Intranasal Delivery of Gene-Edited Microglial Exosomes Improves Neurological Outcomes after Intracerebral Hemorrhage by Regulating Neuroinflammation
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Induction of ICH in Mice
2.3. Cell Culture and Transfection
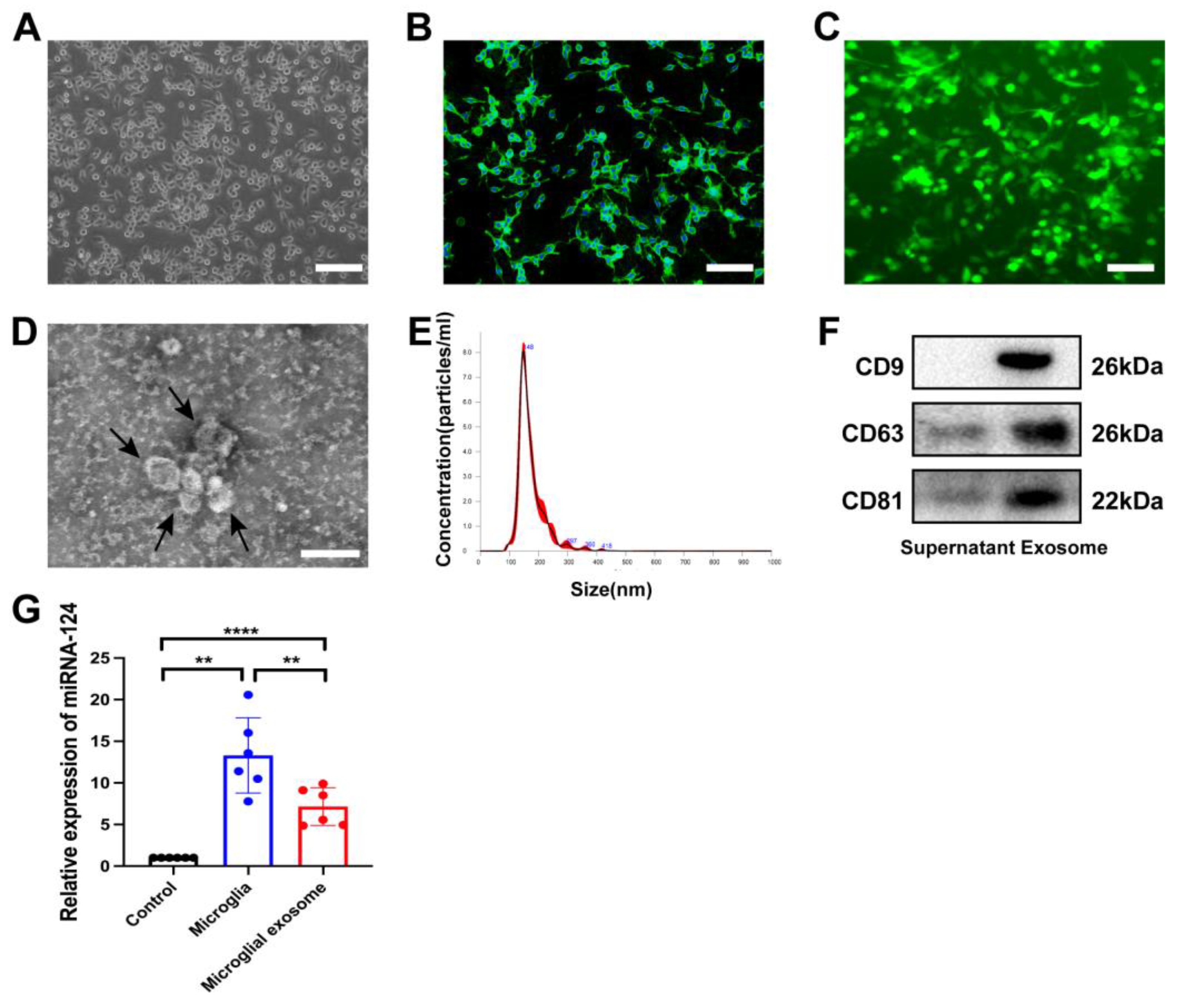
2.4. Exosome Preparation and Identification
2.5. Drug Administration
2.6. Neurological Behavior Assessment
2.7. Hematoma Volume Analysis
2.8. Brain Water Content Assessment
2.9. BBB Permeability
2.10. Immunofluorescence Staining
2.11. Real-Time Polymerase Chain Reaction (RT-PCR)
2.12. Western Blot Analysis
2.13. Flow Cytometry
2.14. Statistical Analysis
3. Results
3.1. Characteristics of miRNA-124-Enriched Microglia-Derived Exosomes
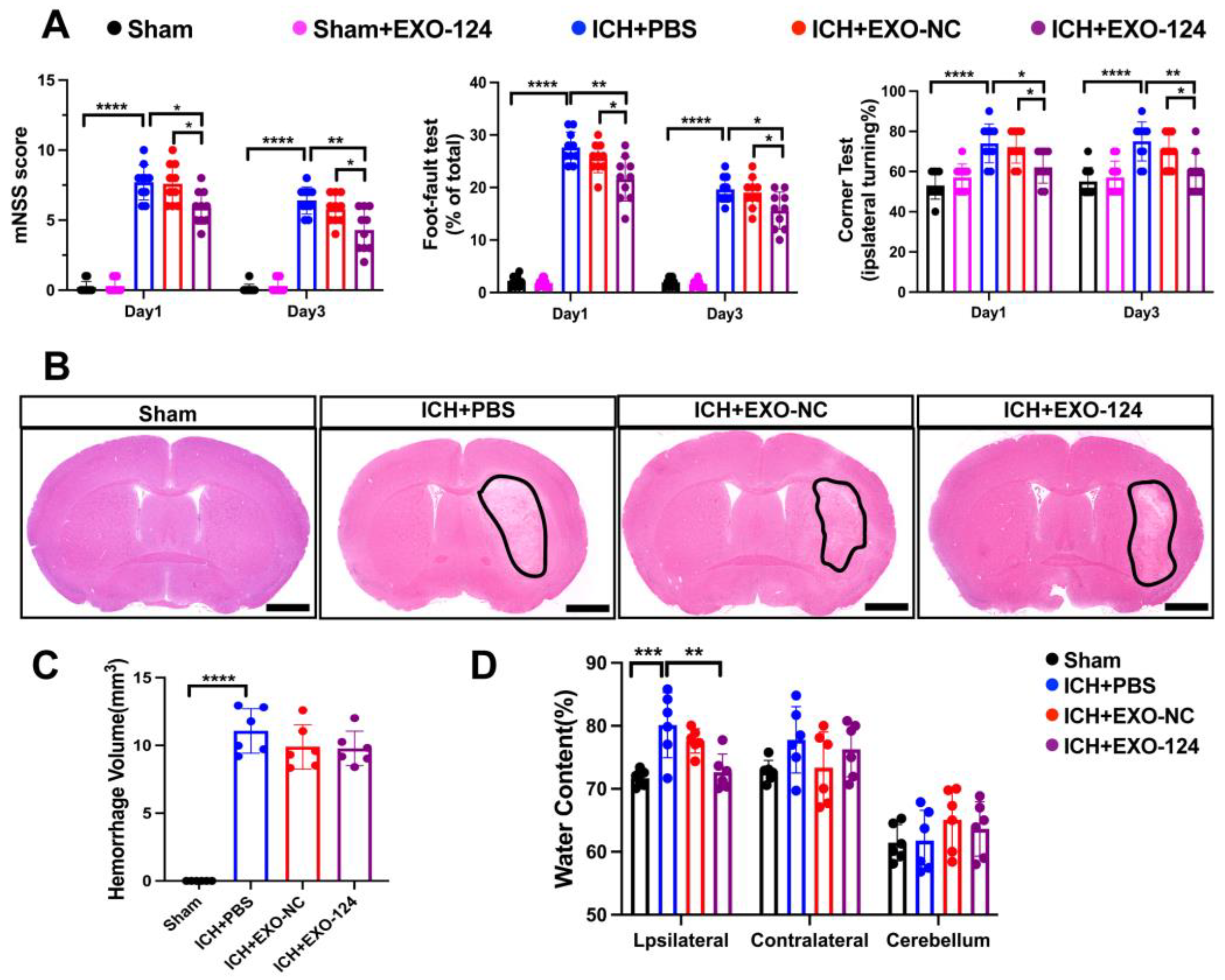
3.2. miRNA-124-Enriched Microglia-Derived Exosomes Attenuate Neurological Deficits, Brain Edema after ICH
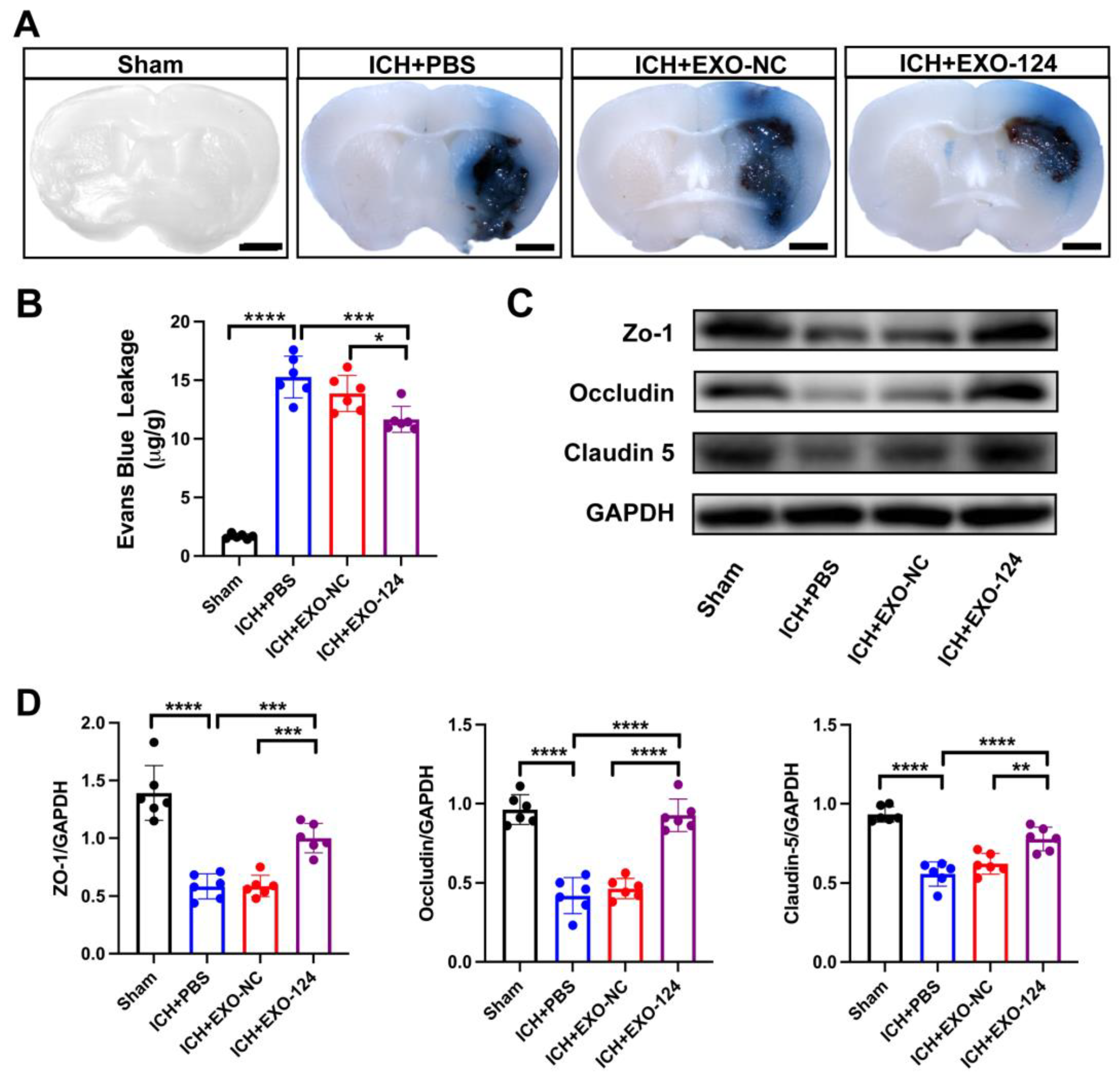
3.3. miRNA-124-Enriched Microglia-Derived Exosomes Attenuate BBB Damage after ICH
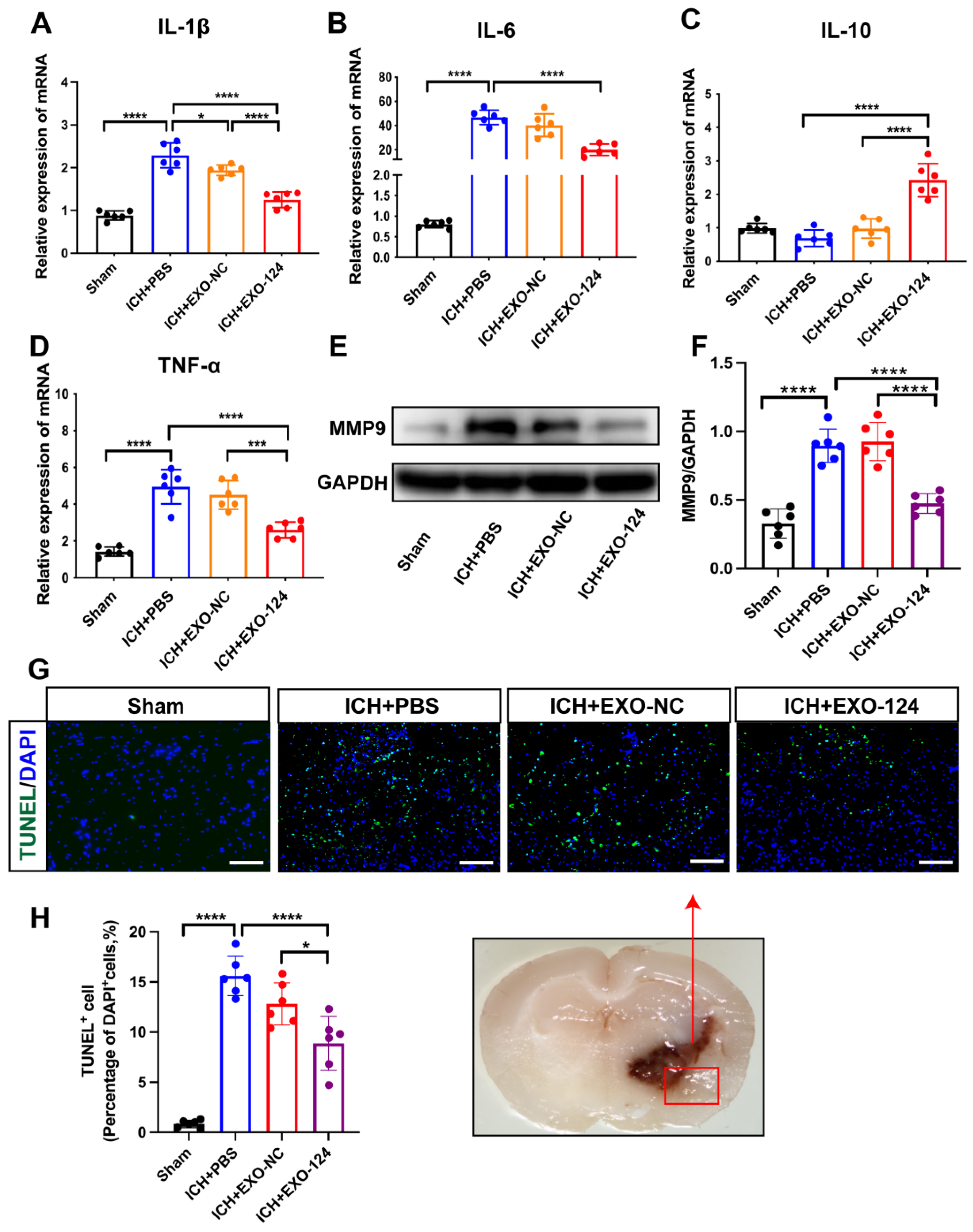
3.4. miRNA-124-Enriched Microglia-Derived Exosomes Reduce Cell Death and Inhibit Brain Inflammation after ICH
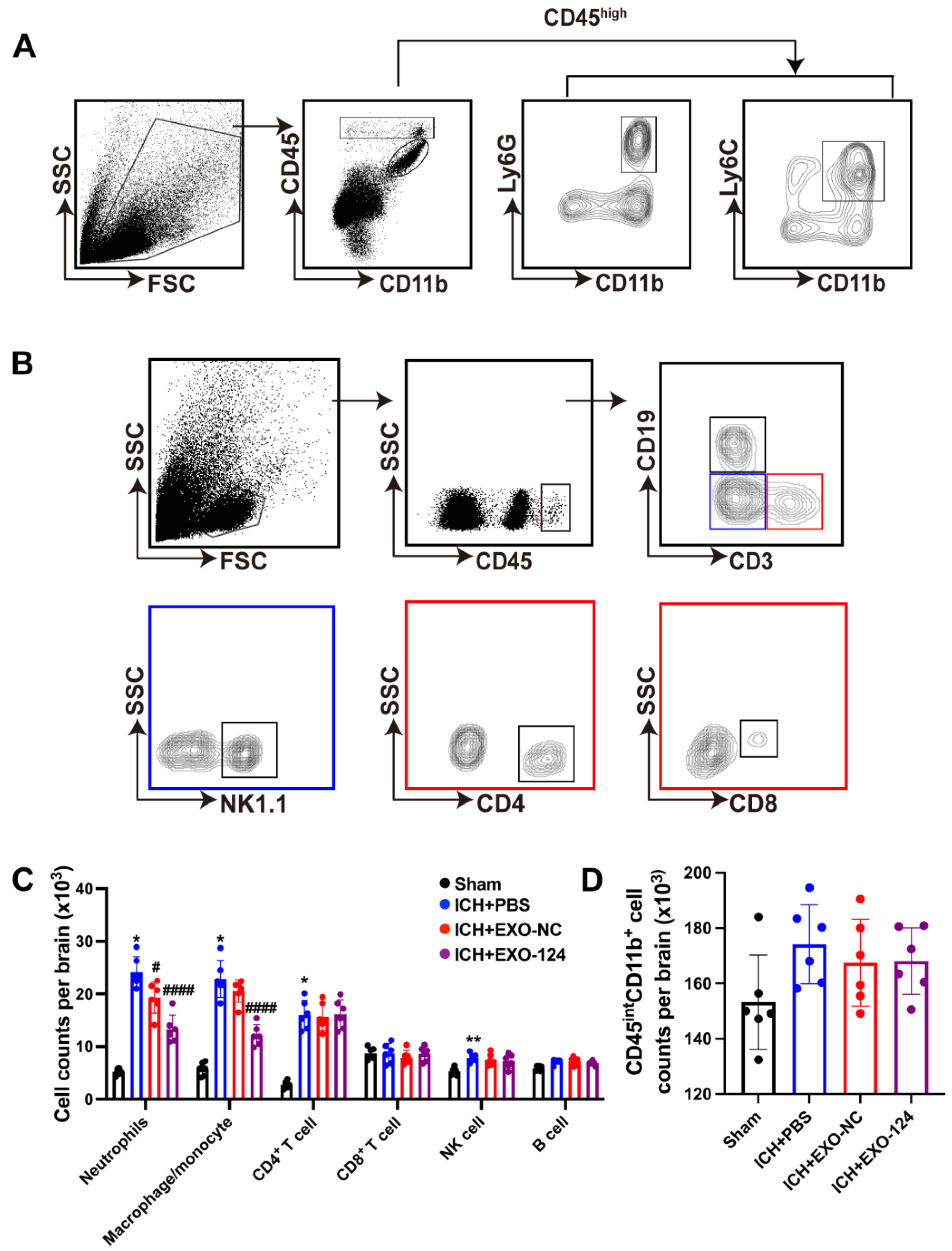
3.5. miRNA-124-Enriched Microglia-Derived Exosomes Reduce Immune Cell Infiltration into the Brain after ICH
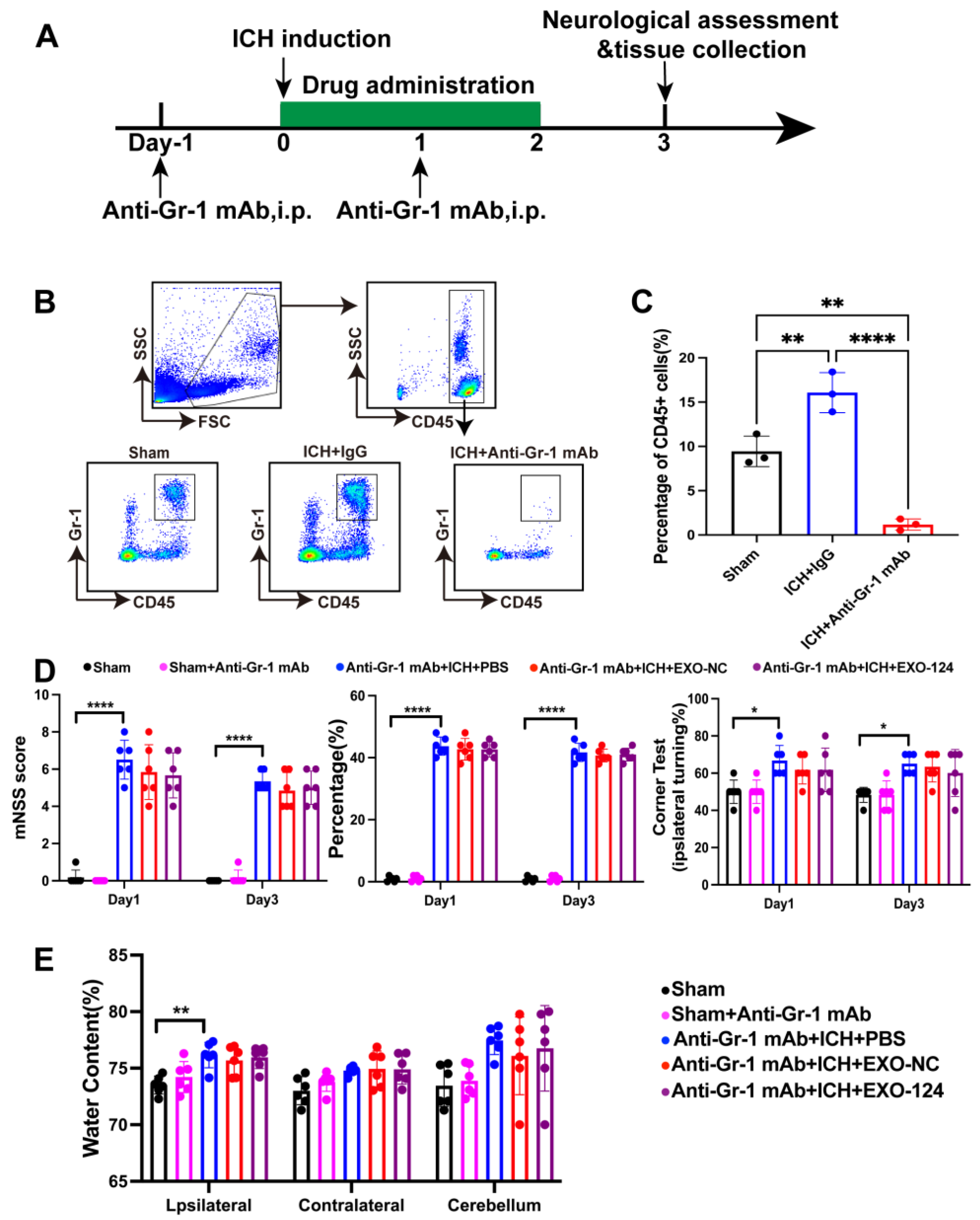
3.6. Gr-1+ Myeloid Cells Are Involved in the Protective Effect of Exo-124
4. Discussion
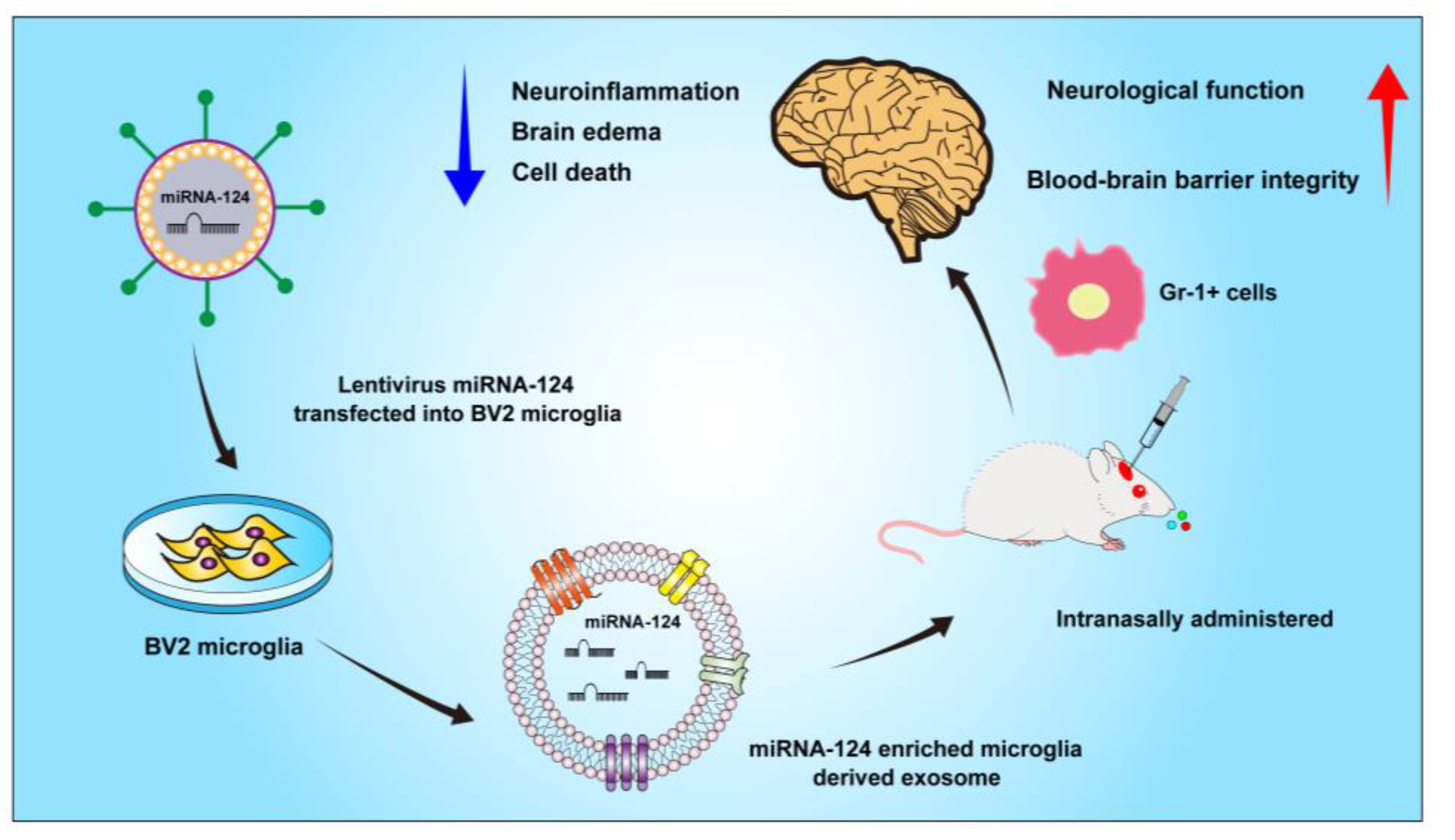
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gross, B.A.; Jankowitz, B.T.; Friedlander, R.M. Cerebral Intraparenchymal Hemorrhage: A Review. JAMA 2019, 321, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Yong, V.W. Neuroinflammation in intracerebral haemorrhage: Immunotherapies with potential for translation. Lancet Neurol. 2020, 19, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Keep, R.F.; Hua, Y.; Xi, G. Intracerebral haemorrhage: Mechanisms of injury and therapeutic targets. Lancet Neurol. 2012, 11, 720–731. [Google Scholar] [CrossRef]
