The Relevance of Serum Macrophage Migration Inhibitory Factor Level and Executive Function in Patients with White Matter Hyperintensity in Cerebral Small Vessel Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Methods
2.2.1. Observational Indexes
2.2.2. Neuropsychological Test
Stroop Test
CTT
2.2.3. Evaluation of Cranial MRI
2.2.4. Determination of Serum MIF Level
2.2.5. Statistical Analysis
3. Results
3.1. Comparison of General Data in the Two Groups
3.2. Comparison of Serum MIF Level, Total Fazekas Scores, and Cognitive Function Assessment in the Two Groups
3.3. Logistic Regression Analysis of WMH-CI in CSVD
3.4. Correlation Analysis of WMH Degree and Cognitive Function
3.5. Correlation Analysis of MIF Level and WMH Degree and Cognitive Function
3.6. The ROC Curve Analysis of the Diagnostic Value of Serum MIF Level and WMH Degree in Predicting CI in Patients with CSVD
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wardlaw, J.M.; Smith, C.; Dichgans, M. Small vessel disease: Mechanisms and clinical implications. Lancet Neurol. 2019, 18, 684–696. [Google Scholar] [CrossRef] [PubMed]
- Azeem, F.; Durrani, R.; Zerna, C.; Smith, E.E. Silent brain infarctions and cognition decline: Systematic review and meta-analysis. J. Neurol. 2020, 267, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Wolters, F.J.; Ikram, M.A. Epidemiology of Vascular Dementia. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Boyle, P.A.; Yu, L.; Wilson, R.S.; Leurgans, S.E.; Schneider, J.A.; Bennett, D.A. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann. Neurol. 2018, 83, 74–83. [Google Scholar] [CrossRef]
- van Uden, I.W.; van der Holst, H.M.; Tuladhar, A.M.; van Norden, A.G.; de Laat, K.F.; Rutten-Jacobs, L.C.; Norris, D.G.; Claassen, J.A.; van Dijk, E.J.; Kessels, R.P.; et al. White Matter and Hippocampal Volume Predict the Risk of Dementia in Patients with Cerebral Small Vessel Disease: The RUN DMC Study. J. Alzheimer’s Dis. 2016, 49, 863–873. [Google Scholar] [CrossRef]
- Salvadori, E.; Brambilla, M.; Cova, I.; Pomati, S.; Pantoni, L. Cognitive evaluation in cerebral small vessel disease: Towards an evidence-based identification of the reference standards. Part 1. A systematic review and qualitative data synthesis. J. Neurol. 2021, 268, 4563–4572. [Google Scholar] [CrossRef]
- Tozer, D.J.; Zeestraten, E.; Lawrence, A.J.; Barrick, T.R.; Markus, H.S. Texture Analysis of T1-Weighted and Fluid-Attenuated Inversion Recovery Images Detects Abnormalities That Correlate with Cognitive Decline in Small Vessel Disease. Stroke 2018, 49, 1656–1661. [Google Scholar] [CrossRef]
- Lampe, L.; Kharabian-Masouleh, S.; Kynast, J.; Arelin, K.; Steele, C.J.; Löffler, M.; Witte, A.V.; Schroeter, M.L.; Villringer, A.; Bazin, P.L. Lesion location matters: The relationships between white matter hyperintensities on cognition in the healthy elderly. J. Cereb. Blood Flow Metab. 2019, 39, 36–43. [Google Scholar] [CrossRef]
- Park, S.H.; Sohn, M.K.; Jee, S.; Yang, S.S. The Characteristics of Cognitive Impairment and Their Effects on Functional Outcome After Inpatient Rehabilitation in Subacute Stroke Patients. Ann. Rehabil. Med. 2017, 41, 734–742. [Google Scholar] [CrossRef]
- Seo, E.H.; Kim, H.; Lee, K.H.; Choo, I.H. Altered Executive Function in Pre-Mild Cognitive Impairment. J. Alzheimer’s Dis. 2016, 54, 933–940. [Google Scholar] [CrossRef]
