Agomelatine: A Potential Multitarget Compound for Neurodevelopmental Disorders
Abstract
1. Introduction
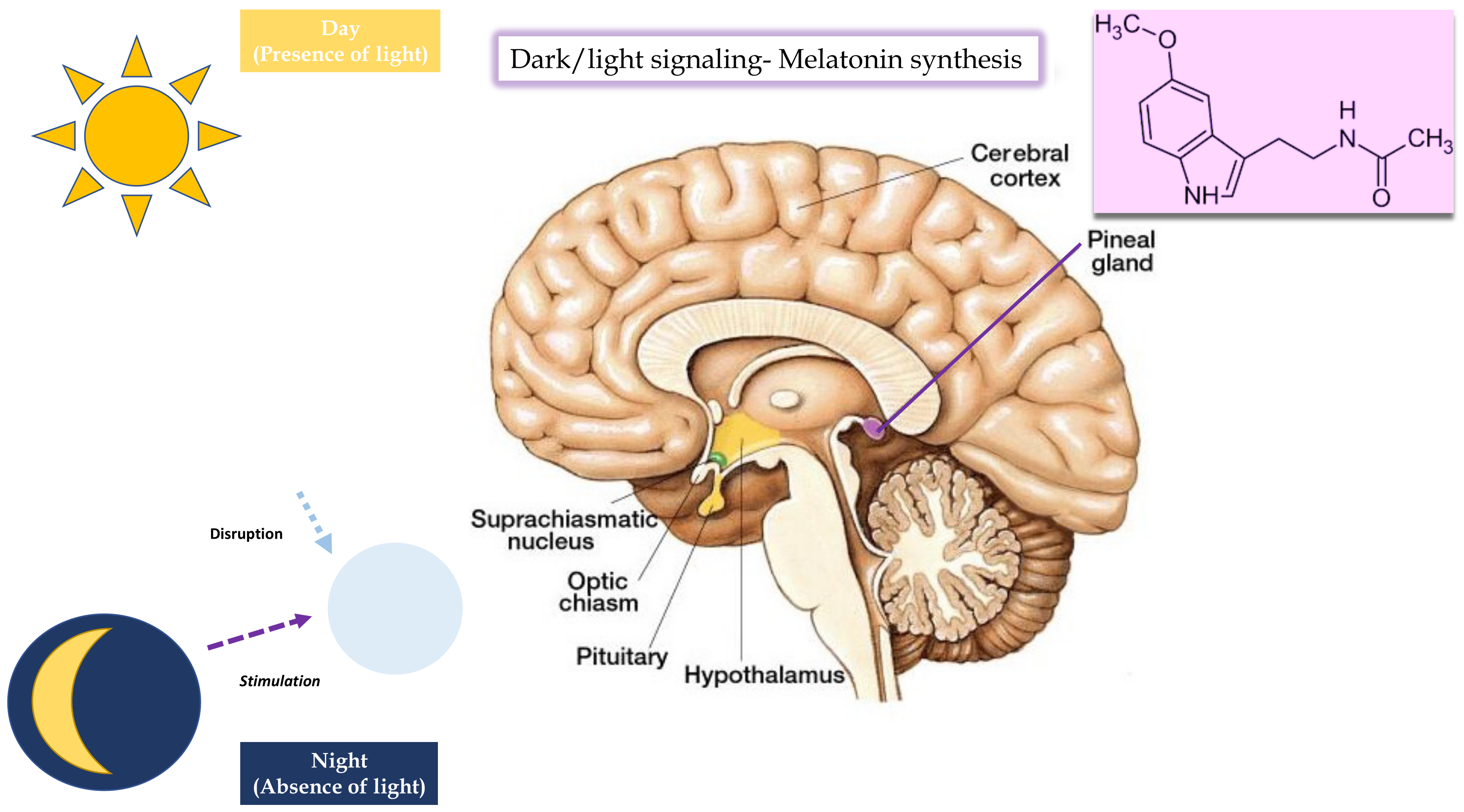
AGM Pharmacology
2. Clinical Use of Agomelatine in ADHD and ASD
2.1. ADHD
2.2. ASD
3. Potential Effects of AGM in Neurodevelopmental Disorders
3.1. Melatoninergic Action
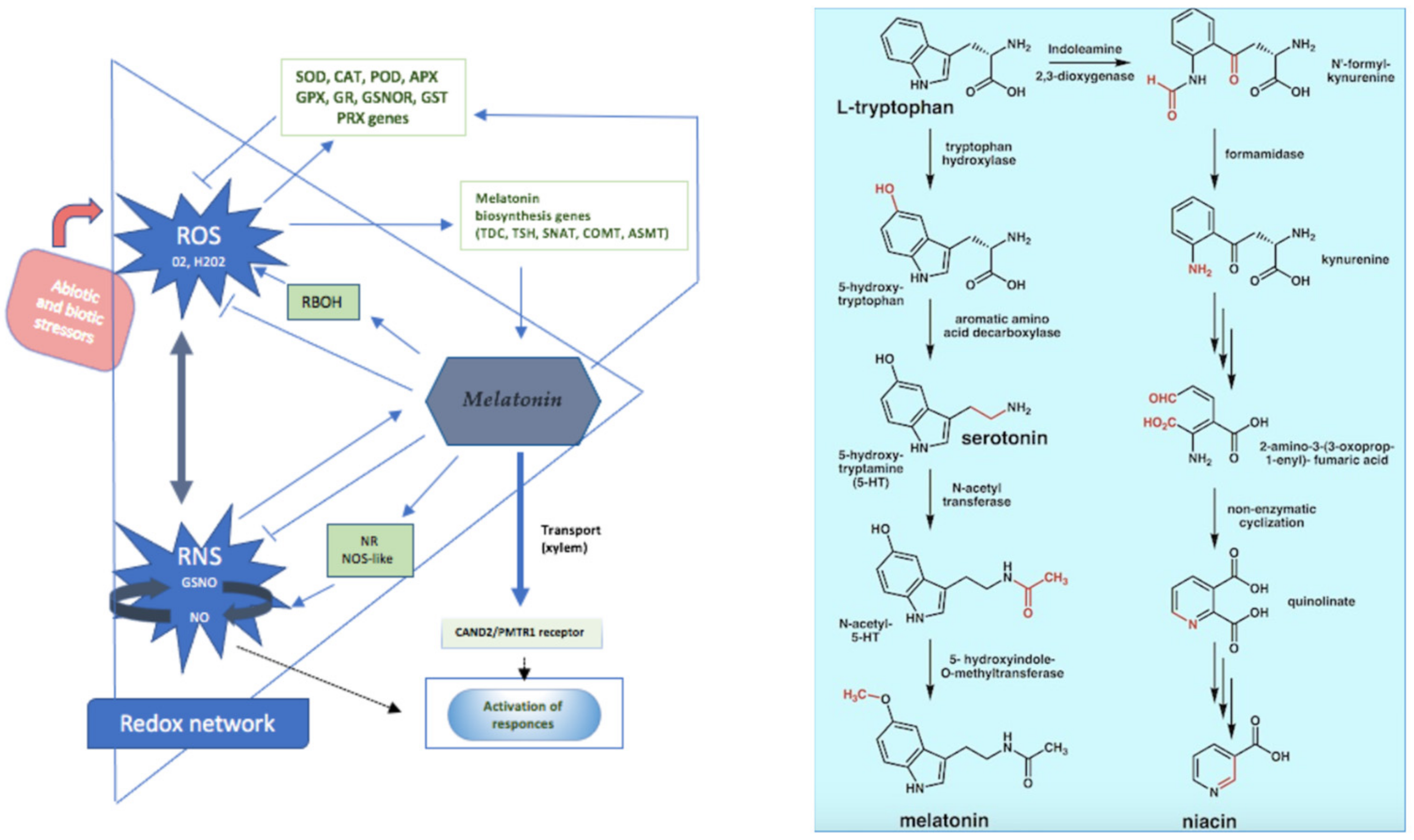
3.2. Anti-Inflammatory and Oxidative Stress Action
3.3. Neurotrophic, Anti-Glutamatergic, and Anxiolytic Actions
3.4. 5-HT2C Antagonist Action
4. Potential Therapeutic Role of AGM against SARS-CoV-2
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Bodinat, C.; Guardiola-Lemaitre, B.; Mocaër, E.; Renard, P.; Muñoz, C.; Millan, M.J. AGM, the First Melatonergic Antidepressant: Discovery, Characterization and Development. Nat. Rev. Drug Discov. 2010, 9, 628–642. [Google Scholar] [CrossRef] [PubMed]
- Buoli, M.; Grassi, S.; Serati, M.; Altamura, A.C. AGM for the Treatment of Generalized Anxiety Disorder. Expert Opin. Pharmacother. 2017, 18, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Pae, C.-U. AGM: A New Option for Treatment of Depression? Expert Opin. Pharmacother. 2014, 15, 443–447. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Conti, C.M.; Marini, S.; Ferri, F.; Iasevoli, F.; Valchera, A.; Fornaro, M.; Cavuto, M.; Srinivasan, V.; Perna, G.; et al. Is There a Role for AGM in the Treatment of Anxiety Disorders? A Review of Published Data. Int. J. Immunopathol. Pharmacol. 2013, 26, 299–304. [Google Scholar] [CrossRef] [PubMed]
- De Berardis, D.; Fornaro, M.; Serroni, N.; Campanella, D.; Rapini, G.; Olivieri, L.; Srinivasan, V.; Iasevoli, F.; Tomasetti, C.; De Bartolomeis, A.; et al. AGM beyond Borders: Current Evidences of Its Efficacy in Disorders Other than Major Depression. Int. J. Mol. Sci. 2015, 16, 1111–1130. [Google Scholar] [CrossRef]
- Fornaro, M.; McCarthy, M.J.; De Berardis, D.; De Pasquale, C.; Tabaton, M.; Martino, M.; Colicchio, S.; Cattaneo, C.I.; D’Angelo, E.; Fornaro, P. Adjunctive AGM Therapy in the Treatment of Acute Bipolar II Depression: A Preliminary Open Label Study. Neuropsychiatr. Dis. Treat. 2013, 9, 243–251. [Google Scholar] [CrossRef]
- Gahr, M. AGM in the Treatment of Major Depressive Disorder: An Assessment of Benefits and Risks. Curr. Neuropharmacol. 2014, 12, 287–398. [Google Scholar] [CrossRef]
- Millan, M.J.; Gobert, A.; Lejeune, F.; Dekeyne, A.; Newman-Tancredi, A.; Pasteau, V.; Rivet, J.-M.; Cussac, D. The Novel Melatonin Agonist AGM (S20098) Is an Antagonist at 5-Hydroxytryptamine2C Receptors, Blockade of Which Enhances the Activity of Frontocortical Dopaminergic and Adrenergic Pathways. J. Pharmacol. Exp. Ther. 2003, 306, 954–964. [Google Scholar] [CrossRef]
- Sansone, R.A.; Sansone, L.A. AGM: A Novel Antidepressant. Innov. Clin. Neurosci. 2011, 8, 10–14. [Google Scholar]
- Cipriani, A.; Furukawa, T.A.; Salanti, G.; Chaimani, A.; Atkinson, L.Z.; Ogawa, Y.; Leucht, S.; Ruhe, H.G.; Turner, E.H.; Higgins, J.P.T.; et al. Comparative Efficacy and Acceptability of 21 Antidepressant Drugs for the Acute Treatment of Adults with Major Depressive Disorder: A Systematic Review and Network Meta-Analysis. Lancet 2018, 391, 1357–1366. [Google Scholar] [CrossRef]
- Potměšil, P. What Combinations of AGM with Other Antidepressants Could Be Successful during the Treatment of Major Depressive Disorder or Anxiety Disorders in Clinical Practice? Ther. Adv. Psychopharmacol. 2019, 9, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.E.; Goodman, M.H. A Systematic Review of Case Studies Testing a Melatonergic Agonist/ 5HT2c Antagonist for Individuals with Obsessive Compulsive Disorder. J. Anxiety Disord. 2020, 69, 102173. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J.; Ahokas, A.; Jarema, M.; Avedisova, A.S.; Vavrusova, L.; Chaban, O.; Gruget, C.; Olivier, V.; Picarel-Blanchot, F.; de Bodinat, C. Efficacy and Safety of AGM (10 or 25 Mg/Day) in Non-Depressed out-Patients with Generalized Anxiety Disorder: A 12-Week, Double- Blind, Placebo-Controlled Study. