in the lateral and medial prefrontal cortex (L/MPFC). Over three years, the high social media use group also showed a stronger reduction in the cortical thickness of the lateral prefrontal cortex and reduced surface area of the temporal parietal junction (TPJ). While such findings are consistent with the researchers’ hypothesis that more excessive SNS use should result in accelerated thinning of regions involved in processing social information (e.g., TPJ) and cognitive control (e.g., LPFC), these associations did not remain significant after correcting for false discovery rate (FDR). Thus, the findings only provide limited evidence that more excessive SNS use might be directly responsible for developmental changes in brain structure.
In the only study using DTI to assess white matter connectivity, He et al. [57] found increased mean diffusivity (MD) in the body and splenium of the corpus callosum in more excessive social media users. While this finding is consistent with those reported in other addictive disorders, the authors note that the results did not reach significance when a more conservative whole-brain voxel-wise analysis (using Tract Based Spatial Statistics; TBSS) was applied. However, the whole-brain analysis did reveal that more excessive SNS use was associated with increased MD in the forceps minor and ventral semantic path. The forceps minor is a white matter bundle that connects the two frontal lobes and thus increased MD in this region is indicative of inter-hemispheric communication deficits between the frontal lobes. Given the important role the frontal lobes play in cognitive control and reward processing, it seems plausible that more problematic SNS users might exhibit less efficient connectivity in these regions. However, since the study by He, Turel and Bechara [57] is so far the only study investigating white matter microstructure in SNS users, and also given the relatively small sample size employed (n = 20), more research is required to validate these findings. Furthermore, TBSS is not without limitations as there is potential for bias in anatomical specificity and accuracy during the skeleton projection step. Future studies should consider using more reliable methods to achieve a more comprehensive understanding of potential alterations in white matter connectivity underlying problematic SNS use.

Table 1.
Summary of structural MRI studies.
Table 1.
Summary of structural MRI studies.
| Authors (Year) | Sample | Mean Age | PSNSU | SNS Assessment Tool | Design | Main Results | Quality Assessment |
|---|---|---|---|---|---|---|---|
| Achterberg et al. (2022) [56] |
|
|
|
|
|
|
|
| He, Turel and Bechara (2017) [49] a |
|
|
|
|
|
|
|
| He et al. (2017) [47] |
|
|
|
|
|
|
|
| He et al. (2018) [57] a |
|
|
|
|
|
|
|
| Lee et al. (2019) [35] b |
|
|
|
|
|
|
|
| Montag et al. (2017) [46] |
|
|
|
|
|
|
|
| Montag et al. (2018) [48] |
|
|
|
|
|
|
|
| Turel et al. (2018a) [53] |
|
|
|
|
|
|
|
| Turel et al. (2018b) [54] |
|
|
|
|
|
|
|
Note. PSNSU refers to studies that investigated the neural correlates of problematic/compulsive SNS use. a He, Turel and Bechara [49] and He et al. [57] used the same sample of Facebook users (n = 20) as a task-based fMRI study by Turel et al. [63]. b Lee et al. [35] used the same sample of smartphone users (n = 88) as a resting-state fMRI study by Lee et al. [36]. Although SNS use was not assessed directly, participants who used smartphones primarily for other purposes (e.g., gaming) were excluded.
3.2. Resting-State fMRI Studies
Six studies were identified that used resting-state fMRI data to investigate the neural correlates of SNS use. Two studies (with the same sample) recruited high vs. low SNS users based on time spent using three popular Chinese SNSs (Weibo, TikTok and Kwai), one study employed a sample with problematic SNS users, one study employed a sample of Facebook users, another study employed a sample of participants with varying levels of mobile technology engagement and a final study employed a sample of Weibo users. The mean age of participants ranged from 20.9 to 25.7. Three studies were rated as moderate quality and three as high quality. A summary of the included resting-state fMRI studies is provided in Table 2.
In the only study to measure resting-state fMRI data in a sample of problematic SNS users, Lee et al. [36] employed functional connectivity analysis at seeds in the dorsal attention network (DAN) and the ventral attention network (VAN). No significant differences between problematic SNS users and healthy controls emerged when using functional connectivity analysis with VAN seeds. However, when using seeds in the DAN, problematic SNS users were shown to have stronger functional connectivity between the right intraparietal sulcus and the right middle occipital gyrus. Because the middle occipital gyrus plays an important role in sensory processing the authors suggested that abnormal connectivity between this region and the DAN may interfere with attentional control processes in problematic SNS users. In addition, when compared to controls functional connectivity between the right frontal eye field and the right dorsolateral prefrontal cortex (DLPFC) was weaker in problematic SNS users. Given the critical role of the DLPFC in exerting executive control, a less efficient control network may consequently result in the inability to manage the amount of time spent on SNSs resulting in more problematic use.
In a study employing a sample of Facebook users, Meshi et al. [64] investigated whether resting-state fMRI data correlated with the frequency users shared personal information on Facebook. Participants completed a self-related sharing assessment [65] and scores were correlated with functional connectivity using seeds at the medial prefrontal cortex (MPFC), central precuneus (CP), caudal anterior cingulate cortex (cACC), and the ventral striatum. No significant correlations were observed when using seeds at the cACC and ventral striatum. However, intrinsic functional connectivity between the MPFC and the right DLPFC, as well as between the CP and right DLPFC, were both positively correlated with self-related sharing. Additionally, connectivity between the CP and the left lateral OFC was positively correlated with self-related sharing, although connectivity between the CP and the left anterior temporal pole (ATP) was negatively correlated with self-related sharing. Increased functional connectivity of both the MPFC and CP to other brain regions is in line with the suggested view that these regions play a role in self-referential processing [66]. In particular, since the DLPFC is critical for executive functioning and working memory, stronger functional connectivity to this region might indicate a greater ability to hold self-related information in working memory, in turn facilitating self-related sharing.
A study by Wilmer et al. [37] investigated functional connectivity in two dissociable pathways stemming from the ventral striatum. While higher connectivity between the ventral striatum and the ventromedial prefrontal cortex (VMPFC) was associated with higher mobile technology engagement (including increased social media use and more frequent status updates), connectivity between the ventral striatum and DLPFC was associated with reduced mobile technology engagement. The researchers suggest that these findings corroborate the notion of the ventral striatum-VMPFC pathway being involved in reward processing, thus resulting in greater sensitivity to rewards obtain through mobile technologies. Similarly, the association between increased functional connectivity in the ventral striatum-DLPFC pathway and reduced mobile media engagement is consistent with this networks role in exerting executive control over behaviour, resulting in more controlled use of mobile technologies.
Using seeds at the medial prefrontal cortex (MPFC), dorsomedial prefrontal cortex (DMPFC) and temporoparietal junction (TPJ), Zhang and Mo [67] correlated functional connectivity with participant’s reposting rate in an experimental paradigm that simulated Weibo use. During the task, completed after the scanning session, participants were shown a series of 90 Weibo messages of either positive, negative or neutral valence. After reading each Weibo message participants had to decide whether to ‘repost’ or ‘not repost’ the message. It was shown that overall participants preferred to repost negative, compared to positive or neutral messages. The repost rate of negative messages was positively correlated with functional connectivity between left TPJ and left middle frontal lobe, as well as between the left TPJ and right insula. Additionally, functional connectivity between left DMPFC and medial OFC was also shown to be positively correlated with the repost rate of negative messages. Conversely, the repost rate of positive messages was positively correlated with functional connectivity between the right TPJ and right superior temporal lobe. Finally, the repost rate of neutral messages showed a positive correlation with the functional connectivity between left TPJ and left ventrolateral OFC, in addition to the functional connectivity between bilateral DMPFC. The finding of increased functional connectivity between the TPJ and other regions being associated with an increased repost rate across all message types was argued to corroborate the important role that the TPJ plays in social communication.
A recent study by Hu et al. [68] used dynamic functional network connectivity analysis to investigate the brain dynamics of reading SNS posts on a smartphone. Resting-state fMRI was first recorded at baseline before participants were assigned to either a social media or science fiction reading task, which took place outside of the scanner. Immediately after the reading task participants underwent a second fMRI session. Reading social media posts was shown to decrease functional connectivity between the default mode network (DMN) and frontoparietal network (FPN), but increased connectivity between the DMN and visual network. It is suggested that since a main function of the DMN is mind wandering [69], SNSs might stimulate the visual network to induce mind wandering, resulting in increased inattention after SNS use. In another study by the same group and recruiting from the same pool of participants, Hu et al. [70] used a longitudinal design to investigate changes in functional connectivity after a month of excessive SNS use. The results showed that the functional connectivity of light SNS users was more similar to that of heavy users after they completed the four-week period of increased SNS use. Using inter-subject correlation analysis, increased SNS use was shown to have a widespread impact on almost all brain networks and functional connectivity between regions that were most affected were associated with selective attention. The study is the first to indicate that more excessive SNS use can result in alterations of cerebral functional connectivity when assessed longitudinally, and results therefore warn of potential neurobiological consequences attributable to just four weeks of excessive SNS use.

Table 2.
Summary of resting-state fMRI studies.
Table 2.
Summary of resting-state fMRI studies.
| Authors (Year) | Sample | Mean Age | PSNSU | SNS Assessment Tool | Design | Main Results | Quality Assessment |
|---|---|---|---|---|---|---|---|
| Hu, Cui et al. (2022) [68] |
|
|
|
|
|
|
|
| Hu, Yu et al. (2022) [70] |
|
|
|
|
|
|
|
| Lee et al. (2021) [36] a |
|
|
|
|
|
|
|
| Meshi et al. (2016) [64] |
|
|
|
|
|
|
|
| Wilmer et al. (2019) [37] |
|
|
|
|
|
|
|
| Zhang and Mo (2016) [67] b |
|
|
|
|
|
|
|
Note. PSNSU refers to studies that investigated the neural correlates of problematic/compulsive SNS use. a Lee et al. [36] used the same sample of smartphone users (n = 88) as a structural MRI study by Lee et al. [35]. Although SNS use was not assessed directly, participants who used smartphones primarily for other purposes (e.g., gaming) were excluded. b Zhang and Mo [67] used the same sample of Weibo users (n = 28) as a task-based fMRI study by Zhang and Qu [72].
