Electrophysiological Evidence Reveals the Asymmetric Transfer from the Right to Left Hemisphere as Key to Reading Proficiency
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Experimental Task and Procedure
2.3. Materials
2.4. Experimental Conditions
2.5. EEG Data Acquisition
2.6. EEG Data Analysis
2.7. Granger Causality Analysis
2.8. Apparatus
3. Results
3.1. Behavioral Data
3.2. EEG Data
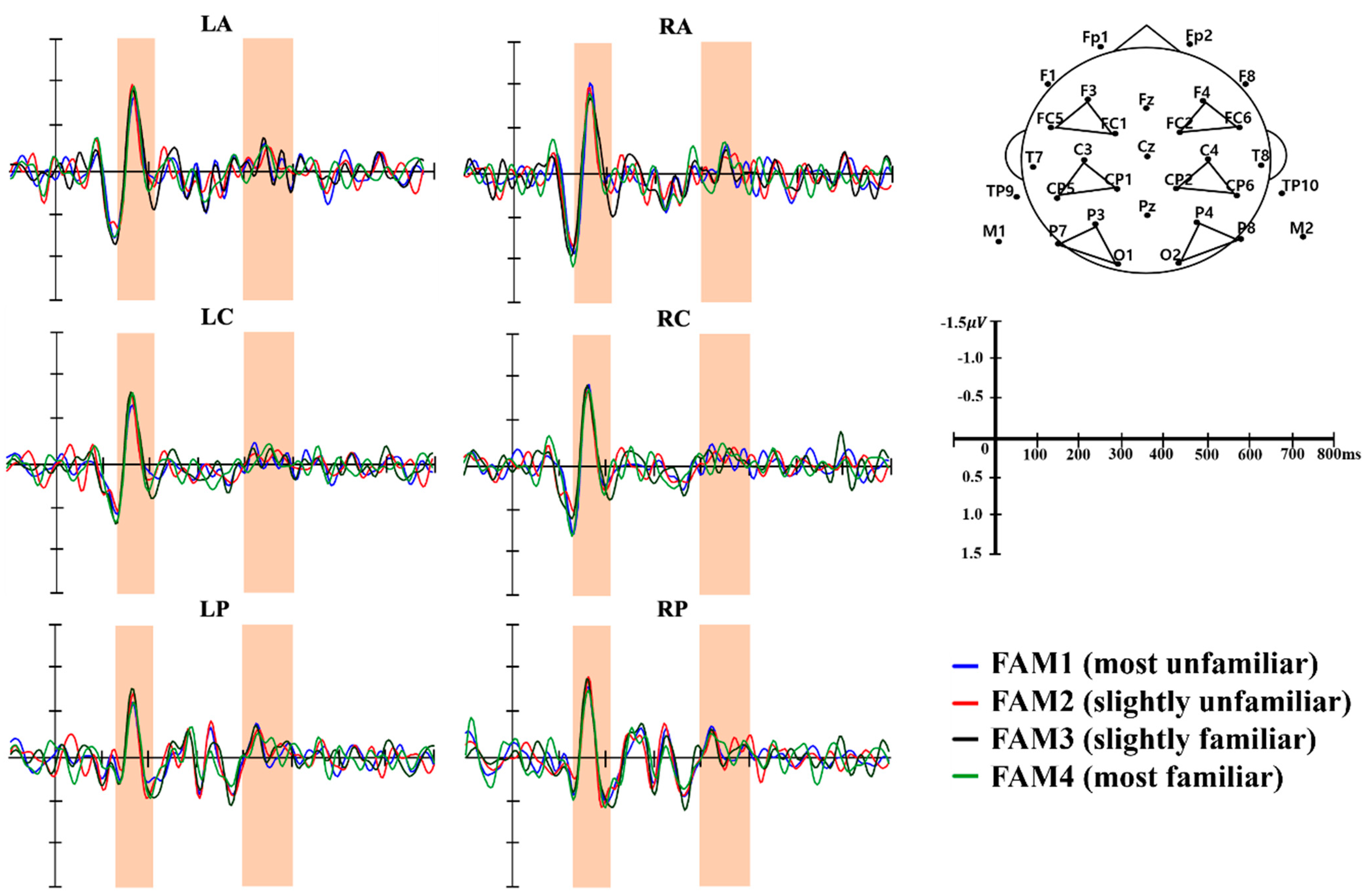
3.2.1. Time-Locked ERP Components
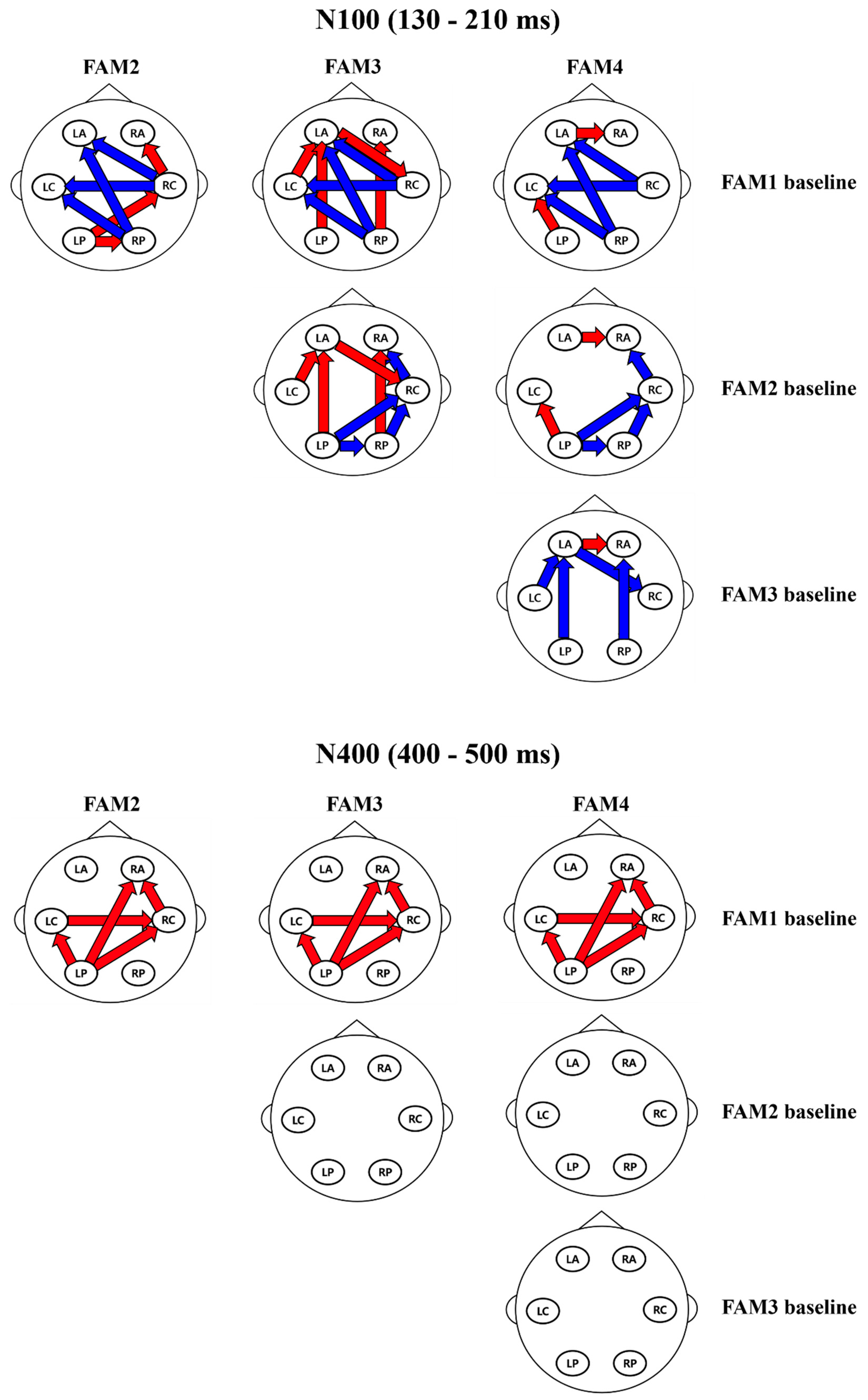
3.2.2. Granger Causality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jonsson, B.; Granberg, C.; Lithner, J. Gaining mathematical understanding: The effects of creative mathematical reasoning and cognitive proficiency. Front. Psychol. 2020, 11, 574366. [Google Scholar] [CrossRef]
- Krizman, J.; Skoe, E.; Marian, V.; Kraus, N. Bilingualism increases neural response consistency and attentional control: Evidence for sensory and cognitive coupling. Brain Lang. 2014, 128, 34–40. [Google Scholar] [CrossRef]
- Kerrigan, L.; Thomas, M.S.; Bright, P.; Filippi, R. Evidence of an advantage in visuo-spatial memory for bilingual compared to monolingual speakers. Biling. Lang. Cogn. 2017, 20, 602–612. [Google Scholar] [CrossRef]
- Schaeffer, D.J.; Krafft, C.E.; Schwarz, N.F.; Chi, L.; Rodrigue, A.L.; Pierce, J.E.; Allison, J.D.; Yanasak, N.E.; Liu, T.; Davis, C.L.; et al. The relationship between uncinate fasciculus white matter integrity and verbal memory proficiency in children. Neuroreport 2014, 25, 921. [Google Scholar] [CrossRef]
- Baker, S.K.; Smolkowski, K.; Katz, R.; Fien, H.; Seeley, J.R.; Kame’Enui, E.J.; Beck, C.T. Reading fluency as a predictor of reading proficiency in low-performing, high-poverty schools. Sch. Psychol. Rev. 2008, 37, 18–37. [Google Scholar] [CrossRef]
- Hudson, R.F.; Torgesen, J.K.; Lane, H.B.; Turner, S.J. Relations among reading skills and sub-skills and text-level reading proficiency in developing readers. Read. Writ. 2012, 25, 483–507. [Google Scholar] [CrossRef]
- Koda, K. The effects of transferred vocabulary knowledge on the development of L2 reading proficiency. Foreign Lang. Ann. 1989, 22, 529–540. [Google Scholar] [CrossRef]
- Yoshizaki, K. Effects of visual familiarity for words on interhemispheric cooperation for lexical processing. Cogn. Brain Res. 2001, 12, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Nam, K. Familiarity with words modulates interhemispheric interactions in visual word recognition. Front. Psychol. 2022, 13, 892858. [Google Scholar] [CrossRef] [PubMed]