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2017, 27, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Stein, D.J.; Khoo, J.-P.; Picarel-Blanchot, F.; Olivier, V.; Van Ameringen, M. Efficacy of AGM 25–50 Mg for the Treatment of Anxious Symptoms and Functional Impairment in Generalized Anxiety Disorder: A Meta-Analysis of Three Placebo-Controlled Studies. Adv. Ther. 2021, 38, 1567–1583. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Deng, H.; Wan, J.; Zhou, Y.; Zhou, Y.; Song, B.; Wang, X. Comparative Remission Rates and Tolerability of Drugs for Generalised Anxiety Disorder: A Systematic Review and Network Meta-Analysis of Double-Blind Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 580858. [Google Scholar] [CrossRef]
- Freiesleben, S.D.; Furczyk, K. A Systematic Review of AGM-Induced Liver Injury. J. Mol. Psychiatry 2015, 3, 4. [Google Scholar] [CrossRef]
- Perlemuter, G.; Cacoub, P.; Valla, D.; Guyader, D.; Saba, B.; Batailler, C.; Moore, K. Characterisation of AGM-Induced Increase in Liver Enzymes: Frequency and Risk Factors Determined from a Pooled Analysis of 7605 Treated Patients. CNS Drugs 2016, 30, 877–888. [Google Scholar] [CrossRef]
- Procedure No. EMEA/H/C/000916. Ema/97539/2009. Available online: https://www.ema.europa.eu/en/documents/assessment-report/thymanax-epar-public-assessment-report_en.pdf (accessed on 5 March 2023).
- Goodwin, G.M.; Emsley, R.; Rembry, S.; Rouillon, F.; AGM Study Group. AGM Prevents Relapse in Patients with Major Depressive Disorder without Evidence of a Discontinuation Syndrome: A 24-Week Randomized, Double-Blind, Placebo-Controlled Trial. J. Clin. Psychiatry 2009, 70, 1128–1137. [Google Scholar] [CrossRef]
- Arango, C.; Buitelaar, J.K.; Fegert, J.M.; Olivier, V.; Pénélaud, P.-F.; Marx, U.; Chimits, D.; Falissard, B.; Barylnik, J.; Birdeanu, L.; et al. Safety and Efficacy of AGM in Children and Adolescents with Major Depressive Disorder Receiving Psychosocial Counselling: A Double-Blind, Randomised, Controlled, Phase 3 Trial in Nine Countries. Lancet Psychiatry 2022, 9, 113–124. [Google Scholar] [CrossRef]
- Niederhofer, H. AGM Treatment with Adolescents with ADHD. J. Atten. Disord. 2012, 16, 530–532. [Google Scholar] [CrossRef]
- Salardini, E.; Zeinoddini, A.; Kohi, A.; Mohammadi, M.-R.; Mohammadinejad, P.; Khiabany, M.; Shahriari, M.; Akhondzadeh, S. AGM as a Treatment for Attention- Deficit/Hyperactivity Disorder in Children and Adolescents: A Double-Blind, Randomized Clinical Trial. J. Child Adolesc. Psychopharmacol. 2016, 26, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Haya Al Mutairi, A.N. AGM Augmenting Partial Stimulant Response in ADHD and Mitigating Stimulant-Induced Insomnia and Anxiety. J. Child Adolesc. Behav. 2015, 3, 1000208. [Google Scholar] [CrossRef]
- Naguy, A.; Alamiri, B. Successful AGM Monotherapy for an Adolescent with Attention Deficit Hyperactivity Disorder and Comorbid Migraine. CNS Spectr. 2020, 27, 134–135. [Google Scholar] [CrossRef] [PubMed]
- Purper-Ouakil, D.; Ramoz, N.; Lepagnol-Bestel, A.-M.; Gorwood, P.; Simonneau, M. Neurobiology of Attention Deficit/Hyperactivity Disorder. Pediatr. Res. 2011, 69, 69R–76R. [Google Scholar] [CrossRef] [PubMed]
- Curatolo, P.; Paloscia, C.; D’Agati, E.; Moavero, R.; Pasini, A. The Neurobiology of Attention Deficit/Hyperactivity Disorder. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2009, 13, 299–304. [Google Scholar] [CrossRef]
- Niederhofer, H. Efficacy of Duloxetine and AGM Does Not Exceed That of Other Antidepressants in Patients with Autistic Disorder: Preliminary Results in 3 Patients. Prim. Care Companion CNS Disord. 2011, 13, PCC.10l01038. [Google Scholar] [CrossRef]
- Naguy, A.; Tajali, A.A. AGM Addressing Behavioural Facets in Autism. J. Psychiatry 2015, 18. [Google Scholar] [CrossRef]
- Ballester, P.; Martínez, M.J.; Inda, M.-M.; Javaloyes, A.; Richdale, A.L.; Muriel, J.; Belda, C.; Toral, N.; Morales, D.; Fernández, E.; et al. Evaluation of AGM for the Treatment of Sleep Problems in Adults with Autism Spectrum Disorder and Co-Morbid Intellectual Disability. J. Psychopharmacol. 2019, 33, 1395–1406. [Google Scholar] [CrossRef]