- Han, R.; Luo, J.; Shi, Y.; Yao, Y.; Hao, J. PD-L1 (Programmed Death Ligand 1) Protects Against Experimental Intracerebral Hemorrhage–Induced Brain Injury. Stroke 2017, 48, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Shao, Y.; Fang, Y.; Lu, J.; Zheng, J.; Xu, S.; Wu, H.; Sun, Z.; Yu, J.; Chen, S.; et al. The Changes of Leukocytes in Brain and Blood After Intracerebral Hemorrhage. Front. Immunol. 2021, 12, 617163. [Google Scholar] [CrossRef]
- Tschoe, C.; Bushnell, C.D.; Duncan, P.W.; Alexander-Miller, M.A.; Wolfe, S.Q. Neuroinflammation after Intracerebral Hemorrhage and Potential Therapeutic Targets. J. Stroke 2020, 22, 29–46. [Google Scholar] [CrossRef]
- Guo, M.; Wang, J.; Zhao, Y.; Feng, Y.; Han, S.; Dong, Q.; Cui, M.; Tieu, K. Microglial exosomes facilitate α-synuclein transmission in Parkinson’s disease. Brain 2020, 143, 1476–1497. [Google Scholar] [CrossRef]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Escudier, B.; Dorval, T.; Chaput, N.; André, F.; Caby, M.-P.; Novault, S.; Flament, C.; Leboulaire, C.; Borg, C.; Amigorena, S.; et al. Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: Results of thefirst phase I clinical trial. J. Transl. Med. 2005, 3, 10. [Google Scholar] [CrossRef]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef]
- Hill, A.F. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci. 2019, 39, 9269–9273. [Google Scholar] [CrossRef]
- Wang, H.; Sui, H.; Zheng, Y.; Jiang, Y.; Shi, Y.; Liang, J.; Zhao, L. Curcumin-primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the Tau protein through the AKT/GSK-3beta pathway. Nanoscale 2019, 11, 7481–7496. [Google Scholar] [CrossRef] [PubMed]
- Cone, A.S.; Yuan, X.; Sun, L.; Duke, L.C.; Vreones, M.P.; Carrier, A.N.; Kenyon, S.M.; Carver, S.R.; Benthem, S.D.; Stimmell, A.C.; et al. Mesenchymal stem cell-derived extracellular vesicles ameliorate Alzheimer’s disease-like phenotypes in a preclinical mouse model. Theranostics 2021, 11, 8129–8142. [Google Scholar] [CrossRef]
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019, 110, 3173–3182. [Google Scholar] [CrossRef]