- Ellis, A.W.; Brysbaert, M. Split fovea theory and the role of the two cerebral hemispheres in reading: A review of the evidence. Neuropsychologia 2010, 48, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.D.; Thierry, G.; Démonet, J.F.; Roberts, M.; Nazir, T. ERP evidence for the split fovea theory. Brain Res. 2007, 1185, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Mohr, B.; Endrass, T.; Hauk, O.; Pulvermüller, F. ERP correlates of the bilateral redundancy gain for words. Neuropsychologia 2007, 45, 2114–2124. [Google Scholar] [CrossRef] [PubMed]
- Hauk, O.; Pulvermüller, F. Effects of word length and frequency on the human event-related potential. Clin. Neurophysiol. 2004, 115, 1090–1103. [Google Scholar] [CrossRef] [PubMed]
- Sereno, S.C.; Rayner, K.; Posner, M.I. Establishing a time-line of word recognition: Evidence from eye movements and event-related potentials. Neuroreport 1998, 9, 2195–2200. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar] [CrossRef]
- Brown, C.M.; Hagoort, P.; Keurs, M.T. Neurophysiological signatures of visual lexical processing: Open-and closed-class words. J. Cogn. Neurosci. 1999, 11, 261–281. [Google Scholar] [CrossRef]
- Lien, M.C.; Allen, P.A.; Crawford, C. Electrophysiological evidence of different loci for case-mixing and word frequency effects in visual word recognition. Psychon. Bull. Rev. 2012, 19, 677–684. [Google Scholar] [CrossRef]
- Polich, J.; Donchin, E. P300 and the word frequency effect. Electroencephalogr. Clin. Neurophysiol. 1988, 70, 33–45. [Google Scholar] [CrossRef]
- Rugg, M.D. Event-related brain potentials dissociate repetition effects of high-and low-frequency words. Mem. Cogn. 1990, 18, 367–379. [Google Scholar] [CrossRef]
- Van Petten, C.; Kutas, M. Interactions between sentence context and word frequencyinevent-related brainpotentials. Mem. Cogn. 1990, 18, 380–393. [Google Scholar] [CrossRef]
- Nowicka, A.; Tacikowski, P. Transcallosal transfer of information and functional asymmetry of the human brain. Laterality 2011, 16, 35–74. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Koo, M.M.; Kim, J.H.; Nam, K.C. The Research for Language Information Processing of Bilateral Hemispheres on Korean Noun Eojeol: Visual Half-field Study. Korean J. Cogn. Biol. Psychol. 2020, 32, 29–53. [Google Scholar] [CrossRef]
- Kim, S.; Lee, C.; Nam, K. The examination of the visual-perceptual locus in hemispheric laterality of the word length effect using Korean visual word. Laterality 2022, 27, 485–512. [Google Scholar] [CrossRef]
- Kang, B.M.; Kim, H.G. Korean Usage Frequency: Sejong Surface and Semantic Analysis Corpus Based on 15 Million Eojeols; Research Institute of Korean Studies, Korea University: Andong, Republic of Korea, 2009. [Google Scholar]