- Ballester, P.; Martínez, M.J.; Javaloyes, A.; Hernández, L.; Peiró, A.M. Agomelatine Effectiveness in Sleep Disturbances in Autism Spectrum Disorder. Clin. Ther. 2015, 37, e132–e133. [Google Scholar] [CrossRef]
- Kumar, H.; Sharma, B.M.; Sharma, B. Benefits of AGM in Behavioral, Neurochemical and Blood Brain Barrier Alterations in Prenatal Valproic Acid Induced Autism Spectrum Disorder. Neurochem. Int. 2015, 91, 34–45. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- White, S.W.; Roberson-Nay, R. Anxiety, Social Deficits, and Loneliness in Youth with Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, K.; Godbout, R. Melatonin and Comorbidities in Children with Autism Spectrum Disorder. Curr. Dev. Disord. Rep. 2018, 5, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Hollocks, M.J.; Lerh, J.W.; Magiati, I.; Meiser-Stedman, R.; Brugha, T.S. Anxiety and Depression in Adults with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Psychol. Med. 2019, 49, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Gentile, I.; Zappulo, E.; Militerni, R.; Pascotto, A.; Borgia, G.; Bravaccio, C. Etiopathogenesis of Autism Spectrum Disorders: Fitting the Pieces of the Puzzle Together. Med. Hypotheses 2013, 81, 26–35. [Google Scholar] [CrossRef]
- Giana, G.; Romano, E.; Porfirio, M.C.; D’Ambrosio, R.; Giovinazzo, S.; Troianiello, M.; Barlocci, E.; Travaglini, D.; Granstrem, O.; Pascale, E.; et al. Detection of Auto-Antibodies to DAT in the Serum: Interactions with DAT Genotype and Psycho-Stimulant Therapy for ADHD. J. Neuroimmunol. 2015, 278, 212–222. [Google Scholar] [CrossRef]
- Santos, P.; Herrmann, A.P.; Elisabetsky, E.; Piato, A. Anxiolytic Properties of Compounds That Counteract Oxidative Stress, Neuroinflammation, and Glutamatergic Dysfunction: A Review. Rev. Bras. Psiquiatr. Sao Paulo Braz. 1999 2019, 41, 168–178. [Google Scholar] [CrossRef]
- Savino, R.; Carotenuto, M.; Polito, A.N.; Di Noia, S.; Albenzio, M.; Scarinci, A.; Ambrosi, A.; Sessa, F.; Tartaglia, N.; Messina, G. Analyzing the Potential Biological Determinants of Autism Spectrum Disorder: From Neuroinflammation to the Kynurenine Pathway. Brain Sci. 2020, 10, 631. [Google Scholar] [CrossRef]
- Frye, R.E.; Delatorre, R.; Taylor, H.; Slattery, J.; Melnyk, S.; Chowdhury, N.; James, S.J. Redox Metabolism Abnormalities in Autistic Children Associated with Mitochondrial Disease. Transl. Psychiatry 2013, 3, e273. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Melatonin in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis: Review. Dev. Med. Child Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.; Klempin, F.; Babu, H.; Benítez-King, G.; Kempermann, G. Melatonin Modulates Cell Survival of New Neurons in the Hippocampus of Adult Mice. Neuropsychopharmacology 2009, 34, 2180–2191. [Google Scholar] [CrossRef]
- Lacivita, E.; Perrone, R.; Margari, L.; Leopoldo, M. Targets for Drug Therapy for Autism Spectrum Disorder: Challenges and Future Directions. J. Med. Chem. 2017, 60, 9114–9141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Couture, J. A Review of the Pathophysiology, Etiology, and Treatment of Attention-Deficit Hyperactivity Disorder (ADHD). Ann. Pharmacother. 2014, 48, 209–225. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.J.; Coffey, B.J. Attention-Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment, 3rd Ed. J. Clin. Psychiatry 2008, 69, 1023. [Google Scholar] [CrossRef]
- Cortese, S.; Brown, T.E.; Corkum, P.; Gruber, R.; O’Brien, L.M.; Stein, M.; Weiss, M.; Owens, J. Assessment and Management of Sleep Problems in Youths with Attention- Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 784–796. [Google Scholar] [CrossRef] [PubMed]
- on behalf of Lombardy ADHD Group; Reale, L.; Bartoli, B.; Cartabia, M.; Zanetti, M.; Costantino, M.A.; Canevini, M.P.; Termine, C.; Bonati, M. Comorbidity Prevalence and Treatment Outcome in Children and Adolescents with ADHD. Eur. Child Adolesc. Psychiatry 2017, 26, 1443–1457. [Google Scholar] [CrossRef]