3.3. Task-Based fMRI Studies
The 13 task-based fMRI studies identified in the literature search used a range of different experimental tasks, thus making it difficult to directly compare neural activity between studies. Two studies used cognitive control tasks (Emotional Stroop and Go/No-Go), two studies used a self-retrieval/self-concept paradigm, another used a description task, and the remaining eight studies all used variations of a cue reactivity task or a paradigm that simulated SNS use. The specific SNS platform under investigation also varied between studies. Four studies employed a sample with problematic SNS users, with the remaining studies investigating either Facebook, Instagram, TikTok, Weibo or general SNS users. The mean age of participants ranged from 16.8 to 26.1. Four studies were rated as moderate quality and nine as high quality. A summary of the included task-based fMRI studies is provided in Table 3.
Dieter et al. [38] investigated neural differences in emotional inhibitory control processing between individuals with a gaming addiction (n = 13) or social network addiction (n = 12) and healthy controls (n = 23). During fMRI the participants completed an Emotional Stroop Task with four categories of positive, negative, neutral and socially anxious words. Behavioural results revealed no significant difference in the reaction times to emotional words between healthy controls and internet addicts (gaming and social network addicts). Similarly, and in contrast to what was hypothesised, the fMRI data revealed no significant difference in dorsal ACC activation between controls and specific internet addicts during socially anxious word blocks. Nonetheless, it was shown that compared to individuals with a social network addiction, participants with an internet gaming addiction exhibited reduced activation in left middle and superior temporal gyrus during socially anxious words. However, all other between group comparisons of the subgroups revealed no significant differences in activation. The findings were interpreted as social anxiety-related alterations in response inhibition playing a larger role in internet gaming addiction rather than SNS addiction, whereby gaming might represent a coping strategy to avoid face to face interactions. The only other study to employ an established reaction time task investigated inhibitory control using a Facebook-specific Go/No-Go paradigm. In this study, Turel et al. [63] observed that bilateral ventral striatum activity during Facebook-Go trials was positively correlated with addiction scores. However, contrary to the hypothesis, activity in regions that make up the brain’s inhibitory system (prefrontal cortex) during Facebook-No-Go trials were not negatively correlated with addiction severity. This pattern of results suggests that on the one hand, similar to other addictive disorders, Facebook users with more addiction-like symptoms exhibit a hyperactive amygdala-striatal (impulsive) brain system, whereas on the other hand they do not have a hypoactive prefrontal inhibitory system, which is dissimilar to other addictions.
Alterations of ventral striatal activity have also been reported in other fMRI studies investigating SNS use. One study employing a sample of Facebook users recorded neural activity whilst participants completed a ‘description task’ [73]. Here, participants received a description (e.g., intelligent) that appeared with a picture of themselves or another person, with the descriptions ostensibly provided by another participant. The findings revealed that when receiving gains in reputation (vs. observing the gains in reputation of another) activity in the left NAc (a component of the ventral striatum) predicted more intense Facebook use. Furthermore, Meshi, Morawetz and Heekeren [73] found that left NAc activity in response to monetary rewards did not predict more intense Facebook use, indicating that individual sensitivity of the NAc in processing social information relevant to the self (rather than its sensitivity to reward more generally) influences the use of Facebook.
Sherman and colleagues also observed alterations in NAc activity in their studies which analysed data from the same sample of teenaged Instagram users. In their experiment, participants completed a paradigm that simulated Instagram use, in which they viewed a series of photos (including some of their own photos) with varying numbers of ‘likes’. Each image was ostensibly rated by other participants and appeared with either a high or low like count. After viewing each image participants had to decide themselves whether to like the picture or not. In their first paper which analysed data from 32 teenagers (mean age = 16.8), Sherman et al. [74] reported increased bilateral NAc activation when participants viewed their own photos that had received many (compared to few) likes. Additionally, when participants viewed ‘risky’ photos (e.g., images depicting cannabis use) they exhibited hypoactivity in regions implicated in cognitive control (e.g., dorsal ACC, bilateral PFC, and lateral parietal cortex). In their later paper, the authors included data from an additional 26 university students (mean age = 19.9) who underwent the same procedure [75]. The findings revealed that university students displayed a similar pattern of results, showing increased activity in bilateral NAc when viewing their own photos with many (vs. few) likes. While this effect replicated the previous study using an older sample, unlike the teenaged Instagram users, the university students did not display reduced activity in regions involved in cognitive control whilst viewing risky images. In the last paper published by this group, Sherman et al. [76] analysed neural activity (in the same sample of Instagram users) when participants decided to like another’s photo. Consistent with their hypothesis liking an image was associated with increased activity in the ventral striatum and VMPFC, as well as a range of other structures involved in reward, salience processing and executive function. The NAc/ventral striatum therefore appears to be a key region involved in facilitating SNS use by becoming hyperactive when receiving peer feedback (e.g., obtaining likes), providing peer evaluation (e.g., distributing likes) and responding to SNS cues (e.g., during SNS-Go trials in the Go/No-Go task).
In a similar experiment to that of Sherman and colleagues, Nasser et al. [77] employed a sample of problematic (n = 15) and non-problematic (n = 15) Instagram users who completed a paradigm that mimicked Instagram use whilst undergoing fMRI. During the task participants viewed a series of Instagram photos that were categorised as either risky (e.g., a selfie whilst driving), neutral (e.g., greyscale image of an inanimate object), or positive (i.e., photos from the participants own Instagram account), and each appeared with either a high or low number of ‘likes’. After viewing each image participants also had to decide whether to ‘like’ or ‘pass’ the photo. Behaviourally it was found that problematic Instagram users liked significantly more risky pictures than the control group. Analysis of the fMRI data also revealed that compared to non-problematic Instagram users, problematic users exhibited increased bilateral precuneus activation when viewing risky images. Increased precuneus activity in more problematic users is assumed to reflect the role this region plays in assigning salience and responding to habit-forming stimuli [78], which is corroborated by the behavioural results (as problematic users were more responsive to risky images). Additionally, activity in the right MPFC when viewing risky photos was negatively correlated with Instagram addiction scores, suggesting that more problematic users might experience decision making/cognitive control deficits when viewing addiction-related cues.
As well as investigating peer feedback in the form of SNS ‘likes’, other studies have investigated neural reactivity to receiving comments on SNS posts. In a task simulating the use of Facebook, adolescent SNS users had to post controversial statements (e.g., “abortions should be illegal”) to a Facebook group and received either positive or negative comments on their post from peers [79]. When compared to a control condition (i.e., posting neutral statements and receiving neutral feedback) trials containing emotionally valenced statements elicited activation in the MPFC, precuneus and PCC. Additionally, receiving negative (vs. positive) peer feedback was associated with increased activity in the ventrolateral PFC, MPFC, and anterior insula, whereas viewing positive comments was associated with greater activity in regions including the posterior insula, TPJ, precuneus and PCC. However, the amount participants used SNSs in the real world did not interact with these effects.
Similarly, fMRI cue-reactivity paradigms have also been utilised to investigate the neural regions involved in decisions to repost content on microblogging platforms (e.g., Weibo), where short passages of text are typically shared by users. Analysing data from the same sample as a previous resting-state fMRI study [67], Zhang and Qu [72] investigated task-based neural activity whilst participants completed a paradigm simulating Weibo use. When reposting emotionally valenced (vs. neutral) messages participants showed increased activity in regions implicated in the emotion and cognitive control systems (e.g., DLPFC, insula, precuneus and TPJ), suggesting that these messages recruit more cognitive and emotional resources. Behaviourally participants were more likely to repost negatively valenced messages, which was also reflected by an increase in TPJ activation. Since the TPJ is known to play a key role in social communication and mentalising, these findings also imply that this region is involved in decisions to promulgate negative information online.
In another fMRI study using an SNS exposure paradigm, 30 TikTok users were shown six personalised recommended TikTok videos (based on user-specific preferences) that were extracted from their own account as well as six generalised recommended videos for new users [80]. Watching personalised compared to generalised recommended TikTok videos resulted in increased activation in regions of the default mode network (DMN; including the bilateral superior and middle temporal gyri, temporal pole, ventral PCC, MPFC, and angular gyrus) as well as in the left dorsal lateral and inferior frontal regions, anterior thalamus and cerebellum. In addition, voxel-wise psychophysiological interaction analysis was used to assess task-related connectivity changes between seeds in the DMN and other regions. The analysis revealed increased connectivity between the DMN seeds and regions including the visual network, primary auditory cortex, and middle frontal gyrus, but reduced connectivity with the cingulate cortex, cuneus and inferior parietal lobe when watching personalised (vs. generalised) videos. Finally, activation in regions implicated in addiction and reward learning (ventral tegmental area, substantia nigra and NAc) were also explored using ROI analyses. While the substantia nigra was activated by both personalised and generalised videos, increased ventral tegmental activation was specific to watching personalised videos. Interestingly, however, and contrasting with an important role for the NAc in other SNS use behaviours, the NAc was deactivated (although not significantly) when viewing both video types. In an extension of this work, the same group published further analyses of the same dataset using graph theory to investigate the functional connectivity between seven networks [81]. Results revealed that viewing personalised videos increased connectivity in the DAN-VAN-DMN pathway, whereas both video types resulted in reduced coupling between the salience network (i.e., anterior insula and dorsal ACC) and VAN as well as between two subsystems in the DMN. Taken together, these findings support a role of the DMN in self-relevant information processing, whereby regions in the DMN are activated by user-specific content and may also facilitate prolonged SNS use through increased coupling of the DMN to visual and auditory pathways, but reduced coupling to regions in the control network. The authors suggest that the results are also consistent with roles for the substantia nigra in saliency-coding and ventral tegmental area in reward-value coding.