- Povroznik, J.M.; Ozga, J.E.; Vonder Haar, C.; Engler-Chiurazzi, E.B. Executive (dys)function after stroke: Special considerations for behavioral pharmacology. Behav. Pharmacol. 2018, 29, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Zanon Zotin, M.C.; Sveikata, L.; Viswanathan, A.; Yilmaz, P. Cerebral small vessel disease and vascular cognitive impairment: From diagnosis to management. Curr. Opin. Neurol. 2021, 34, 246–257. [Google Scholar] [CrossRef] [PubMed]
- Kapasi, A.; DeCarli, C.; Schneider, J.A. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017, 134, 171–186. [Google Scholar] [CrossRef]
- Liu, Y.C.; Tsai, Y.H.; Tang, S.C.; Liou, H.C.; Kang, K.H.; Liou, H.H.; Jeng, J.S.; Fu, W.M. Cytokine MIF Enhances Blood-Brain Barrier Permeability: Impact for Therapy in Ischemic Stroke. Sci. Rep. 2018, 8, 743. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.S.; Chen, W.; Liu, S.; Zhang, Y.Y.; Li, X.H. Serum macrophage migration inhibitory factor levels are associated with infarct volumes and long-term outcomes in patients with acute ischemic stroke. Int. J. Neurosci. 2017, 127, 539–546. [Google Scholar] [CrossRef]
- Oikonomidi, A.; Tautvydaitė, D.; Gholamrezaee, M.M.; Henry, H.; Bacher, M.; Popp, J. Macrophage Migration Inhibitory Factor is Associated with Biomarkers of Alzheimer’s Disease Pathology and Predicts Cognitive Decline in Mild Cognitive Impairment and Mild Dementia. J. Alzheimer’s Dis. 2017, 60, 273–281. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Laing, K.K.; Simoes, S.; Baena-Caldas, G.P.; Lao, P.J.; Kothiya, M.; Igwe, K.C.; Chesebro, A.G.; Houck, A.L.; Pedraza, L.; Hernández, A.I.; et al. Cerebrovascular disease promotes tau pathology in Alzheimer’s disease. Brain Commun. 2020, 2, fcaa132. [Google Scholar] [CrossRef]
- Bos, D.; Wolters, F.J.; Darweesh, S.K.L.; Vernooij, M.W.; de Wolf, F.; Ikram, M.A.; Hofman, A. Cerebral small vessel disease and the risk of dementia: A systematic review and meta-analysis of population-based evidence. Alzheimer’s Dement. 2018, 14, 1482–1492. [Google Scholar] [CrossRef]
- Yu, J.; Li, J.; Huang, X. The Beijing version of the Montreal Cognitive Assessment as a brief screening tool for mild cognitive impairment: A community-based study. BMC Psychiatry 2012, 12, 156. [Google Scholar] [CrossRef]
- Tremblay, M.P.; Potvin, O.; Belleville, S.; Bier, N.; Gagnon, L.; Blanchet, S.; Domingues, N.S.; Gaudreau, G.; Macoir, J.; Hudon, C. The Victoria Stroop Test: Normative Data in Quebec-French Adults and Elderly. Arch. Clin. Neuropsychol. 2016, 31, 926–933. [Google Scholar] [CrossRef]
- Galor, N.; Wilf, M.; Plotnik, M. Developing multiple shortened forms of virtual reality-based color trails test. Appl. Neuropsychol. Adult 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gaudreau, A.S.; Macoir, J.; Hudon, C. Normative data for the Color Trails Test in middle-aged and elderly Quebec-French people. Appl. Neuropsychol. Adult 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fazekas, F.; Chawluk, J.B.; Alavi, A.; Hurtig, H.I.; Zimmerman, R.A. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. AJR Am. J. Roentgenol. 1987, 149, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Li, X.; Teng, Z.; Li, X.; Jin, W.; Lv, P.Y. Homocysteine is Associated with the Development of Cerebral Small Vessel Disease: Retrospective Analyses from Neuroimaging and Cognitive Outcomes. J. Stroke Cerebrovasc. Dis. 2020, 29, 105393. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Ge, X.; Du, J.; Wang, Y.; Sun, Y.; Han, X.; Ding, W.; Cao, M.; Xu, Q.; Zhou, Y. Characterizing the Penumbras of White Matter Hyperintensities and Their Associations with Cognitive Function in Patients with Subcortical Vascular Mild Cognitive Impairment. Front. Neurol. 2019, 10, 348. [Google Scholar] [CrossRef]