- Andrade, C. Stahl′s Essential Psychopharmacology: Neuroscientific Basis and Practical Applications. Mens Sana Monogr. 2010, 8, 146. [Google Scholar] [CrossRef]
- Maenner, M.J.; Shaw, K.A.; Baio, J.; Washington, A.; Patrick, M.; DiRienzo, M.; Christensen, D.L.; Wiggins, L.D.; Pettygrove, S. Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2016. MMWR Surveill. Summ. 2020, 69, 1–12. [Google Scholar] [CrossRef]
- DeFilippis, M.; Wagner, K.D. Management of Treatment-Resistant Depression in Children and Adolescents. Paediatr. Drugs 2014, 16, 353–361. [Google Scholar] [CrossRef]
- Goodyer, I.M. Editorial Perspective: Antidepressants and the Depressed Adolescent. Child Adolesc. Ment. Health 2018, 23, 137–140. [Google Scholar] [CrossRef]
- Merikangas, K.R.; He, J.-P.; Burstein, M.; Swanson, S.A.; Avenevoli, S.; Cui, L.; Benjet, C.; Georgiades, K.; Swendsen, J. Lifetime Prevalence of Mental Disorders in U.S. Adolescents: Results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 980–989. [Google Scholar] [CrossRef]
- Amray, A.N.; Munir, K.; Jahan, N.; Motiwala, F.B.; Naveed, S. Psychopharmacology of Pediatric Anxiety Disorders: A Narrative Review. Cureus 2019, 11, e5487. [Google Scholar] [CrossRef] [PubMed]
- Kessler, R.C.; Petukhova, M.; Sampson, N.A.; Zaslavsky, A.M.; Wittchen, H.-U. Twelve-Month and Lifetime Prevalence and Lifetime Morbid Risk of Anxiety and Mood Disorders in the United States. Int. J. Methods Psychiatr. Res. 2012, 21, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Blackburn, T.P. Depressive Disorders: Treatment Failures and Poor Prognosis over the Last 50 Years. Pharmacol. Res. Perspect. 2019, 7, e00472. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Nanovska, S.; Regen, W.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Reynolds, C.F.; Riemann, D. Sleep and Mental Disorders: A Meta-Analysis of Polysomnographic Research. Psychol. Bull. 2016, 142, 969–990. [Google Scholar] [CrossRef]
- Landmann, N.; Kuhn, M.; Piosczyk, H.; Feige, B.; Baglioni, C.; Spiegelhalder, K.; Frase, L.; Riemann, D.; Sterr, A.; Nissen, C. The Reorganisation of Memory during Sleep. Sleep Med. Rev. 2014, 18, 531–541. [Google Scholar] [CrossRef]
- Landmann, N.; Kuhn, M.; Maier, J.-G.; Spiegelhalder, K.; Baglioni, C.; Frase, L.; Riemann, D.; Sterr, A.; Nissen, C. REM Sleep and Memory Reorganization: Potential Relevance for Psychiatry and Psychotherapy. Neurobiol. Learn. Mem. 2015, 122, 28–40. [Google Scholar] [CrossRef]
- Gordon, A.M.; Chen, S. The Role of Sleep in Interpersonal Conflict: Do Sleepless Nights Mean Worse Fights? Soc. Psychol. Personal. Sci. 2014, 5, 168–175. [Google Scholar] [CrossRef]
- Guadagni, V.; Burles, F.; Ferrara, M.; Iaria, G. The Effects of Sleep Deprivation on Emotional Empathy. J. Sleep Res. 2014, 23, 657–663. [Google Scholar] [CrossRef]
- Harvey, A.G.; Murray, G.; Chandler, R.A.; Soehner, A. Sleep Disturbance as Transdiagnostic: Consideration of Neurobiological Mechanisms. Clin. Psychol. Rev. 2011, 31, 225–235. [Google Scholar] [CrossRef]
- Harvey, A.G. A Transdiagnostic Approach to Treating Sleep Disturbance in Psychiatric Disorders. Cogn. Behav. Ther. 2009, 38 (Suppl. 1), 35–42. [Google Scholar] [CrossRef]
- Marotta, R.; Risoleo, M.C.; Messina, G.; Parisi, L.; Carotenuto, M.; Vetri, L.; Roccella, M. The Neurochemistry of Autism. Brain Sci. 2020, 10, 163. [Google Scholar] [CrossRef] [PubMed]
- Alfano, C.A.; Beidel, D.C.; Turner, S.M.; Lewin, D.S. Preliminary Evidence for Sleep Complaints among Children Referred for Anxiety. Sleep Med. 2006, 7, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Ritvo, E.R.; Ritvo, R.; Yuwiler, A.; Brothers, A.; Freeman, B.J.; Plotkin, S. Elevated Daytime Melatonin Concentrations in Autism: A Pilot Study. Eur. Child Adolesc. Psychiatry 1993, 2, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Laino, D.; D’Alonzo, R.; Mencarelli, A.; Di Genova, L.; Fattorusso, A.; Argentiero, A.; Mencaroni, E. Pediatric Sleep Disturbances and Treatment with Melatonin. J. Transl. Med. 2019, 17, 77. [Google Scholar] [CrossRef] [PubMed]