- Delorme, A.; Makeig, S. EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J. Neurosci. Methods 2004, 134, 9–21. [Google Scholar] [CrossRef]
- Winkler, I.; Haufe, S.; Tangermann, M. Automatic classification of artifactual ICA-components for artifact removal in EEG signals. Behav. Brain Funct. 2011, 7, 30. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B (Methodol.) 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Groppe, D.M.; Urbach, T.P.; Kutas, M. Mass univariate analysis of event-related brain potentials/fields I: A critical tutorial review. Psychophysiology 2001, 48, 1711–1725. [Google Scholar] [CrossRef] [PubMed]
- Granger, C.W. Investigating causal relations by econometric models and cross-spectral methods. Econometrica 1969, 27, 424–438. [Google Scholar] [CrossRef]
- Seth, A. Granger causality. Scholarpedia 2007, 2, 1667. [Google Scholar] [CrossRef]
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Gow Jr, D.W.; Segawa, J.A.; Ahlfors, S.P.; Lin, F.H. Lexical influences on speech perception: A Granger causality analysis of MEG and EEG source estimates. Neuroimage 2008, 43, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Hawelka, S.; Gagl, B.; Wimmer, H. A dual-route perspective on eye movements of dyslexic readers. Cognition 2010, 115, 367–379. [Google Scholar] [CrossRef]
- Yap, M.J.; Balota, D.A.; Sibley, D.E.; Ratcliff, R. Individual differences in visual word recognition: Insights from the English Lexicon Project. J. Exp. Psychol. Hum. Percept. Perform. 2012, 38, 53. [Google Scholar] [CrossRef] [PubMed]
- Aboitiz, F.; López, J.; Montiel, J. Long distance communication in the human brain: Timing constraints for inter-hemispheric synchrony and the origin of brain lateralization. Biol. Res. 2003, 36, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Aboitiz, F.; Montiel, J. One hundred million years of interhemispheric communication: The history of the corpus callosum. Braz. J. Med. Biol. Res. 2003, 36, 409–420. [Google Scholar] [CrossRef]
- Miniussi, C.; Girelli, M.; Marzi, C.A. Neural site of the redundant target effect: Neurophysiological evidence. J. Cogn. Neurosci. 1998, 10, 216–230. [Google Scholar] [CrossRef]
- Mohr, B.; Pulvermüller, F. Redundancy gains and costs in cognitive processing: Effects of short stimulus onset asynchronies. J. Exp. Psychol. Learn. Mem. Cogn. 2002, 28, 1200. [Google Scholar] [CrossRef]
- Allen, M. Models of hemispheric specialization. Psychol. Bull. 1983, 93, 73–104. [Google Scholar] [CrossRef]
- Coles, G.S.; Goldstein, L. Hemispheric EEG activation and literacy development. International Journal of Clinical Neuropsychology 1985, 7, 3–7. [Google Scholar]