- Wei, M.; Li, C.; Yan, Z.; Hu, Z.; Dong, L.; Zhang, J.; Wang, X.; Li, Y.; Zhang, H. Activated Microglia Exosomes Mediated miR-383-3p Promotes Neuronal Necroptosis Through Inhibiting ATF4 Expression in Intracerebral Hemorrhage. Neurochem. Res. 2021, 46, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Gong, F.; Ge, X.; Lv, C.; Huang, C.; Feng, S.; Zhou, Z.; Rong, Y.; Wang, J.; Ji, C.; et al. Neuron-derived exosomes-transmitted miR-124-3p protect traumatically injured spinal cord by suppressing the activation of neurotoxic microglia and astrocytes. J. Nanobiotechnol. 2020, 18, 105. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Huang, S.; Yin, Z.; Zhu, J.; Ge, X.; Han, Z.; Tan, J.; Zhang, S.; Zhao, J.; Chen, F.; et al. Increases in miR-124-3p in Microglial Exosomes Confer Neuroprotective Effects by Targeting FIP200-Mediated Neuronal Autophagy Following Traumatic Brain Injury. Neurochem. Res. 2019, 44, 1903–1923. [Google Scholar] [CrossRef]
- Shenoy, A.; Blelloch, R.H. Regulation of microRNA function in somatic stem cell proliferation and differentiation. Nat. Rev. Mol. Cell Biol. 2014, 15, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Raza, U.; Zhang, J.D.; Sahin, O. MicroRNAs: Master regulators of drug resistance, stemness, and metastasis. J. Mol. Med. 2014, 92, 321–336. [Google Scholar]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased miR-124-3p in microglial exosomes following traumatic brain injury inhibits neuronal inflammation and contributes to neurite outgrowth via their transfer into neurons. FASEB J. 2018, 32, 512–528. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Guo, M.; Hu, T.; Li, W.; Huang, S.; Yin, Z.; Li, Y.; Chen, F.; Zhu, L.; Kang, C.; et al. Increased Microglial Exosomal miR-124-3p Alleviates Neurodegeneration and Improves Cognitive Outcome after rmTBI. Mol. Ther. 2020, 28, 503–522. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, D.; Wang, H.; Wan, Z.; Luo, Q.; Zhong, Y.; Yin, M.; Qing, Z.; Li, Z.; Bao, B. MiR-21-5p/dual-specificity phosphatase 8 signalling mediates the anti-inflammatory effect of haem oxygenase-1 in aged intracerebral haemorrhage rats. Aging Cell 2019, 18, e13022. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Hohjoh, H.; Fukuoka, M.; Sato, W.; Oki, S.; Tomi, C.; Yamaguchi, H.; Kondo, T.; Takahashi, R.; Yamamura, T. Circulating exosomes suppress the induction of regulatory T cells via let-7i in multiple sclerosis. Nat. Commun. 2018, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gao, X.; Zhang, W.; Liu, M.; Han, Z.; Li, M.; Lei, P.; Liu, Q. Microglial replacement in the aged brain restricts neuroinflammation following intracerebral hemorrhage. Cell Death Dis. 2022, 13, 33. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Shi, S.X.; Yao, N.; Cheng, X.; Guo, A.; Zhu, Z.; Zhang, X.; Liu, Q. Brain transforms natural killer cells that exacerbate brain edema after intracerebral hemorrhage. J. Exp. Med. 2020, 217, e20200213. [Google Scholar] [CrossRef]
- Yin, Z.; Han, Z.; Hu, T.; Zhang, S.; Ge, X.; Huang, S.; Wang, L.; Yu, J.; Li, W.; Wang, Y.; et al. Neuron-derived exosomes with high miR-21-5p expression promoted polarization of M1 microglia in culture. Brain Behav. Immun. 2020, 83, 270–282. [Google Scholar] [CrossRef] [PubMed]
- Otero-Ortega, L.; Gómez de Frutos, M.C.; Laso-García, F.; Rodríguez-Frutos, B.; Medina-Gutiérrez, E.; Lopez, J.A.; Vazquez, J.; Díez-Tejedor, E.; Gutiérrez-Fernández, M. Exosomes promote restoration after an experimental animal model of intracerebral hemorrhage. J. Cereb. Blood Flow Metab. 2018, 38, 767–779. [Google Scholar] [CrossRef]
- Maroto, R.; Zhao, Y.; Jamaluddin, M.; Popov, V.L.; Wang, H.; Kalubowilage, M.; Zhang, Y.; Luisi, J.; Sun, H.; Culbertson, C.T.; et al. Effects of storage temperature on airway exosome integrity for diagnostic and functional analyses. J. Extracell. Vesicles 2017, 6, 1359478. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Wang, D.; Gao, X.; Li, S.; Cheng, X.; Shen, Y.; Li, S.; Jia, Q.; Liu, Q. Inhibition of exosome release augments neuroinflammation following intracerebral hemorrhage. FASEB J. 2021, 35, e21617. [Google Scholar] [CrossRef]
- Condamine, T.; Kumar, V.; Ramachandran, I.R.; Youn, J.-I.; Celis, E.; Finnberg, N.; El-Deiry, W.S.; Winograd, R.; Vonderheide, R.H.; English, N.R.; et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R–mediated apoptosis. J. Clin. Investig. 2014, 124, 2626–2639. [Google Scholar] [CrossRef]