- Lalanne, S.; Fougerou-Leurent, C.; Anderson, G.M.; Schroder, C.M.; Nir, T.; Chokron, S.; Delorme, R.; Claustrat, B.; Bellissant, E.; Kermarrec, S.; et al. Melatonin: From Pharmacokinetics to Clinical Use in Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 1490. [Google Scholar] [CrossRef] [PubMed]
- Grivas, T.B.; Savvidou, O.D. Melatonin the “Light of Night” in Human Biology and Adolescent Idiopathic Scoliosis. Scoliosis 2007, 2, 6. [Google Scholar] [CrossRef]
- Sadeh, A. Sleep and Melatonin in Infants: A Preliminary Study. Sleep 1997, 20, 185–191. [Google Scholar]
- Rossignol, D.A.; Frye, R.E. Mitochondrial Dysfunction in Autism Spectrum Disorders: A Systematic Review and Meta-Analysis. Mol. Psychiatry 2012, 17, 290–314. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.; Maes, M. Interactions of Tryptophan and Its Catabolites with Melatonin and the Alpha 7 Nicotinic Receptor in Central Nervous System and Psychiatric Disorders: Role of the Aryl Hydrocarbon Receptor and Direct Mitochondria Regulation. Int. J. Tryptophan Res. IJTR 2017, 10, 1178646917691738. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Stone, T.W. The Kynurenine Pathway and the Brain: Challenges, Controversies and Promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-Kynurenine Pathway Enzymes Are Therapeutic Target for Neuropsychiatric Diseases: Focus on Cell Type Differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Wigner, P.; Synowiec, E.; Jóźwiak, P.; Czarny, P.; Bijak, M.; Barszczewska, G.; Białek, K.; Szemraj, J.; Gruca, P.; Papp, M.; et al. The Changes of Expression and Methylation of Genes Involved in Oxidative Stress in Course of Chronic Mild Stress and Antidepressant Therapy with AGM. Genes 2020, 11, 644. [Google Scholar] [CrossRef] [PubMed]
- Wigner, P.; Czarny, P.; Synowiec, E.; Bijak, M.; Talarowska, M.; Galecki, P.; Szemraj, J.; Sliwinski, T. Variation of Genes Encoding KAT1, AADAT and IDO1 as a Potential Risk of Depression Development. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2018, 52, 95–103. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Redox Regulation and the Autistic Spectrum: Role of Tryptophan Catabolites, Immuno-Inflammation, Autoimmunity and the Amygdala. Curr. Neuropharmacol. 2014, 12, 148–167. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.F. Quest for Biomarkers of Treatment-Resistant Depression: Shifting the Paradigm toward Risk. Front. Psychiatry 2013, 4, 57. [Google Scholar] [CrossRef]
- Zhang, X.; Gainetdinov, R.R.; Beaulieu, J.-M.; Sotnikova, T.D.; Burch, L.H.; Williams, R.B.; Schwartz, D.A.; Krishnan, K.R.R.; Caron, M.G. Loss-of-Function Mutation in Tryptophan Hydroxylase-2 Identified in Unipolar Major Depression. Neuron 2005, 45, 11–16. [Google Scholar] [CrossRef]
- Chumboatong, W.; Khamchai, S.; Tocharus, C.; Govitrapong, P.; Tocharus, J. AGM Exerts an Anti-Inflammatory Effect by Inhibiting Microglial Activation Through TLR4/NLRP3 Pathway in PMCAO Rats. Neurotox. Res. 2022, 40, 259–266. [Google Scholar] [CrossRef]
- Cankara, F.N.; Günaydın, C.; Çelik, Z.B.; Şahin, Y.; Pekgöz, Ş.; Erzurumlu, Y.; Gülle, K. AGM Confers Neuroprotection against Cisplatin-Induced Hippocampal Neurotoxicity. Metab. Brain Dis. 2021, 36, 339–349. [Google Scholar] [CrossRef]
- Manda, K.; Reiter, R.J. Melatonin Maintains Adult Hippocampal Neurogenesis and Cognitive Functions after Irradiation. Prog. Neurobiol. 2010, 90, 60–68. [Google Scholar] [CrossRef]
- Markham, A.; Bains, R.; Franklin, P.; Spedding, M. Changes in Mitochondrial Function Are Pivotal in Neurodegenerative and Psychiatric Disorders: How Important Is BDNF? Br. J. Pharmacol. 2014, 171, 2206–2229. [Google Scholar] [CrossRef]
- Sasaki, R.; Kojima, S.; Onishi, H. Do Brain-Derived Neurotrophic Factor Genetic Polymorphisms Modulate the Efficacy of Motor Cortex Plasticity Induced by Non-Invasive Brain Stimulation? A Systematic Review. Front. Hum. Neurosci. 2021, 15, 742373. [Google Scholar] [CrossRef] [PubMed]