In a study by Leménager et al. [82], participants completed a self-retrieval paradigm in which they rated the extent to which various self-concept-related characteristics described their self, ideal self, and gaming avatar (created by them), whilst undergoing fMRI. The sample consisted of pathological internet gamers (n = 19), pathological SNS users (n = 19) and healthy controls (n = 19). It was found that individuals with a gaming addiction exhibited increased left angular gyrus activation when reflecting on the characteristics of their avatar, whereas individuals with an SNS addiction exhibited striatal hypoactivations whilst making self (vs. ideal)-reflections. The authors suggest that the finding of reduced activity in the dorsal striatum in participants with an SNS addiction compared to healthy controls might indicate that self (vs. ideal)-reflections are less rewarding for SNS addicts. This in turn might suggest that these individuals experience deficits in emotion regulation that could be the result of (or the perception of) social feedback received online. A more recent study used a similar self-concept paradigm in which participants had to make self-judgements on academic, physical and prosocial traits from their own perspective and from the perspective of others [83]. It was shown that individuals who reported less SNS use made more positive ratings from self-judgements vs. reflected-peer-judgements and more excessive SNS use was linked to increased MPFC activity during self-judgements and particularly during physical (vs. academic and prosocial) self-judgements. Nonetheless, longitudinal assessments of clinical symptoms, prosocial behaviour and self-concept clarity (at 1-and 2-year follow up) indicated no long-term effects of SNS use or altered MPFC activity. Thus, while the findings are consistent with the MPFC’s core role in the self-referential cognition network and suggest that excessive SNS use might modulate activity in this region during self-judgements resulting in more intensified self-refection processes, there is no evidence that this leads to negative long-term consequences.

Table 3.
Summary of task-based fMRI studies.
Table 3.
Summary of task-based fMRI studies.
| Authors (Year) | Sample | Mean Age | PSNSU | SNS Assessment Tool | Design | Main Results | Quality Assessment |
|---|---|---|---|---|---|---|---|
| Dieter et al. (2017) [38] |
|
|
|
|
|
|
|
| Leménager et al. (2016) [82] |
|
|
|
|
|
|
|
| Meshi, Morawetz and Heekeren (2013) [73] |
|
|
|
|
|
|
|
| Nasser et al. (2020) [77] |
|
|
|
|
|
|
|
| Peters et al. (2021) [83] |
|
|
|
|
|
|
|
| Sherman et al. (2016) [74] a |
|
|
|
|
|
|
|
| Sherman, Greenfield et al. (2018) [75] a |
|
|
|
|
|
|
|
| Sherman, Hernandez et al. (2018) [76] a |
|
|
|
|
|
|
|
| Su, Zhou, Gong et al. (2021) [80] b |
|
|
|
|
|
|
|
| Su, Zhou, Wang et al. (2021) [81] b |
|
|
|
|
|
|
|
| Turel et al. (2014) [63] c |
|
|
|
|
|
|
|
| Wikman et al. (2022) [79] |
|
|
|
|
|
|
|
| Zhang and Qu (2020) [72] d |
|
|
|
|
|
|
|
Note. PSNSU refers to studies that investigated the neural correlates of problematic/compulsive SNS use. a Sherman et al. [74,75,76] used the same sample of high school students (n = 32) and Sherman et al. [75,76] used the same sample of university students (n = 26). b Su et al. [79,80] used the same sample of TikTok users (n = 30). While this study administered an assessment of ‘TikTok addiction’, scores were not correlated with brain activity. c Turel et al. [63] used the same sample of Facebook users (n = 20) as two structural MRI studies by He, Turel and Bechara [49] and He et al. [57]. d Zhang and Qu [72] used the same sample of Weibo users (n = 28) as a resting-state fMRI study by Zhang and Mo [67].
4. Discussion
The present article sought to systematically review existing research employing MRI methods to investigate the neural underpinnings of SNS use. In general, MRI studies investigating ‘SNS addiction’ are relatively scarce and a larger number of MRI studies have investigated either internet gaming or non-specific internet use addiction (e.g., 44 MRI studies investigating internet gaming disorder were identified in a recent systematic review; [87]). Below, we evaluate the main findings in relation to the key brain regions implicated in SNS use/addiction.
4.1. Ventral Striatum/Nucleus Accumbens
The ventral striatum, and in particular the NAc within the ventral striatum, appears to be a key region involved in the use of SNSs. The NAc is known to play a central role in the brain’s ‘reward circuit’ and is responsible for regulating motivation for drug-seeking behaviour in addiction [88]. Neuroimaging studies of substance use addictions have consistently shown greater ventral striatum/NAc activity in addicts presented with addiction-related cues [89,90] and there is also evidence that NAc activity is correlated with increased cravings in problem gamblers [21]. Therefore, MRI studies investigating SNS use might reasonably expect neuroadaptations of the ventral striatum/NAc to be associated with excessive/problematic SNS use or responses to SNS cues. Indeed, there is evidence that problematic SNS users show heightened activity in the ventral striatum when responding to SNS cues [63] and even regular users show greater activity in this region when performing tasks that simulate SNS use especially when the task involves cues related to social reward (e.g., ‘likes’) [73,74,75]. Furthermore, higher SNS engagement may also be characterised by reduced functional connectivity between the ventral striatum and DLPFC (reflecting reduced control) but increased connectivity in the ventral striatum-ventral medial PFC ‘reward pathway’ [37]. Multiple structural MRI studies have also found associations between more excessive/problematic SNS use and reduced ventral striatum volume. Montag et al. [46] provides the most compelling evidence for reduced NAc GMV in more excessive SNS users, since the study used a comparatively large sample size and employed an objective measure of SNS usage. Such a finding is also consistent with the substance addiction literature where reduced volume of structures within the brain’s reward system are typically observed in addicted individuals [91,92]. More excessive/problematic SNS use therefore seems to be characterised by reduced ventral striatum volume but heightened activity in this region in response to SNS cues or social rewards related to SNS use.
4.2. Prefrontal Cortex
The prefrontal cortex can be subdivided into three broad structures that comprise the lateral, medial and orbitofrontal regions. The lateral prefrontal regions are thought to be responsible for implementing cognitive control and executive functions such as working memory, selective attention and planning, while the medial prefrontal regions play a role in motivation, decision making and self-referential processing [93]. Due to its many connections with the limbic system the orbitofrontal cortex plays a vital role in emotional behaviour and reward processes and is responsible for forming reward expectations as well as representing reward value and subjective pleasantness [94]. The functions of the PFC are thus clearly relevant to the development of addiction and various structural and functional abnormalities of prefrontal regions have been documented in the substance use addiction literature [95]. Such abnormalities are thought to be responsible for the impaired inhibitory control and increased cravings/impulsivity experienced during addiction. Multiple studies identified in the present review have also implicated prefrontal regions in the use and problematic use of SNSs.
Nonetheless, evidence of structural PFC abnormalities being associated with SNS use is scarce. It is well established that drugs of abuse are associated with grey matter atrophy in the PFC [96,97,98] and there is also evidence that behavioural addictions such as gambling disorder are related to reduced cortical thickness in frontal regions [99]. Yet, only one study has reported such a relationship (reduced GMV of the right orbitofrontal cortex) in problematic SNS users [35]. However, this study did employ a large sample size when compared to the other structural MRI studies identified in this review. Notably, one study found that adolescents with more excessive SNS use showed higher cortical thickness in the lateral and medial PFC [56]. This finding is not entirely inconsistent with other addictive disorders since increased GMV in the right prefrontal cortex has also been observed in problem gamblers and is suggested to be a neuroadaptation in response to the increased cognitive demands required to control gambling impulses [100]. However, in their longitudinal study, Achterberg et al. [56] also reported accelerated cortical thinning in the lateral PFC in excessive SNS users, although this finding did not survive FDR correction. Thus, to date there is only weak evidence to suggest that SNS use is associated with structural changes in the PFC.
A larger number of studies have reported functional alterations of the PFC at rest and during task performance. The OFC has been implicated in only a few functional MRI studies, none of which correlated activity with excessive or problematic SNS use. Instead, increased functional connectivity between the OFC and central precuneus appears to be associated with sharing personal information online [64], while stronger connections to the dorsomedial PFC and temporal parietal junction facilitates SNS reposting [67]. Sherman et al. [76] also reported increased OFC activity when participants ‘liked’ another’s Instagram post. Thus, while abnormal OFC activity/connectivity has not been associated with more problematic SNS use, this region does seem to play an important role in active SNS engagement (e.g., posting, sharing and ‘liking’ SNS content).
The lateral prefrontal cortex (particularly the dorsolateral prefrontal cortex; DLPFC) has also been shown to be involved in SNS use behaviours. It is likely that the DLPFC is recruited to manage the cognitive and emotional demands of sharing personal and emotionally valenced content online [64,72]. Given the key role the DLPFC plays in implementing cognitive control [50], it is also unsurprising that more efficient connectivity with the ventral striatum is associated with lower SNS engagement (improved control) [37] while reduced connectivity with the dorsal attention network is associated with more problematic use (reduced control) [36]. Limited inhibitory control abilities in more problematic SNS users may also be reflected by reduced white matter integrity in the forceps minor (a white matter bundle connecting the lateral and medial surfaces of the frontal lobes) [57]. However, contrary to what might be expected, fMRI studies employing tasks of executive function have reported no abnormalities in PFC activation for problematic SNS users [38,63].