- Ren, H.; Kong, Y.; Liu, Z.; Zang, D.; Yang, X.; Wood, K.; Li, M.; Liu, Q. Selective NLRP3 (Pyrin Domain–Containing Protein 3) Inflammasome Inhibitor Reduces Brain Injury After Intracerebral Hemorrhage. Stroke 2018, 49, 184–192. [Google Scholar] [CrossRef]
- Long, X.; Yao, X.; Jiang, Q.; Yang, Y.; He, X.; Tian, W.; Zhao, K.; Zhang, H. Astrocyte-derived exosomes enriched with miR-873a-5p inhibit neuroinflammation via microglia phenotype modulation after traumatic brain injury. J. Neuroinflamm. 2020, 17, 89. [Google Scholar] [CrossRef]
- Wu, S.; Han, J.; Yao, N.; Li, Y.; Zhang, F.; Shi, Y.; Shi, F.; Li, Z. Activation of P2X4 receptor exacerbates acute brain injury after intracerebral hemorrhage. CNS Neurosci. Ther. 2022, 28, 1008–1018. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ren, H.; Sheth, K.N.; Shi, F.; Liu, Q. A TSPO ligand attenuates brain injury after intracerebral hemorrhage. FASEB J. 2017, 31, 3278–3287. [Google Scholar] [CrossRef]
- Shi, K.; Li, H.; Chang, T.; He, W.; Kong, Y.; Qi, C.; Li, R.; Huang, H.; Zhu, Z.; Zheng, P.; et al. Bone marrow hematopoiesis drives multiple sclerosis progression. Cell 2022, 185, 2234–2247.e17. [Google Scholar] [CrossRef]
- Perets, N.; Betzer, O.; Shapira, R.; Brenstein, S.; Angel, A.; Sadan, T.; Ashery, U.; Popovtzer, R.; Offen, D. Golden Exosomes Selectively Target Brain Pathologies in Neurodegenerative and Neurodevelopmental Disorders. Nano Lett. 2019, 19, 3422–3431. [Google Scholar] [CrossRef] [PubMed]
- Nadeau, C.A.; Dietrich, K.; Wilkinson, C.M.; Crawford, A.M.; George, G.N.; Nichol, H.K.; Colbourne, F. Prolonged Blood-Brain Barrier Injury Occurs After Experimental Intracerebral Hemorrhage and Is Not Acutely Associated with Additional Bleeding. Transl. Stroke Res. 2019, 10, 287–297. [Google Scholar] [CrossRef]
- Morotti, A.; Phuah, C.-L.; Anderson, C.D.; Jessel, M.J.; Schwab, K.; Ayres, A.M.; Pezzini, A.; Padovani, A.; Gurol, E.; Viswanathan, A.; et al. Leukocyte Count and Intracerebral Hemorrhage Expansion. Stroke 2016, 47, 1473–1478. [Google Scholar] [CrossRef]