- Abellaneda-Pérez, K.; Martin-Trias, P.; Cassé-Perrot, C.; Vaqué-Alcázar, L.; Lanteaume, L.; Solana, E.; Babiloni, C.; Lizio, R.; Junqué, C.; Bargalló, N.; et al. BDNF Val66Met Gene Polymorphism Modulates Brain Activity Following RTMS-Induced Memory Impairment. Sci. Rep. 2022, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Cheeran, B.; Talelli, P.; Mori, F.; Koch, G.; Suppa, A.; Edwards, M.; Houlden, H.; Bhatia, K.; Greenwood, R.; Rothwell, J.C. A Common Polymorphism in the Brain-Derived Neurotrophic Factor Gene (BDNF) Modulates Human Cortical Plasticity and the Response to RTMS. J. Physiol. 2008, 586, 5717–5725. [Google Scholar] [CrossRef] [PubMed]
- Su, B.; Ji, Y.-S.; Sun, X.; Liu, X.-H.; Chen, Z.-Y. Brain-Derived Neurotrophic Factor (BDNF)-Induced Mitochondrial Motility Arrest and Presynaptic Docking Contribute to BDNF- Enhanced Synaptic Transmission. J. Biol. Chem. 2014, 289, 1213–1226. [Google Scholar] [CrossRef]
- Penrod, R.D.; Kumar, J.; Smith, L.N.; McCalley, D.; Nentwig, T.B.; Hughes, B.W.; Barry, G.M.; Glover, K.; Taniguchi, M.; Cowan, C.W. Activity-regulated cytoskeleton-associated protein (Arc/Arg3.1) regulates anxiety- and novelty-related behaviors. Genes Brain Behavior. 2019, 18, e12561. [Google Scholar] [CrossRef]
- Lim, C.K.; Essa, M.M.; de Paula Martins, R.; Lovejoy, D.B.; Bilgin, A.A.; Waly, M.I.; Al-Farsi, Y.M.; Al-Sharbati, M.; Al-Shaffae, M.A.; Guillemin, G.J. Altered Kynurenine Pathway Metabolism in Autism: Implication for Immune-Induced Glutamatergic Activity: Altered Kynurenine Pathway Metabolism in ASD. Autism Res. 2016, 9, 621–631. [Google Scholar] [CrossRef]
- Musazzi, L.; Racagni, G.; Popoli, M. Stress, Glucocorticoids and Glutamate Release: Effects of Antidepressant Drugs. Neurochem. Int. 2011, 59, 138–149. [Google Scholar] [CrossRef]
- Casanova, M.F.; Shaban, M.; Ghazal, M.; El-Baz, A.S.; Casanova, E.L.; Sokhadze, E.M. Ringing Decay of Gamma Oscillations and Transcranial Magnetic Stimulation Therapy in Autism Spectrum Disorder. Appl. Psychophysiol. Biofeedback 2021, 46, 161–173. [Google Scholar] [CrossRef]
- Sonmez, A.I.; Almorsy, A.; Ramsey, L.B.; Strawn, J.R.; Croarkin, P.E. Novel Pharmacological Treatments for Generalized Anxiety Disorder: Pediatric Considerations. Depress. Anxiety 2020, 37, 747–759. [Google Scholar] [CrossRef]
- Harvey, B.H.; Regenass, W.; Dreyer, W.; Möller, M. Social Isolation Rearing-Induced Anxiety and Response to AGM in Male and Female Rats: Role of Corticosterone, Oxytocin, and Vasopressin. J. Psychopharmacol. Oxf. Engl. 2019, 33, 640–646. [Google Scholar] [CrossRef]
- McClung, C.A. How Might Circadian Rhythms Control Mood? Let Me Count the Ways. Biol. Psychiatry 2013, 74, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Floden, D.; Vallesi, A.; Stuss, D.T. Task Context and Frontal Lobe Activation in the Stroop Task. J. Cogn. Neurosci. 2011, 23, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Koresh, O.; Kozlovsky, N.; Kaplan, Z.; Zohar, J.; Matar, M.A.; Cohen, H. The Long-Term Abnormalities in Circadian Expression of Period 1 and Period 2 Genes in Response to Stress Is Normalized by AGM Administered Immediately after Exposure. Eur. Neuropsychopharmacol. J. Eur. Coll. Neuropsychopharmacol. 2012, 22, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.T.; Frith, C.D.; Büchel, C.; Nobre, A.C. Orienting Attention in Time: Behavioural and Neuroanatomical Distinction between Exogenous and Endogenous Shifts. Neuropsychologia 2000, 38, 808–819. [Google Scholar] [CrossRef]
- Davidson, R.J.; Pizzagalli, D.; Nitschke, J.B.; Putnam, K. Depression: Perspectives from Affective Neuroscience. Annu. Rev. Psychol. 2002, 53, 545–574. [Google Scholar] [CrossRef]