Finally, the medial PFC has been implicated in a range of fMRI studies. Increased functional connectivity between the medial PFC and ventral striatum in more excessive SNS users [37] and increased medial PFC activity in response to viewing SNS photos with many ‘likes’ [74,75] could potentially reflect an increased motivation to pursue SNS rewards. However, more problematic SNS users have been found to show reduced medial PFC activity when viewing cues depicting risky behaviours which might indicate impaired decision making abilities [77]. Other studies have also supported a key role for the medial PFC in the self-referential cognition network. Reduced medial PFC activity when making self-reflections might indicate deficits in self-referential cognition as a result of excessive SNS use [82]. Although, one study found increased medial PFC activity during self-judgements in more excessive users, which had no relationship with long-term negative outcomes [83]. Increased medial PFC activity when viewing personalised SNS videos [80] or when receiving negative SNS comments [79] may also reflect the processing of content relevant to the self.
4.3. Amygdala
The amygdala is another key structure implicated in the development of substance use addictions. The amygdala is engaged in emotional and motivational processes and forms a crucial component of the brain’s arousal/stress systems which are thought to play a key role in facilitating the transition to drug dependence and maintenance [101]. Meta-analyses of drug cue-reactivity studies have found cue-induced amygdala activity to be one of the most robust findings in the substance addiction literature [90,102], and rodent models have also shown that activating the amygdala intensifies motivation for drug consumption [103]. By contrast, no existing research has found an association between amygdala activity and problematic SNS use (although cue-reactivity studies are limited). Nonetheless, Sherman et al. [76] did find increased amygdala activity in regular SNS users when ‘liking’ another’s post, which is consistent with the amygdala’s role in motivation and reward expectancy (‘liking’ another’s post may in turn increase the likelihood of an individual reciprocating ‘likes’ on a future post, intensifying reward expectancy). Structurally, two studies reported an association between reduced amygdala volume and more excessive/problematic SNS use [47,49]. This finding is in keeping with observations in substance use disorders [52,104,105,106], where reduced amygdala volume is suggested to be responsible for increased susceptibility to emotion dysregulation, resulting in a reduced ability to manage cravings. Thus, in SNS use, the amygdala appears to play a role in decisions to distribute positive feedback to others, while reduced amygdala size may be predictive of more intense or harmful SNS use.
4.4. Cingulate Cortex
The cingulate cortex, principally the ACC (a central node of the cognitive control network; 50) but also the posterior cingulate cortex (PCC), has been widely implicated in substance use addictions [90,107,108]. Structural MRI studies have also identified reduced ACC volume in both substance use [109,110] and behavioural addictions (e.g., internet gaming disorder; [111]) reflecting diminished cognitive control abilities. In the present review, two studies were identified which found significant correlations between ACC volume and problematic SNS use. Montag et al. [48] found reduced subgenual ACC volume in more addicted SNS users, whereas He, Turel and Bechara [49] found an association between increased dorsal ACC volume and higher addiction severity. While this might indicate specific functions for ACC subregions in addiction, the finding of increased dorsal ACC volume in more problematic SNS users is still not consistent with the wider addiction literature. Indeed, neural deficits in the dorsal ACC have been proposed to constitute a hallmark neurocognitive deficit underlying addictive disorders [112] and stimulating the rostrodorsal ACC can help suppress alcohol cravings in individuals with alcohol use disorder [113]. In line with this, Sherman et al. [74] found that teenaged Instagram users showed deactivations of regions in the cognitive control network (including the dorsal ACC), when viewing Instagram posts depicting risky (vs. neutral) behaviours ostensibly posted by peers, which suggests disinhibited cognitive control in response to highly salient SNS cues. Although this effect appears to be age-specific [75], potentially reflecting an immature capacity for cognitive control during adolescence. The finding of increased ACC activity when ‘liking’ a peers Instagram post [76] also dovetails with evidence of an integral role for the ACC in linking motivational outcomes to behaviour [114]. Nonetheless, in contrast to what was hypothesised, neither of the two fMRI studies employing cognitive control tasks observed alterations of ACC activity in problematic SNS users [38,63], which is therefore inconsistent with similar studies investigating substance use [115] and other behavioural addictions [116].
The PCC constitutes a central structure of the default mode network (DMN), a neural network that is maximally activated at rest [117,118]. The DMN has been found to show increased activation and altered functional connectivity with various other regions (including regions in the dorsal and ventral attention networks) when viewing personalised TikTok videos and after reading SNS microblogs [68,80,81]. Such findings imply that passively consuming SNS content (particularly personalised content) may activate a neural state similar to that when mind wandering, which may facilitate prolonged use through modulating attention and inducing absent-minded scrolling/browsing of SNS feeds. Modifications of DMN functional connectivity have also been observed in substance use addictions which are proposed to facilitate cravings and relapse [119]. The DMN is thought to be predominantly engaged during phases of withdrawal and preoccupation in addiction and is thus active at the expense of the cognitive control network, limiting its capacity to manage cravings and prevent relapse.
4.5. Precuneus
The precuneus, a region which neighbours the PCC, is another core hub of the DMN and is therefore also implicated in studies demonstrating alterations of DMN activity and functional connectivity when viewing SNS content [68,80,81]. The precuneus is also thought to play an important role in a range of complex tasks including visuo-spatial imagery, memory retrieval and self-referential cognition [120]. As previously discussed, enhanced coupling between the precuneus and prefrontal regions appears to be associated with increased sharing of personal information on SNSs, whereas increased connectivity between the precuneus and anterior temporal pole (ATP) is negatively associated with self-related sharing [64]. The findings are therefore consistent with a role for the precuneus in self-referential cognition since enhanced connectivity with prefrontal regions is necessary to hold self-related information in working memory. In contrast, the ATP is engaged when thinking about the mental states of others [121], and thus thinking about how others might react to a post may inhibit frequent self-disclosures on SNSs. Other research has shown that the precuneus is also engaged during decisions to repost emotionally valenced messages online [72,79] and when viewing posts with many (vs. few) likes [74], suggesting that these SNS behaviours trigger self-relevance processing. As reviewed above, only one study implicated the precuneus in problematic SNS use during exposure to Instagram photos, suggesting a role for this region in cue reactivity and the transmission of visual information to motivational systems [77]. Meta-analyses have also found that cue-induced precuneus activity is associated with substance use [23,122] and behavioural addictions [123].
4.6. Temporal Parietal Junction
One function of the temporal parietal junction (TPJ) is to form a key node of the mentalising network, which enables cognitive empathy and perspective taking, allowing us to interpret the mental states of others [124]. Given that mentalising (or other functions of the TPJ) are not directly implicated in substance use or addictive behaviours, it is unsurprising that TPJ activation is rarely reported in addiction research. One study has linked reduced amygdala-TPJ connectivity to neuroticism, a personality trait which can influence addiction severity [125]. Nonetheless, the TPJ may be more relevant in the development of behavioural addictions that involve social communication and interpreting another’s mental state (e.g., SNS use). Increased grey matter density of the TPJ has been shown to be positively correlated with internet addiction [126], yet the only structural MRI study identified in the present review to implicate the TPJ found a stronger decrease in the surface area of this region in more excessive adolescent SNS users over a period of three years [56]. However, such disparities may reflect unique age-related differences between measures of grey matter morphology [127]. Furthermore, while Achterberg et al. [56] argued that stronger reductions in the surface area of the TPJ in more excessive SNS users was consistent with their hypothesis that SNS use would lead to accelerated maturation of brain regions important for social processing, they also noted that the correlation was weak and did not survive a correction for multiple tests. As reviewed above, the TPJ has also been implicated in regular (non-addicted) SNS reposting behaviours [67,72]. Thus, when reposting SNS messages (especially emotionally valenced messages) the TPJ may play an important role in enabling an understanding of how such messages will be viewed from another’s perspective, while excessive SNS use (at least during adolescence) may accelerate cortical thinning in this region, potentially contributing to a lack of impulse control [128].
4.7. Insula
A single structural MRI study has implicated the insula in problematic SNS use [54], indicating that grey matter deficits in this region may result in increased impulsivity and a preference for immediate gains in problematic users. This is consistent with the role of the insula in decision making and delaying gratification. Grey matter deficits of the insula have also been proposed to constitute an important structural marker of drug addictions. For example, in one study, both cocaine and heroin dependent patients were shown to have reduced grey matter in the posterior insula when compared to healthy controls [129]. The importance of the insula for addiction processes is further supported by evidence that damage to this region can cause cigarette smokers to spontaneously quit without relapse [130] and insular reactivity to addiction-related cues can predict relapse [131]. This is because the insula is thought to be responsible for forming conscious representations of the homeostatic imbalance caused by withdrawal which manifests as urges or cravings for drug consumption [55]. Despite its important role in the maintenance of substance use addictions, research is yet to associate functional alterations of the insula with more problematic SNS use. Nonetheless, as reviewed above, in regular users, insula activity and its connectivity with the TPJ has been associated with reposting emotionally valenced messages, ‘liking’ SNS photos and receiving valenced SNS comments [67,72,76,79]. Such patterns of activity are also consistent with a role for the insula in detecting salient stimuli and modulating emotional responses [132].
4.8. Other Observations
As well as identifying brain regions associated with SNS use, the present review also highlights methodological issues that future research should address. Firstly, seven different scales were employed across the identified articles to assess problematic/compulsive SNS use. The inclusion of certain diagnostic criteria assessed in some of these scales (e.g., escape/mood management criterion) have also been criticised for lacking clinical validity and pathologising normal use motives [133,134]. Thus, there is a need to establish consensus regarding the symptomatology of problematic SNS use and for diagnostic criteria to be assessed consistently across studies in order to improve comparability. Furthermore, the majority of studies that assess excessive SNS use have relied on self-reports of use intensity. Recent evidence suggests that individuals are often inaccurate in reporting the duration of their SNS use, displaying a tendency to overestimate their usage intensity [135,136]. Future research should aim to utilize objective measures of SNS use to ensure that excessive use behaviours are reliably assessed. Moreover, existing neuroimaging research is limited with large heterogeneity among studies. As such more MRI studies employing more consistent methods are required to establish firm conclusions about the neural underpinnings of excessive/problematic SNS use.