- Minagawa-Kawai, Y.; Cristià, A.; Dupoux, E. Cerebral lateralization and early speech acquisition: A developmental scenario. Dev. Cogn. Neurosci. 2011, 1, 217–232. [Google Scholar] [CrossRef]
- Bishop, D.V.M.; Hardiman, M.J.; Barry, J.G. Auditory deficit as a consequence rather than endophenotype of specific language impairment: Electrophysiological evidence. PLoS ONE 2012, 7, e35851. [Google Scholar] [CrossRef]
- Horowitz-Kraus, T.; Buck, C.; Dorrmann, D. Altered neural circuits accompany lower performance during narrative comprehension in children with reading difficulties: An fMRI study. Ann. Dyslexia 2016, 66, 301–318. [Google Scholar] [CrossRef]
- Power, J.D.; Cohen, A.L.; Nelson, S.M.; Wig, G.S.; Barnes, K.A.; Church, J.A.; Vogel, A.C.; Laumann, T.O.; Miezin, F.M.; Schlaggar, B.L.; et al. Functional network organization of the human brain. Neuron 2011, 72, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Saralegui, I.; Ontañón, J.M.; Fernandez-Ruanova, B.; Garcia-Zapirain, B.; Basterra, A.; Sanz-Arigita, E.J. Reading networks in children with dyslexia compared to children with ocular motility disturbances revealed by fMRI. Front. Hum. Neurosci. 2014, 8, 936. [Google Scholar] [CrossRef] [PubMed]
- Fernandino, L.; Iacoboni, M.; Zaidel, E. The effects of bilateral presentations on lateralized lexical decision. Brain Cogn. 2007, 64, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Yovel, G.; Levy, J.; Grabowecky, M.; Paller, K.A. Neural correlates of the left-visual-field superiority in face perception appear at multiple stages of face processing. J. Cogn. Neurosci. 2003, 15, 462–474. [Google Scholar] [CrossRef]
- Yovel, G.; Tambini, A.; Brandman, T. The asymmetry of the fusiform face area is a stable individual characteristic that underlies the left-visual-field superiority for faces. Neuropsychologia 2008, 46, 3061–3068. [Google Scholar] [CrossRef]
- Dehaene, S.; Pegado, F.; Braga, L.W.; Ventura, P.; Filho, G.N.; Jobert, A.; Dehaene-Lambertz, G.; Kolinsky, R.; Morais, J.; Cohen, L. How learning to read changes the cortical networks for vision and language. Science 2010, 330, 1359–1364. [Google Scholar] [CrossRef]
- Dehaene, S.; Cohen, L.; Morais, J.; Kolinsky, R. Illiterate to literate: Behavioural and cerebral changes induced by reading acquisition. Nat. Rev. Neurosci. 2015, 16, 234–244. [Google Scholar] [CrossRef]
- Sadato, N.; Pascual-Leone, A.; Grafman, J.; Ibañez, V.; Deiber, M.P.; Dold, G.; Hallett, M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature 1996, 380, 526–528. [Google Scholar] [CrossRef] [PubMed]