- Casey, B.J.; Castellanos, F.X.; Giedd, J.N.; Marsh, W.L.; Hamburger, S.D.; Schubert, A.B.; Vauss, Y.C.; Vaituzis, A.C.; Dickstein, D.P.; Sarfatti, S.E.; et al. Implication of Right Frontostriatal Circuitry in Response Inhibition and Attention-Deficit/Hyperactivity Disorder. J. Am. Acad. Child Adolesc. Psychiatry 1997, 36, 374–383. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Giedd, J.N.; Marsh, W.L.; Hamburger, S.D.; Vaituzis, A.C.; Dickstein, D.P.; Sarfatti, S.E.; Vauss, Y.C.; Snell, J.W.; Lange, N.; et al. Quantitative Brain Magnetic Resonance Imaging in Attention- Deficit Hyperactivity Disorder. Arch. Gen. Psychiatry 1996, 53, 607–616. [Google Scholar] [CrossRef]
- Filipek, P.A.; Semrud-Clikeman, M.; Steingard, R.J.; Renshaw, P.F.; Kennedy, D.N.; Biederman, J. Volumetric MRI Analysis Comparing Subjects Having Attention-Deficit Hyperactivity Disorder with Normal Controls. Neurology 1997, 48, 589–601. [Google Scholar] [CrossRef]
- Mataró, M.; Garcia-Sánchez, C.; Junqué, C.; Estévez-González, A.; Pujol, J. Magnetic Resonance Imaging Measurement of the Caudate Nucleus in Adolescents with Attention-Deficit Hyperactivity Disorder and Its Relationship with Neuropsychological and Behavioral Measures. Arch. Neurol. 1997, 54, 963–968. [Google Scholar] [CrossRef]
- Silberstein, R.B.; Farrow, M.; Levy, F.; Pipingas, A.; Hay, D.A.; Jarman, F.C. Functional Brain Electrical Activity Mapping in Boys with Attention-Deficit/Hyperactivity Disorder. Arch. Gen. Psychiatry 1998, 55, 1105–1112. [Google Scholar] [CrossRef]
- Cheng, W.; Rolls, E.T.; Gu, H.; Zhang, J.; Feng, J. Autism: Reduced Connectivity between Cortical Areas Involved in Face Expression, Theory of Mind, and the Sense of Self. Brain J. Neurol. 2015, 138 Pt 5, 1382–1393. [Google Scholar] [CrossRef]
- Kosaka, H.; Omori, M.; Munesue, T.; Ishitobi, M.; Matsumura, Y.; Takahashi, T.; Narita, K.; Murata, T.; Saito, D.N.; Uchiyama, H.; et al. Smaller Insula and Inferior Frontal Volumes in Young Adults with Pervasive Developmental Disorders. NeuroImage 2010, 50, 1357–1363. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Sokhadze, E.; Opris, I.; Wang, Y.; Li, X. Autism Spectrum Disorders: Linking Neuropathological Findings to Treatment with Transcranial Magnetic Stimulation. Acta Paediatr. Oslo Nor. 1992 2015, 104, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Bauman, M.L.; Kemper, T.L. Neuroanatomic Observations of the Brain in Autism: A Review and Future Directions. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2005, 23, 183–187. [Google Scholar] [CrossRef]
- Niederhofer, H. Treating ADHD with AGM. J. Atten. Disord. 2012, 16, 346–348. [Google Scholar] [CrossRef]
- Sara, S.J. The Locus Coeruleus and Noradrenergic Modulation of Cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Hou, Y.; Shen, J.; Huang, Y.; Martin, W.; Cheng, F. Network-Based Drug Repurposing for Novel Coronavirus 2019-NCoV/SARS-CoV-2. Cell Discov. 2020, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.A.; Hennig, A.; Martino, D. Relationship between COVID 19 and Movement Disorders: A Narrative Review. Eur. J. Neurol. 2021, 29, 1243–1253. [Google Scholar] [CrossRef]
- Anderson, G.; Reiter, R.J. Melatonin: Roles in Influenza, COVID-19, and Other Viral Infections. Rev. Med. Virol. 2020, 30, e2109. [Google Scholar] [CrossRef]
- Yadalam, P.K.; Balaji, T.M.; Varadarajan, S.; Alzahrani, K.J.; Al-Ghamdi, M.S.; Baeshen, H.A.; Alfarhan, M.F.A.; Khurshid, Z.; Bhandi, S.; Jagannathan, R.; et al. Assessing the Therapeutic Potential of AGM, Ramelteon, and Melatonin against SARS-CoV-2. Saudi J. Biol. Sci. 2022, 29, 3140–3150. [Google Scholar] [CrossRef]
- Feitosa, E.L.; Júnior, F.T.D.S.S.; Nery Neto, J.A.D.O.; Matos, L.F.L.; Moura, M.H.D.S.; Rosales, T.O.; De Freitas, G.B.L. COVID-19: Rational Discovery of the Therapeutic Potential of Melatonin as a SARS-CoV-2 Main Protease Inhibitor. Int. J. Med. Sci. 2020, 17, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C.; et al. Angiotensin-Converting Enzyme 2 Is a Functional Receptor for the SARS Coronavirus. Nature 2003, 426, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 Spike Receptor-Binding Domain Bound to the ACE2 Receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Cecon, E.; Izabelle, C.; Poder, S.L.; Real, F.; Zhu, A.; Tu, L.; Ghigna, M.R.; Klonjkowski, B.; Bomsel, M.; Jockers, R.; et al. Therapeutic Potential of Melatonin and Melatonergic Drugs on K18 HACE2 Mice Infected with SARS-CoV-2. J. Pineal Res. 2022, 72, e12772. [Google Scholar] [CrossRef] [PubMed]