Additionally, the majority of research to date has focused on investigating the neural correlates of SNS use in adolescents and young adults. While this seems reasonable given that younger populations have increased exposure to SNSs and are thought to be more vulnerable to experiencing negative consequences [137], future research may also wish to assess whether excessive/problematic SNS use in older adults is associated with similar brain changes. Furthermore, many of the studies that implicate regions of the cognitive control network in the use of SNSs have inferred that deficits to these regions may be responsible for the development of more problematic or compulsive SNS use. Yet, neither of the two task-based fMRI studies employing cognitive control tasks have found evidence of dysfunctional activity within the cognitive control network in more problematic users [38,63]. This lack of evidence is surprising since impaired activity of the cognitive control network during tasks of executive functions has been consistently observed in individuals with substance use disorders and other behavioural addictions [112,138]. Thus, more task-based fMRI studies employing behavioural paradigms (e.g., Go/No-Go, Stroop, and Approach/Avoidance) are needed to better understand the cognitive/motivational processes underpinning SNS use and overuse. Moreover, longitudinal research is scarce and existing studies report mixed results. While only subtle differences in brain morphology have been attributed to excessive SNS use [56], some studies have shown widespread functional connectivity changes as a result of increased SNS use [70]. Yet, other research suggests that abnormal neural activity associated with increased SNS use has no long-term effects on clinical symptoms [83]. As such, it cannot yet be concluded that excessive/problematic SNS use is responsible for significant neuroadaptations that represent a disease process, as is observed in substance use disorders. Alternatively pre-existing neural abnormalities may be responsible for facilitating the development of more compulsive or problematic forms of SNS use.
While our review does not directly inform the treatment of problematic SNS use, our findings highlight the important role of (social) reward and its associated brain structures in driving this behaviour. Incorporating elements into intervention packages that focus on controlled and sensible seeking of SNS rewards (e.g., through limiting exposure to SNS notifications or disabling the ‘like’ function) may thus constitute a viable avenue to tackle problematic SNS use.
5. Conclusions
In sum, our systematic review of MRI studies investigating SNS use suggests that more excessive/problematic use does share some important neural similarities to substance abuse. In particular, the existing evidence leads us to conclude that problematic use may be characterised by: (1) reduced GMV of the ventral striatum, amygdala, subgenual anterior cingulate cortex, orbitofrontal cortex and posterior insula; (2) increased ventral striatum and precuneus activity in response to SNS cues; (3) abnormal functional connectivity between middle occipital gyrus and DLPFC and the dorsal attention network; (4) impaired white matter integrity in the corpus callosum and forceps minor. In regular users, regions including the temporal parietal junction, ventral striatum, precuneus, insula and frontal lobes have been implicated in active SNS engagement (i.e., sharing/reposting/‘liking’ SNS content), while altered functional connectivity of the default mode network is associated with passive consumption of SNS content (particularly personalised recommended videos). Nonetheless, given that these regions are not consistently implicated across studies, and considering the large heterogeneity of current studies (e.g., in the type of SNS under investigation, the scales used to assess SNS use/addiction, and the experimental tasks employed), our conclusions should remain tentative. To date, the majority of research investigating the neural correlates of problematic/compulsive SNS use are structural MRI studies, and thus more fMRI research is required to elucidate the functional alterations that might underpin a ‘SNS addiction’. Furthermore, most existing neuroimaging research is limited by small sample sizes, recruiting from student populations, and employing self-reports of usage intensity. More large-scale and longitudinal research employing objective measures of SNS use are essential to establish stronger conclusions regarding the neurobiological effects of SNSs.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/2076-3425/13/5/787/s1, Table S1: Quality assessment ratings for structural MRI studies adapted from EPHPP criteria; Table S2. Quality assessment ratings for resting state fMRI studies adapted from EPHPP criteria; Table S3. Quality assessment ratings for task-based fMRI studies adapted from EPHPP criteria.
Author Contributions
Conceptualization, M.W. and N.I.; methodology, M.W.; validation, M.W. and N.I., data curation, M.W.; writing—original draft preparation, M.W.; writing—review and editing, N.I.; visualization, M.W.; supervision, N.I. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Economic and Social Research Council through a NINE-DTP doctoral studentship (ES/P000762/1). The funder played no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit this article for publication.
Data Availability Statement
Not applicable to this article as no new data were created or analysed. The supporting information can be found in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- DataReportal. Digital 2012: Global Digital Overview. Available online: https://datareportal.com/reports/digital-2012-global-digital-overview (accessed on 18 May 2022).
- DataReportal. Digital 2022: Global Overview Report. Available online: https://datareportal.com/reports/digital-2022-global-overview-report (accessed on 18 May 2022).
- Statista. Daily Time Spent on Social Networking by Internet Users Worldwide from 2012 to 2020. Available online: https://www.statista.com/statistics/433871/daily-social-media-usage-worldwide/ (accessed on 18 May 2022).
- Kelly, Y.; Zilanawala, A.; Booker, C.; Sacker, A. Social media use and adolescent mental health: Findings from the UK Millennium Cohort Study. EClinicalMedicine 2018, 6, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Twenge, J.M.; Joiner, T.E.; Rogers, M.L.; Martin, G.N. Increases in depressive symptoms, suicide-related outcomes, and suicide rates among US adolescents after 2010 and links to increased new media screen time. Clin. Psychol. Sci. 2018, 6, 3–17. [Google Scholar] [CrossRef]
- Twenge, J.M.; Martin, G.N.; Campbell, W.K. Decreases in psychological well-being among American adolescents after 2012 and links to screen time during the rise of smartphone technology. Emotion 2018, 18, 765–780. [Google Scholar] [CrossRef]
- Orben, A.; Przybylski, A.K. The association between adolescent well-being and digital technology use. Nat. Hum. Behav. 2019, 3, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Vuorre, M.; Orben, A.; Przybylski, A.K. There is no evidence that associations between adolescents’ digital technology engagement and mental health problems have increased. Clin. Psychol. Sci. 2021, 9, 823–835. [Google Scholar] [CrossRef]
- Cheng, C.; Lau, Y.-C.; Chan, L.; Luk, J.W. Prevalence of social media addiction across 32 nations: Meta-analysis with subgroup analysis of classification schemes and cultural values. Addict. Behav. 2021, 117, 106845. [Google Scholar] [CrossRef] [PubMed]
- Johannes, N.; Dora, J.; Rusz, D. Social smartphone apps do not capture attention despite their perceived high reward value. Collabra Psychol. 2019, 5, 14. [Google Scholar] [CrossRef]
- Thomson, K.; Hunter, S.C.; Butler, S.H.; Robertson, D.J. Social media ‘addiction’: The absence of an attentional bias to social media stimuli. J. Behav. Addict. 2021, 10, 302–313. [Google Scholar] [CrossRef]
- Wilcockson, T.D.; Osborne, A.M.; Ellis, D.A. Digital detox: The effect of smartphone abstinence on mood, anxiety, and craving. Addict. Behav. 2019, 99, 106013. [Google Scholar] [CrossRef]
- Wadsley, M.; Ihssen, N. The roles of implicit approach motivation and explicit reward in excessive and problematic use of social networking sites. PLoS ONE 2022, 17, e0264738. [Google Scholar] [CrossRef] [PubMed]
- Billieux, J.; Schimmenti, A.; Khazaal, Y.; Maurage, P.; Heeren, A. Are we overpathologizing everyday life? A tenable blueprint for behavioral addiction research. J. Behav. Addict. 2015, 4, 119–123. [Google Scholar] [CrossRef]
- Kardefelt-Winther, D. Commentary on: Are we overpathologizing everyday life? A tenable blueprint for behavioral addiction research: Problems with atheoretical and confirmatory research approaches in the study of behavioral addictions. J. Behav. Addict. 2015, 4, 126–129. [Google Scholar] [CrossRef] [PubMed]
- Maraz, A.; Király, O.; Demetrovics, Z. Commentary on: Are we overpathologizing everyday life? A tenable blueprint for behavioral addiction research: The diagnostic pitfalls of surveys: If you score positive on a test of addiction, you still have a good chance not to be addicted. J. Behav. Addict. 2015, 4, 151–154. [Google Scholar] [CrossRef]