- Mracsko, E.; Javidi, E.; Na, S.-Y.; Kahn, A.; Liesz, A.; Veltkamp, R. Leukocyte invasion of the brain after experimental intracerebral hemorrhage in mice. Stroke 2014, 45, 2107–2114. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tian, W.; Chen, X.; Xu, H.; Dai, W.; Zhang, Y.; Wu, X.; Yu, W.; Tian, J.; Su, D. Peripheral Neutrophils-Derived Matrix Metallopeptidase-9 Induces Postoperative Cognitive Dysfunction in Aged Mice. Front. Aging Neurosci. 2022, 14, 683295. [Google Scholar] [CrossRef]
- Qin, Z.; Wang, P.-Y.; Su, D.-F.; Liu, X. miRNA-124 in Immune System and Immune Disorders. Front. Immunol. 2016, 7, 406. [Google Scholar] [CrossRef]
- Yu, A.; Zhang, T.; Duan, H.; Pan, Y.; Zhang, X.; Yang, G.; Wang, J.; Deng, Y.; Yang, Z. MiR-124 contributes to M2 polarization of microglia and confers brain inflammatory protection via the C/EBP-α pathway in intracerebral hemorrhage. Immunol. Lett. 2017, 182, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Gomes, C.; Vaz, A.R.; Brites, D. Exploring New Inflammatory Biomarkers and Pathways during LPS-Induced M1 Polarization. Mediat. Inflamm. 2016, 2016, 6986175. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chopp, M. Exosome Therapy for Stroke. Stroke 2018, 49, 1083–1090. [Google Scholar] [CrossRef]
- Potolicchio, I.; Carven, G.J.; Xu, X.; Stipp, C.; Riese, R.J.; Stern, L.J.; Santambrogio, L. Proteomic analysis of microglia-derived exosomes: Metabolic role of the aminopeptidase CD13 in neuropeptide catabolism. J. Immunol. 2005, 175, 2237–2243. [Google Scholar] [CrossRef]
- Sardar Sinha, M.; Ansell-Schultz, A.; Civitelli, L.; Hildesjö, C.; Larsson, M.; Lannfelt, L.; Ingelsson, M.; Hallbeck, M. Alzheimer’s disease pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018, 136, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef]
- Gan, L.; Li, Z.; Lv, Q.; Huang, W. Rabies virus glycoprotein (RVG29)-linked microRNA-124-loaded polymeric nanoparticles inhibit neuroinflammation in a Parkinson’s disease model. Int. J. Pharm. 2019, 567, 118449. [Google Scholar] [CrossRef]