- Brusco, L.I.; Cruz, P.; Cangas, A.V.; Rojas, C.G.; Vigo, D.E.; Cardinali, D.P. Efficacy of Melatonin in Non-Intensive Care Unit Patients with COVID-19 Pneumonia and Sleep Dysregulation. Melatonin Res. 2021, 4, 173–188. [Google Scholar] [CrossRef]
- Savino, R.; Polito, A.N.; Arcidiacono, G.; Poliseno, M.; Lo Caputo, S. Neuropsychiatric Disorders in Pediatric Long COVID-19: A Case Series. Brain Sci. 2022, 12, 514. [Google Scholar] [CrossRef]
- de Sousa Moreira, J.L.; Barbosa, S.M.B.; Vieira, J.G.; Chaves, N.C.B.; Felix, E.B.G.; Feitosa, P.W.G.; da Cruz, I.S.; da Silva, C.G.L.; Neto, M.L.R. The Psychiatric and Neuropsychiatric Repercussions Associated with Severe Infections of COVID-19 and Other Coronaviruses. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110159. [Google Scholar] [CrossRef]
| Authors | Year | Article Type | Age (Year) | Number of Patients | Gender | Diagnosis/Comorbidity | Dose of Agomelatine |
|---|---|---|---|---|---|---|---|
| Niederhofer H., et al. [21] | 2012 | Placebo-controlled study | 17–19 years old | 10 | M:F = 8:2 | Severe ADHD | 25 mg/day |
| Naguy A. and Al-tajali A. [22] | 2015 | Case report | 13 years old | 1 | F | Severe ADHD | 25 mg/day |
| Salardini E., et al. [23] | 2016 | Double-blind randomized controlled trial | 6–15 years old | 54 | Not available | Severe ADHD | 15 mg/day in patients with weight ≥30 kg and 25 mg/day in patients with weight ≥45 kg |
| Naguy A. and Alamiri B. [24] | 2020 | Case report | 15 years old | 1 | F | Severe ADHD/migraine | 25 mg/day |
| Authors | Year | Article Type | Age (Year) | Number of Patients | Gender | Diagnosis/Comorbidity | Dose of Agomelatine |
|---|---|---|---|---|---|---|---|
| Niederhofer H., et al. [27] | 2011 | Case report: 10 week clinical trial | Adults | 2:1 | M | Severe ASD/ID | 25 mg/day |
| Naguy A. and Ali Al Tajali [28] | 2015 | Case report | 10 years old | 1 | M | Severe ASD/behavioral disorder and insomnia | 25 mg/day |
| Ballester P., et al. [29] | 2015 | Randomized, cross-double-blind, multicenter study | 30–32 years old | 25 | M:F = 20:5 | Severe ASD | 25 mg/day |
| Ballester P., et al. [30] | 2019 | Cross-sectional, randomized, triple-blind, placebo-controlled study | 35 ± 12 years old | 23 | M:F = 19:4 | ASD/ID and sleep disturbance | 25 mg/day |
| Kumar H., et al. [31] | 2015 | Study on animal models | / | / | / | Autism VPA-induced | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savino, R.; Polito, A.N.; Marsala, G.; Ventriglio, A.; Di Salvatore, M.; De Stefano, M.I.; Valenzano, A.; Marinaccio, L.; Bellomo, A.; Cibelli, G.; et al. Agomelatine: A Potential Multitarget Compound for Neurodevelopmental Disorders. Brain Sci. 2023, 13, 734. https://doi.org/10.3390/brainsci13050734
Savino R, Polito AN, Marsala G, Ventriglio A, Di Salvatore M, De Stefano MI, Valenzano A, Marinaccio L, Bellomo A, Cibelli G, et al. Agomelatine: A Potential Multitarget Compound for Neurodevelopmental Disorders. Brain Sciences. 2023; 13(5):734. https://doi.org/10.3390/brainsci13050734
Chicago/Turabian StyleSavino, Rosa, Anna Nunzia Polito, Gabriella Marsala, Antonio Ventriglio, Melanie Di Salvatore, Maria Ida De Stefano, Anna Valenzano, Luigi Marinaccio, Antonello Bellomo, Giuseppe Cibelli, and et al. 2023. "Agomelatine: A Potential Multitarget Compound for Neurodevelopmental Disorders" Brain Sciences 13, no. 5: 734. https://doi.org/10.3390/brainsci13050734
APA StyleSavino, R., Polito, A. N., Marsala, G., Ventriglio, A., Di Salvatore, M., De Stefano, M. I., Valenzano, A., Marinaccio, L., Bellomo, A., Cibelli, G., Monda, M., Monda, V., Messina, A., Polito, R., Carotenuto, M., & Messina, G. (2023). Agomelatine: A Potential Multitarget Compound for Neurodevelopmental Disorders. Brain Sciences, 13(5), 734. https://doi.org/10.3390/brainsci13050734