- Koob, G.F.; Le Moal, M. Drug abuse: Hedonic homeostatic dysregulation. Science 1997, 278, 52–58. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The neural basis of drug craving: An incentive-sensitization theory of addiction. Brain Res. Rev. 1993, 18, 247–291. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.S.; Volkow, N.D.; Kassed, C.A.; Chang, L. Imaging the addicted human brain. Sci. Pract. Perspect. 2007, 3, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Goudriaan, A.E.; De Ruiter, M.B.; Van Den Brink, W.; Oosterlaan, J.; Veltman, D.J. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: An fMRI study. Addict. Biol. 2010, 15, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Limbrick-Oldfield, E.H.; Mick, I.; Cocks, R.; McGonigle, J.; Sharman, S.; Goldstone, A.P.; Stokes, P.; Waldman, A.; Erritzoe, D.; Bowden-Jones, H. Neural substrates of cue reactivity and craving in gambling disorder. Transl. Psychiatry 2017, 7, e992. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Fellows, L.; Small, D.; Dagher, A. Food and drug cues activate similar brain regions: A meta-analysis of functional MRI studies. Physiol. Behav. 2012, 106, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, J.M.; Versace, F.; Robinson, J.D.; Minnix, J.A.; Lam, C.Y.; Cui, Y.; Brown, V.L.; Cinciripini, P.M. Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. Neuroimage 2012, 60, 252–262. [Google Scholar] [CrossRef]
- Quaglieri, A.; Mari, E.; Boccia, M.; Piccardi, L.; Guariglia, C.; Giannini, A.M. Brain network underlying executive functions in gambling and alcohol use disorders: An activation likelihood estimation meta-analysis of fMRI studies. Brain Sci. 2020, 10, 353. [Google Scholar] [CrossRef]
- van Holst, R.J.; de Ruiter, M.B.; van den Brink, W.; Veltman, D.J.; Goudriaan, A.E. A voxel-based morphometry study comparing problem gamblers, alcohol abusers, and healthy controls. Drug Alcohol Depend. 2012, 124, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Meshi, D.; Tamir, D.I.; Heekeren, H.R. The emerging neuroscience of social media. Trends Cogn. Sci. 2015, 19, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Ihssen, N.; Wadsley, M. A Reward and Incentive-Sensitization Perspective on Compulsive Use of Social Networking Sites–Wanting but not Liking Predicts Checking Frequency and Problematic Use Behavior. Addict. Behav. 2021, 116, 106808. [Google Scholar] [CrossRef]
- Berridge, K.C.; Robinson, T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016, 71, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Dong, G.; Huang, J.; Du, X. Enhanced reward sensitivity and decreased loss sensitivity in Internet addicts: An fMRI study during a guessing task. J. Psychiatr. Res. 2011, 45, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Qin, W.; Wang, G.; Zeng, F.; Zhao, L.; Yang, X.; Liu, P.; Liu, J.; Sun, J.; von Deneen, K.M. Microstructure abnormalities in adolescents with internet addiction disorder. PLoS ONE 2011, 6, e20708. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lin, F.-C.; Du, Y.-S.; Zhao, Z.-M.; Xu, J.-R.; Lei, H. Gray matter abnormalities in Internet addiction: A voxel-based morphometry study. Eur. J. Radiol. 2011, 79, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Fernandez, O. How has internet addiction research evolved since the advent of internet gaming disorder? An overview of cyberaddictions from a psychological perspective. Curr. Addict. Rep. 2015, 2, 263–271. [Google Scholar] [CrossRef]
- Tashjian, S.M.; Galván, A. Dorsolateral prefrontal cortex response to negative tweets relates to executive functioning. Soc. Cogn. Affect. Neurosci. 2020, 15, 775–787. [Google Scholar] [CrossRef]
- Radke, S.; Jankowiak, K.; Tops, S.; Abel, T.; Habel, U.; Derntl, B. Neurobiobehavioral responses to virtual social rejection in females—Exploring the influence of oxytocin. Soc. Cogn. Affect. Neurosci. 2021, 16, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Namkoong, K.; Lee, J.; Lee, B.O.; Jung, Y.C. Lateral orbitofrontal gray matter abnormalities in subjects with problematic smartphone use. J. Behav. Addict. 2019, 8, 404–411. [Google Scholar] [CrossRef]
- Lee, D.; Lee, J.; Namkoong, K.; Jung, Y.-C. Altered functional connectivity of the dorsal attention network among problematic social network users. Addict. Behav. 2021, 116, 106823. [Google Scholar] [CrossRef] [PubMed]
- Wilmer, H.H.; Hampton, W.H.; Olino, T.M.; Olson, I.R.; Chein, J.M. Wired to be connected? Links between mobile technology engagement, intertemporal preference and frontostriatal white matter connectivity. Soc. Cogn. Affect. Neurosci. 2019, 14, 367–379. [Google Scholar] [CrossRef]
- Dieter, J.; Hoffmann, S.; Mier, D.; Reinhar, I.; Beutel, M.; Vollstadt-Klein, S.; Kiefer, F.; Mann, K.; Lemenager, T. The role of emotional inhibitory control in specific internet addiction—An fMRI study. Behav. Brain Res. 2017, 324, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kuss, D.J.; Griffiths, M.D. Internet and gaming addiction: A systematic literature review of neuroimaging studies. Brain Sci. 2012, 2, 347–374. [Google Scholar] [CrossRef] [PubMed]
- Sharifat, H.; Rashid, A.A.; Suppiah, S. Systematic review of the utility of functional MRI to investigate internet addiction disorder: Recent updates on resting state and task-based fMRI. Malays. J. Med. Health Sci. 2018, 14, 21–33. [Google Scholar]
- Sepede, G.; Tavino, M.; Santacroce, R.; Fiori, F.; Salerno, R.M.; Di Giannantonio, M. Functional magnetic resonance imaging of internet addiction in young adults. World J. Radiol. 2016, 8, 210. [Google Scholar] [CrossRef]
- Yin, T.; Li, Z.; Xiong, J.; Lan, L.; Sun, R.; Ren, F.; Zhang, P. Neuroimaging biomarkers of psychogenic erectile dysfunction: Protocol for a systematic review. BMJ Open 2019, 9, e030061. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Ciliska, D.; Dobbins, M.; Micucci, S. A process for systematically reviewing the literature: Providing the research evidence for public health nursing interventions. Worldviews Evid.-Based Nurs. 2004, 1, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Hengstschläger, A.; Sommerlad, A.; Huntley, J. What are the neural correlates of impaired awareness of social cognition and function in dementia? A systematic review. Brain Sci. 2022, 12, 1136. [Google Scholar] [CrossRef]
- McLachlan, E.; Rai, S.; Al-Shihabi, A.; Huntley, J.; Burgess, N.; Howard, R.; Reeves, S. Neuroimaging correlates of false memory in’Alzheimer’s disease: A preliminary systematic review. Psychiatry Res. Neuroimaging 2020, 296, 111021. [Google Scholar] [CrossRef] [PubMed]
- Montag, C.; Markowetz, A.; Blaszkiewicz, K.; Andone, I.; Lachmann, B.; Sariyska, R.; Trendafilov, B.; Eibes, M.; Kolb, J.; Reuter, M.; et al. Facebook usage on smartphones and gray matter volume of the nucleus accumbens. Behav. Brain Res. 2017, 329, 221–228. [Google Scholar] [CrossRef] [PubMed]
- He, Q.H.; Turel, O.; Brevers, D.; Bechara, A. Excess social media use in normal populations is associated with amygdala-striatal but not with prefrontal morphology. Psychiatry Res.-Neuroimaging 2017, 269, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Montag, C.; Zhao, Z.; Sindermann, C.; Xu, L.; Fu, M.; Li, J.; Zheng, X.; Li, K.; Kendrick, K.M.; Dai, J. Internet communication disorder and the structure of the human brain: Initial insights on WeChat addiction. Sci. Rep. 2018, 8, 2155. [Google Scholar] [CrossRef] [PubMed]
- He, Q.H.; Turel, O.; Bechara, A. Brain anatomy alterations associated with Social Networking Site (SNS) addiction. Sci. Rep. 2017, 7, 45064. [Google Scholar] [CrossRef]
- MacDonald, A.W.; Cohen, J.D.; Stenger, V.A.; Carter, C.S. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science 2000, 288, 1835–1838. [Google Scholar] [CrossRef]
- Jentsch, J.D.; Pennington, Z.T. Reward, interrupted: Inhibitory control and its relevance to addictions. Neuropharmacology 2014, 76, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Makris, N.; Gasic, G.P.; Seidman, L.J.; Goldstein, J.M.; Gastfriend, D.R.; Elman, I.; Albaugh, M.D.; Hodge, S.M.; Ziegler, D.A.; Sheahan, F.S. Decreased absolute amygdala volume in cocaine addicts. Neuron 2004, 44, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Turel, O.; He, Q.; Brevers, D.; Bechara, A. Social networking sites use and the morphology of a social-semantic brain network. Soc. Neurosci. 2018, 13, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Turel, O.; He, Q.H.; Brevers, D.; Bechara, A. Delay discounting mediates the association between posterior insular cortex volume and social media addiction symptoms. Cogn. Affect. Behav. Neurosci. 2018, 18, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, N.H.; Bechara, A. The insula and drug addiction: An interoceptive view of pleasure, urges, and decision-making. Brain Struct. Funct. 2010, 214, 435–450. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, M.; Becht, A.; van der Cruijsen, R.; van de Groep, I.H.; Spaans, J.P.; Klapwijk, E.; Crone, E.A. Longitudinal associations between social media use, mental well-being and structural brain development across adolescence. Dev. Cogn. Neurosci. 2022, 54, 101088. [Google Scholar] [CrossRef] [PubMed]