- Ionta, S. Visual neuropsychology in development: Anatomo-functional brain mechanisms of action/perception binding in health and disease. Front. Hum. Neurosci. 2021, 15, 689912. [Google Scholar] [CrossRef] [PubMed]
- Tong, F. Primary visual cortex and visual awareness. Nat. Rev. Neurosci. 2003, 4, 219–229. [Google Scholar] [CrossRef] [PubMed]
- de Haan, E.H.; Cowey, A. On the usefulness of ‘what’and ‘where’pathways in vision. Trends Cogn. Sci. 2011, 15, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Goodale, M.A. Separate visual systems for perception and action: A framework for understanding cortical visual impairment. Dev. Med. Child Neurol. 2013, 55, 9–12. [Google Scholar] [CrossRef]
| Physical Length Variable | Frequency Variable | Semantic Variable | ||||
|---|---|---|---|---|---|---|
| # of Morphemes | # of Syllables | # of Phonemes | # of Strokes | First Syllable Frequency (Log) | # of Objective Meanings | |
| FAM1 | 2.097 | 3.293 | 8.213 | 19.427 | 3.761 | 1.347 |
| (0.336) | (0.487) | (1.509) | (4.919) | (0.485) | (0.688) | |
| FAM2 | 2.081 | 3.24 | 8.053 | 18.96 | 3.677 | 1.653 |
| (0.302) | (0.566) | (1.643) | (4.458) | (0.581) | (1.797) | |
| FAM3 | 2.12 | 3.187 | 8.093 | 19.187 | 3.745 | 1.493 |
| (0.327) | (0.456) | (1.307) | (4.096) | (0.412) | (1.07) | |
| FAM4 | 2.093 | 3.093 | 7.893 | 17.76 | 3.73 | 1.507 |
| (0.293) | (0.597) | (1.737) | (4.526) | (0.468) | (1.095) | |
| Pseudowords | FAM1 | FAM2 | FAM3 | FAM4 | |
|---|---|---|---|---|---|
| RTs (ms) | 536 (49) | 533 (48) | 522 (47) | 508 (48) | 504 (40) |
| ACC (%) | 0.903 (0.089) | 0.924 (0.081) | 0.937 (0.081) | 0.941 (0.072) | 0.959 (0.039) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Kim, J.; Nam, K. Electrophysiological Evidence Reveals the Asymmetric Transfer from the Right to Left Hemisphere as Key to Reading Proficiency. Brain Sci. 2023, 13, 621. https://doi.org/10.3390/brainsci13040621
Kim S, Kim J, Nam K. Electrophysiological Evidence Reveals the Asymmetric Transfer from the Right to Left Hemisphere as Key to Reading Proficiency. Brain Sciences. 2023; 13(4):621. https://doi.org/10.3390/brainsci13040621
Chicago/Turabian StyleKim, Sangyub, Joonwoo Kim, and Kichun Nam. 2023. "Electrophysiological Evidence Reveals the Asymmetric Transfer from the Right to Left Hemisphere as Key to Reading Proficiency" Brain Sciences 13, no. 4: 621. https://doi.org/10.3390/brainsci13040621
APA StyleKim, S., Kim, J., & Nam, K. (2023). Electrophysiological Evidence Reveals the Asymmetric Transfer from the Right to Left Hemisphere as Key to Reading Proficiency. Brain Sciences, 13(4), 621. https://doi.org/10.3390/brainsci13040621