- Peng, H.; Li, Y.; Ji, W.; Zhao, R.; Lu, Z.; Shen, J.; Wu, Y.; Wang, J.; Hao, Q.; Wang, J.; et al. Intranasal Administration of Self-Oriented Nanocarriers Based on Therapeutic Exosomes for Synergistic Treatment of Parkinson’s Disease. ACS Nano 2022, 16, 869–884. [Google Scholar] [CrossRef]
- Kim, M.; Lee, Y.; Lee, M. Hypoxia-specific anti-RAGE exosomes for nose-to-brain delivery of anti-miR-181a oligonucleotide in an ischemic stroke model. Nanoscale 2021, 13, 14166–14178. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, S.; Chang, J.; Wei, J.; Feng, M.; Wang, R. Perihematomal Edema After Intracerebral Hemorrhage: An Update on Pathogenesis, Risk Factors, and Therapeutic Advances. Front. Immunol. 2021, 12, 740632. [Google Scholar] [CrossRef]
- Shi, K.; Tian, D.-C.; Li, Z.-G.; Ducruet, A.F.; Lawton, M.T.; Shi, F.-D. Global brain inflammation in stroke. Lancet Neurol. 2019, 18, 1058–1066. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wan, L.; Luo, Z.; Xie, Y.; Liu, Y.; Huang, T.; Lu, H.; Hu, J. Microglia-Derived Exosomes Improve Spinal Cord Functional Recovery after Injury via Inhibiting Oxidative Stress and Promoting the Survival and Function of Endothelia Cells. Oxid. Med. Cell. Longev. 2021, 2021, 1695087. [Google Scholar] [CrossRef]
- Hao, R.; Hao, R.; Sun, B.; Yang, L.; Ma, C.; Li, S. RVG29-modified microRNA-loaded nanoparticles improve ischemic brain injury by nasal delivery. Drug Deliv. 2020, 27, 772–781. [Google Scholar] [CrossRef]
- You, L.; Wang, J.; Liu, T.; Zhang, Y.; Han, X.; Wang, T.; Guo, S.; Dong, T.; Xu, J.; Anderson, G.J.; et al. Targeted Brain Delivery of Rabies Virus Glycoprotein 29-Modified Deferoxamine-Loaded Nanoparticles Reverses Functional Deficits in Parkinsonian Mice. ACS Nano 2018, 12, 4123–4139. [Google Scholar] [CrossRef] [PubMed]
- Hua, H.; Zhang, X.; Mu, H.; Meng, Q.; Jiang, Y.; Wang, Y.; Lu, X.; Wang, A.; Liu, S.; Zhang, Y.; et al. RVG29-modified docetaxel-loaded nanoparticles for brain-targeted glioma therapy. Int. J. Pharm. 2018, 543, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Xiang, X.; Grizzle, W.; Sun, D.; Zhang, S.; Axtell, R.C.; Ju, S.; Mu, J.; Zhang, L.; Steinman, L.; et al. Treatment of brain inflammatory diseases by delivering exosome encapsulated anti-inflammatory drugs from the nasal region to the brain. Mol. Ther. 2011, 19, 1769–1779. [Google Scholar] [CrossRef]