- He, Q.H.; Turel, O.; Bechara, A. Association of excessive social media use with abnormal white matter integrity of the corpus callosum. Psychiatry Res.-Neuroimaging 2018, 278, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Meerkerk, G.-J.; Van Den Eijnden, R.J.; Vermulst, A.A.; Garretsen, H.F. The compulsive internet use scale (CIUS): Some psychometric properties. Cyberpsychol. Behav. 2009, 12, 1–6. [Google Scholar] [CrossRef]
- Kim, D.; Lee, Y.; Lee, J.; Nam, J.K.; Chung, Y. Development of Korean smartphone addiction proneness scale for youth. PLoS ONE 2014, 9, e97920. [Google Scholar] [CrossRef]
- Montag, C.; Bey, K.; Sha, P.; Li, M.; Chen, Y.F.; Liu, W.Y.; Zhu, Y.K.; Li, C.B.; Markett, S.; Keiper, J. Is it meaningful to distinguish between generalized and specific Internet addiction? Evidence from a cross-cultural study from Germany, Sweden, Taiwan and China. Asia-Pac. Psychiatry 2015, 7, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowski, M.; Altstötter-Gleich, C.; Brand, M. Validation and psychometric properties of a short version of Young’s Internet Addiction Test. Comput. Hum. Behav. 2013, 29, 1212–1223. [Google Scholar] [CrossRef]
- Van Rooij, A.J.; Schoenmakers, T.M.; Vermulst, A.A.; Van Den Eijnden, R.J.; Van De Mheen, D. Online video game addiction: Identification of addicted adolescent gamers. Addiction 2011, 106, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Turel, O.; He, Q.H.; Xue, G.; Xiao, L.; Bechara, A. Examination of neural systems sub-serving facebook “addiction”. Psychol. Rep. 2014, 115, 675–695. [Google Scholar] [CrossRef] [PubMed]
- Meshi, D.; Mamerow, L.; Kirilina, E.; Morawetz, C.; Margulies, D.S.; Heekeren, H.R. Sharing self-related information is associated with intrinsic functional connectivity of cortical midline brain regions. Sci. Rep. 2016, 6, 22491. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, C.J. Narcissism on Facebook: Self-promotional and anti-social behavior. Personal. Individ. Differ. 2012, 52, 482–486. [Google Scholar] [CrossRef]
- Northoff, G.; Heinzel, A.; De Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage 2006, 31, 440–457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Mo, L. Mentalizing and Information Propagation through Social Network: Evidence from a Resting-State-fMRI Study. Front. Psychol. 2016, 7, 1716. [Google Scholar] [CrossRef]
- Hu, B.; Cui, Y.-L.; Yu, Y.; Li, Y.-T.; Yan, L.-F.; Sun, J.-T.; Sun, Q.; Zhang, J.; Wang, W.; Cui, G.-B. Combining Dynamic Network Analysis and Cerebral Carryover Effect to Evaluate the Impacts of Reading Social Media Posts and Science Fiction in the Natural State on the Human Brain. Front. Neurosci. 2022, 16, 66. [Google Scholar] [CrossRef]
- Brewer, J.A.; Worhunsky, P.D.; Gray, J.R.; Tang, Y.-Y.; Weber, J.; Kober, H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. USA 2011, 108, 20254–20259. [Google Scholar] [CrossRef]
- Hu, B.; Yu, Y.; Yan, L.F.; Qi, G.Q.; Wu, D.; Li, Y.T.; Shi, A.P.; Liu, C.X.; Shang, Y.X.; Li, Z.Y. Intersubject correlation analysis reveals the plasticity of cerebral functional connectivity in the long-term use of social media. Hum. Brain Mapp. 2022, 43, 2262–2275. [Google Scholar] [CrossRef]
- Wilmer, H.H.; Chein, J.M. Mobile technology habits: Patterns of association among device usage, intertemporal preference, impulse control, and reward sensitivity. Psychon. Bull. Rev. 2016, 23, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.J.; Qu, C. Emotional, especially negative microblogs are more popular on the web: Evidence from an fMRI study. Brain Imaging Behav. 2020, 14, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Meshi, D.; Morawetz, C.; Heekeren, H.R. Nucleus accumbens response to gains in reputation for the self relative to gains for others predicts social media use. Front. Hum. Neurosci. 2013, 7, 439. [Google Scholar] [CrossRef]
- Sherman, L.E.; Payton, A.A.; Hernandez, L.M.; Greenfield, P.M.; Dapretto, M. The power of the like in adolescence: Effects of peer influence on neural and behavioral responses to social media. Psychol. Sci. 2016, 27, 1027–1035. [Google Scholar] [CrossRef]
- Sherman, L.E.; Greenfield, P.M.; Hernandez, L.M.; Dapretto, M. Peer Influence Via Instagram: Effects on Brain and Behavior in Adolescence and Young Adulthood. Child Dev. 2018, 89, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Sherman, L.E.; Hernandez, L.M.; Greenfield, P.M.; Dapretto, M. What the brain ‘Likes’: Neural correlates of providing feedback on social media. Soc. Cogn. Affect. Neurosci. 2018, 13, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Nasser, N.S.; Sharifat, H.; Rashid, A.A.; Ab Hamid, S.; Rahim, E.A.; Loh, J.L.; Ching, S.M.; Hoo, F.K.; Ismail, S.I.F.; Tyagi, R.; et al. Cue-Reactivity Among Young Adults With Problematic Instagram Use in Response to Instagram-Themed Risky Behavior Cues: A Pilot fMRI Study. Front. Psychol. 2020, 11, 556060. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, S.J.; Ketcherside, A.; McQueeny, T.M.; Dunlop, J.P.; Filbey, F.M. The hyper-sentient addict: An exteroception model of addiction. Am. J. Drug Alcohol Abus. 2015, 41, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Wikman, P.; Moisala, M.; Ylinen, A.; Lindblom, J.; Leikas, S.; Salmela-Aro, K.; Lonka, K.; Güroğlu, B.; Alho, K. Brain Responses to Peer Feedback in Social Media Are Modulated by Valence in Late Adolescence. Front. Behav. Neurosci. 2022, 16, 790478. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhou, H.; Gong, L.Y.; Teng, B.Y.; Geng, F.J.; Hu, Y.Z. Viewing personalized video clips recommended by TikTok activates default mode network and ventral tegmental area. Neuroimage 2021, 237, 118136. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Zhou, H.; Wang, C.; Geng, F.; Hu, Y. Individualized video recommendation modulates functional connectivity between large scale networks. Hum. Brain Mapp. 2021, 42, 5288–5299. [Google Scholar] [CrossRef] [PubMed]
- Leménager, T.; Dieter, J.; Hill, H.; Hoffmann, S.; Reinhard, I.; Beutel, M.; Vollstädt-Klein, S.; Kiefer, F.; Mann, K. Exploring the Neural Basis of Avatar Identification in Pathological Internet Gamers and of Self-Reflection in Pathological Social Network Users. J. Behav. Addict. 2016, 5, 485–499. [Google Scholar] [CrossRef]
- Peters, S.; Van der Cruijsen, R.; van der Aar, L.; Spaans, J.; Becht, A.; Crone, E. Social media use and the not-so-imaginary audience: Behavioral and neural mechanisms underlying the influence on self-concept. Dev. Cogn. Neurosci. 2021, 48, 100921. [Google Scholar] [CrossRef] [PubMed]
- Wölfling, K.; Beutel, M.; Müller, K. Construction of a standardized clinical interview to assess internet addiction: First findings regarding the usefulness of AICA-C. J. Addict. Res. Ther. 2012, 6, 003. [Google Scholar]
- Ellison, N.B.; Steinfield, C.; Lampe, C. The benefits of Facebook “friends:” Social capital and college students’ use of online social network sites. J. Comput.-Mediat. Commun. 2007, 12, 1143–1168. [Google Scholar] [CrossRef]
- Young, K.S. Caught in the Net: How to Recognize the Signs of Internet Addiction—And a Winning Strategy for Recovery; John Wiley & Sons: Hoboken, NJ, USA, 1998. [Google Scholar]
- Gao, X.; Zhang, M.; Yang, Z.; Wen, M.; Huang, H.; Zheng, R.; Wang, W.; Wei, Y.; Cheng, J.; Han, S.; et al. Structural and Functional Brain Abnormalities in Internet Gaming Disorder and Attention-Deficit/Hyperactivity Disorder: A Comparative Meta-Analysis. Front. Psychiatry 2021, 12, 679437. [Google Scholar] [CrossRef]
- Salgado, S.; Kaplitt, M.G. The nucleus accumbens: A comprehensive review. Stereotact. Funct. Neurosurg. 2015, 93, 75–93. [Google Scholar] [CrossRef] [PubMed]
- David, S.P.; Munafò, M.R.; Johansen-Berg, H.; Smith, S.M.; Rogers, R.D.; Matthews, P.M.; Walton, R.T. Ventral striatum/nucleus accumbens activation to smoking-related pictorial cues in smokers and nonsmokers: A functional magnetic resonance imaging study. Biol. Psychiatry 2005, 58, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Kühn, S.; Gallinat, J. Common biology of craving across legal and illegal drugs—A quantitative meta-analysis of cue-reactivity brain response. Eur. J. Neurosci. 2011, 33, 1318–1326. [Google Scholar] [CrossRef]
- Makris, N.; Oscar-Berman, M.; Jaffin, S.K.; Hodge, S.M.; Kennedy, D.N.; Caviness, V.S.; Marinkovic, K.; Breiter, H.C.; Gasic, G.P.; Harris, G.J. Decreased volume of the brain reward system in alcoholism. Biol. Psychiatry 2008, 64, 192–202. [Google Scholar] [CrossRef]
- Seifert, C.L.; Magon, S.; Sprenger, T.; Lang, U.E.; Huber, C.G.; Denier, N.; Vogel, M.; Schmidt, A.; Radue, E.-W.; Borgwardt, S. Reduced volume of the nucleus accumbens in heroin addiction. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 637–645. [Google Scholar] [CrossRef]
- Siddiqui, S.V.; Chatterjee, U.; Kumar, D.; Siddiqui, A.; Goyal, N. Neuropsychology of prefrontal cortex. Indian J. Psychiatry 2008, 50, 202. [Google Scholar]
- Kringelbach, M.L. The human orbitofrontal cortex: Linking reward to hedonic experience. Nat. Rev. Neurosci. 2005, 6, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Z.; Volkow, N.D. Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nat. Rev. Neurosci. 2011, 12, 652–669. [Google Scholar] [CrossRef] [PubMed]