| Primers | Forward Sequences (5′-3′) | Reverse Sequences (5′-3′) |
|---|---|---|
| IL-1β | TCGCAGCAGCACATCAACAAGAG | AGGTCCACGGAAAGCACACAGG |
| IL-6 | ACGCTTCTGGGCCTGTTGTT | CCTGCTGCTGGTGATTCTCT |
| IL-10 | TCCCTGGGTGAGAAGCTGAAGAC | CACCTGCTCCACTGCCTTGC |
| TNF-α | GCCTCTTCTCATTCCTGCTTGTGGG | GTGGTTTGAGTGTGAGGGTCTG |
| GAPDH | GCCAAGGCTGTGGGCAAGGT | TCTCCAGGCGGCACGCAGA |
| miRNA-124 | TCTTTAAGGCACGCGGTG | TATGGTTTTGACGACTGTGTGAT |
| U6 | CTCGCTTCGGCAGCACA | AACGCTTCACGAATTTGCGT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Ge, X.; Wang, C.; Yin, Z.; Jia, Z.; Hu, T.; Li, M.; Wang, D.; Han, Z.; Wang, L.; et al. Intranasal Delivery of Gene-Edited Microglial Exosomes Improves Neurological Outcomes after Intracerebral Hemorrhage by Regulating Neuroinflammation. Brain Sci. 2023, 13, 639. https://doi.org/10.3390/brainsci13040639
Guo M, Ge X, Wang C, Yin Z, Jia Z, Hu T, Li M, Wang D, Han Z, Wang L, et al. Intranasal Delivery of Gene-Edited Microglial Exosomes Improves Neurological Outcomes after Intracerebral Hemorrhage by Regulating Neuroinflammation. Brain Sciences. 2023; 13(4):639. https://doi.org/10.3390/brainsci13040639
Chicago/Turabian StyleGuo, Mengtian, Xintong Ge, Conglin Wang, Zhenyu Yin, Zexi Jia, Tianpeng Hu, Meimei Li, Dong Wang, Zhaoli Han, Lu Wang, and et al. 2023. "Intranasal Delivery of Gene-Edited Microglial Exosomes Improves Neurological Outcomes after Intracerebral Hemorrhage by Regulating Neuroinflammation" Brain Sciences 13, no. 4: 639. https://doi.org/10.3390/brainsci13040639
APA StyleGuo, M., Ge, X., Wang, C., Yin, Z., Jia, Z., Hu, T., Li, M., Wang, D., Han, Z., Wang, L., Xiong, X., Chen, F., & Lei, P. (2023). Intranasal Delivery of Gene-Edited Microglial Exosomes Improves Neurological Outcomes after Intracerebral Hemorrhage by Regulating Neuroinflammation. Brain Sciences, 13(4), 639. https://doi.org/10.3390/brainsci13040639