- Crunelle, C.L.; Kaag, A.M.; Van Wingen, G.; van den Munkhof, H.E.; Homberg, J.R.; Reneman, L.; Van Den Brink, W. Reduced frontal brain volume in non-treatment-seeking cocaine-dependent individuals: Exploring the role of impulsivity, depression, and smoking. Front. Hum. Neurosci. 2014, 8, 7. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Matochik, J.A.; Cadet, J.-L.; London, E.D. Smaller volume of prefrontal lobe in polysubstance abusers: A magnetic resonance imaging study. Neuropsychopharmacology 1998, 18, 243–252. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Sullivan, E.V.; Rosenbloom, M.J.; Mathalon, D.H.; Lim, K.O. A controlled study of cortical gray matter and ventricular changes in alcoholic men over a 5-year interval. Arch. Gen. Psychiatry 1998, 55, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Odlaug, B.L.; Chamberlain, S.R. Reduced cortical thickness in gambling disorder: A morphometric MRI study. Eur. Arch. Psychiatry Clin. Neurosci. 2015, 265, 655–661. [Google Scholar] [CrossRef] [PubMed]
- Koehler, S.; Hasselmann, E.; Wüstenberg, T.; Heinz, A.; Romanczuk-Seiferth, N. Higher volume of ventral striatum and right prefrontal cortex in pathological gambling. Brain Struct. Funct. 2015, 220, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Brain stress systems in the amygdala and addiction. Brain Res. 2009, 1293, 61–75. [Google Scholar] [CrossRef]
- Chase, H.W.; Eickhoff, S.B.; Laird, A.R.; Hogarth, L. The neural basis of drug stimulus processing and craving: An activation likelihood estimation meta-analysis. Biol. Psychiatry 2011, 70, 785–793. [Google Scholar] [CrossRef]
- Warlow, S.M.; Robinson, M.J.; Berridge, K.C. Optogenetic central amygdala stimulation intensifies and narrows motivation for cocaine. J. Neurosci. 2017, 37, 8330–8348. [Google Scholar] [CrossRef] [PubMed]
- Gilman, J.M.; Kuster, J.K.; Lee, S.; Lee, M.J.; Kim, B.W.; Makris, N.; van der Kouwe, A.; Blood, A.J.; Breiter, H.C. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J. Neurosci. 2014, 34, 5529–5538. [Google Scholar] [CrossRef] [PubMed]
- Padula, C.B.; McQueeny, T.; Lisdahl, K.M.; Price, J.S.; Tapert, S.F. Craving is associated with amygdala volumes in adolescent marijuana users during abstinence. Am. J. Drug Alcohol Abus. 2015, 41, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Wrase, J.; Makris, N.; Braus, D.F.; Mann, K.; Smolka, M.N.; Kennedy, D.N.; Caviness, V.S.; Hodge, S.M.; Tang, L.; Albaugh, M. Amygdala volume associated with alcohol abuse relapse and craving. Am. J. Psychiatry 2008, 165, 1179–1184. [Google Scholar] [CrossRef]
- Zakiniaeiz, Y.; Scheinost, D.; Seo, D.; Sinha, R.; Constable, R.T. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. NeuroImage Clin. 2017, 13, 181–187. [Google Scholar] [CrossRef]
- Zhao, Y.; Sallie, S.N.; Cui, H.; Zeng, N.; Du, J.; Yuan, T.; Li, D.; De Ridder, D.; Zhang, C. Anterior cingulate cortex in addiction: New insights for neuromodulation. Neuromodul. Technol. Neural Interface 2021, 24, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Fritz, H.-C.; Wittfeld, K.; Schmidt, C.O.; Domin, M.; Grabe, H.J.; Hegenscheid, K.; Hosten, N.; Lotze, M. Current smoking and reduced gray matter volume—A voxel-based morphometry study. Neuropsychopharmacology 2014, 39, 2594–2600. [Google Scholar] [CrossRef]
- Yang, X.; Tian, F.; Zhang, H.; Zeng, J.; Chen, T.; Wang, S.; Jia, Z.; Gong, Q. Cortical and subcortical gray matter shrinkage in alcohol-use disorders: A voxel-based meta-analysis. Neurosci. Biobehav. Rev. 2016, 66, 92–103. [Google Scholar] [CrossRef]
- Wang, H.; Jin, C.; Yuan, K.; Shakir, T.M.; Mao, C.; Niu, X.; Niu, C.; Guo, L.; Zhang, M. The alteration of gray matter volume and cognitive control in adolescents with internet gaming disorder. Front. Behav. Neurosci. 2015, 9, 64. [Google Scholar] [CrossRef]
- Luijten, M.; Machielsen, M.W.; Veltman, D.J.; Hester, R.; de Haan, L.; Franken, I.H. Systematic review of ERP and fMRI studies investigating inhibitory control and error processing in people with substance dependence and behavioural addictions. J. Psychiatry Neurosci. 2014, 39, 149–169. [Google Scholar] [CrossRef]
- Leong, S.L.; Glue, P.; Manning, P.; Vanneste, S.; Lim, L.J.; Mohan, A.; De Ridder, D. Anterior cingulate cortex implants for alcohol addiction: A feasibility study. Neurotherapeutics 2020, 17, 1287–1299. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The cingulate cortex and limbic systems for emotion, action, and memory. Brain Struct. Funct. 2019, 224, 3001–3018. [Google Scholar] [CrossRef] [PubMed]
- Ames, S.L.; Wong, S.W.; Bechara, A.; Cappelli, C.; Dust, M.; Grenard, J.L.; Stacy, A.W. Neural correlates of a Go/NoGo task with alcohol stimuli in light and heavy young drinkers. Behav. Brain Res. 2014, 274, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Van Holst, R.J.; van Holstein, M.; van Den Brink, W.; Veltman, D.J.; Goudriaan, A.E. Response inhibition during cue reactivity in problem gamblers: An fMRI study. PLoS ONE 2012, 7, e30909. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The brain’s default mode network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef]
- Zhang, R.; Volkow, N.D. Brain default-mode network dysfunction in addiction. Neuroimage 2019, 200, 313–331. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Trimble, M.R. The precuneus: A review of its functional anatomy and behavioural correlates. Brain 2006, 129, 564–583. [Google Scholar] [CrossRef]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef]
- Schacht, J.P.; Anton, R.F.; Myrick, H. Functional neuroimaging studies of alcohol cue reactivity: A quantitative meta-analysis and systematic review. Addict. Biol. 2013, 18, 121–133. [Google Scholar] [CrossRef]
- Starcke, K.; Antons, S.; Trotzke, P.; Brand, M. Cue-reactivity in behavioral addictions: A meta-analysis and methodological considerations. J. Behav. Addict. 2018, 7, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.D.; Frith, U. The neural basis of mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef]
- Dean, S.F.; Fede, S.J.; Diazgranados, N.; Momenan, R. Addiction neurocircuitry and negative affect: A role for neuroticism in understanding amygdala connectivity and alcohol use disorder. Neurosci. Lett. 2020, 722, 134773. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ma, Y.; Zhuang, K.; Chen, Q.; Shi, B.; Qiu, J. Linking temporal-parietal junction to internet addiction tendency: Moderating effect of critical thinking. J. Behav. Addict. 2021, 10, 759–766. [Google Scholar] [CrossRef]
- Gennatas, E.D.; Avants, B.B.; Wolf, D.H.; Satterthwaite, T.D.; Ruparel, K.; Ciric, R.; Hakonarson, H.; Gur, R.E.; Gur, R.C. Age-related effects and sex differences in gray matter density, volume, mass, and cortical thickness from childhood to young adulthood. J. Neurosci. 2017, 37, 5065–5073. [Google Scholar] [CrossRef]
- Pehlivanova, M.; Wolf, D.H.; Sotiras, A.; Kaczkurkin, A.N.; Moore, T.M.; Ciric, R.; Cook, P.A.; de La Garza, A.G.; Rosen, A.F.; Ruparel, K. Diminished cortical thickness is associated with impulsive choice in adolescence. J. Neurosci. 2018, 38, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Gardini, S.; Venneri, A. Reduced grey matter in the posterior insula as a structural vulnerability or diathesis to addiction. Brain Res. Bull. 2012, 87, 205–211. [Google Scholar] [CrossRef]
- Naqvi, N.H.; Rudrauf, D.; Damasio, H.; Bechara, A. Damage to the insula disrupts addiction to cigarette smoking. Science 2007, 315, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Janes, A.; Gilman, J.; Radoman, M.; Pachas, G.; Fava, M.; Evins, A. Revisiting the role of the insula and smoking cue-reactivity in relapse: A replication and extension of neuroimaging findings. Drug Alcohol Depend. 2017, 179, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Brand, M.; Rumpf, H.-J.; King, D.L.; Potenza, M.N.; Wegmann, E. Clarifying terminologies in research on gaming disorder and other addictive behaviors: Distinctions between core symptoms and underlying psychological processes. Curr. Opin. Psychol. 2020, 36, 49–54. [Google Scholar] [CrossRef]
- Kuss, D.J.; Griffiths, M.D.; Pontes, H.M. Chaos and confusion in DSM-5 diagnosis of Internet Gaming Disorder: Issues, concerns, and recommendations for clarity in the field. J. Behav. Addict. 2017, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Burnell, K.; George, M.J.; Kurup, A.R.; Underwood, M.K.; Ackerman, R.A. Associations between self-reports and device-reports of social networking site use: An application of the truth and bias model. Commun. Methods Meas. 2021, 15, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Ernala, S.K.; Burke, M.; Leavitt, A.; Ellison, N.B. How well do people report time spent on Facebook? An evaluation of established survey questions with recommendations. In Proceedings of the 2020 CHI Conference on Human Factors in Computing Systems, Honolulu, HI, USA, 25–30 April 2020; pp. 1–14. [Google Scholar]
- Shannon, H.; Bush, K.; Villeneuve, P.J.; Hellemans, K.G.; Guimond, S. Problematic Social Media Use in Adolescents and Young Adults: Systematic Review and Meta-analysis. JMIR Ment. Health 2022, 9, e33450. [Google Scholar] [CrossRef] [PubMed]
- Moccia, L.; Pettorruso, M.; De Crescenzo, F.; De Risio, L.; Di Nuzzo, L.; Martinotti, G.; Bifone, A.; Janiri, L.; Di Nicola, M. Neural correlates of cognitive control in gambling disorder: A systematic review of fMRI studies. Neurosci. Biobehav. Rev. 2017, 78, 104–116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).