- Ryu, W.S.; Jeong, S.W.; Kim, D.E. Total small vessel disease burden and functional outcome in patients with ischemic stroke. PLoS ONE 2020, 15, e0242319. [Google Scholar] [CrossRef]
- Kynast, J.; Lampe, L.; Luck, T.; Frisch, S.; Arelin, K.; Hoffmann, K.T.; Loeffler, M.; Riedel-Heller, S.G.; Villringer, A.; Schroeter, M.L. White matter hyperintensities associated with small vessel disease impair social cognition beside attention and memory. J. Cereb. Blood Flow Metab. 2018, 38, 996–1009. [Google Scholar] [CrossRef]
- Tuladhar, A.M.; Reid, A.T.; Shumskaya, E.; de Laat, K.F.; van Norden, A.G.; van Dijk, E.J.; Norris, D.G.; de Leeuw, F.E. Relationship between white matter hyperintensities, cortical thickness, and cognition. Stroke 2015, 46, 425–432. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, A.; Tang, J.; Wei, D.; Li, P.; Chen, K.; Wang, Y.; Zhang, Z. Association of white matter integrity and cognitive functions in patients with subcortical silent lacunar infarcts. Stroke 2015, 46, 1123–1126. [Google Scholar] [CrossRef]
- Alber, J.; Alladi, S.; Bae, H.J.; Barton, D.A.; Beckett, L.A.; Bell, J.M.; Berman, S.E.; Biessels, G.J.; Black, S.E.; Bos, I.; et al. White matter hyperintensities in vascular contributions to cognitive impairment and dementia (VCID): Knowledge gaps and opportunities. Alzheimer’s Dement. 2019, 5, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Caunca, M.R.; De Leon-Benedetti, A.; Latour, L.; Leigh, R.; Wright, C.B. Neuroimaging of Cerebral Small Vessel Disease and Age-Related Cognitive Changes. Front. Aging Neurosci. 2019, 11, 145. [Google Scholar] [CrossRef] [PubMed]
- Brugulat-Serrat, A.; Salvadó, G.; Sudre, C.H.; Grau-Rivera, O.; Suárez-Calvet, M.; Falcon, C.; Sánchez-Benavides, G.; Gramunt, N.; Fauria, K.; Cardoso, M.J.; et al. Patterns of white matter hyperintensities associated with cognition in middle-aged cognitively healthy individuals. Brain Imaging Behav. 2020, 14, 2012–2023. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, A.; Baldereschi, M.; Lamassa, M.; Bovis, F.; Inzitari, M.; Solfrizzi, V.; Panza, F.; Galluzzo, L.; Scafato, E.; Inzitari, D. Daily Function as Predictor of Dementia in Cognitive Impairment, No Dementia (CIND) and Mild Cognitive Impairment (MCI): An 8-Year Follow-Up in the ILSA Study. J. Alzheimer’s Dis. 2016, 53, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, X.; Li, Q.; Lu, C.; Li, S. The relevance of serum macrophage migratory inhibitory factor and cognitive dysfunction in patients with cerebral small vascular disease. Front. Aging Neurosci. 2023, 15, 1083818. [Google Scholar] [CrossRef]
- Asare, Y.; Schmitt, M.; Bernhagen, J. The vascular biology of macrophage migration inhibitory factor (MIF). Expression and effects in inflammation, atherogenesis and angiogenesis. Thromb. Haemost. 2013, 109, 391–398. [Google Scholar] [CrossRef]
- Sinitski, D.; Kontos, C.; Krammer, C.; Asare, Y.; Kapurniotu, A.; Bernhagen, J. Macrophage Migration Inhibitory Factor (MIF)-Based Therapeutic Concepts in Atherosclerosis and Inflammation. Thromb. Haemost. 2019, 119, 553–566. [Google Scholar] [CrossRef]
- Burger-Kentischer, A.; Goebel, H.; Seiler, R.; Fraedrich, G.; Schaefer, H.E.; Dimmeler, S.; Kleemann, R.; Bernhagen, J.; Ihling, C. Expression of macrophage migration inhibitory factor in different stages of human atherosclerosis. Circulation 2002, 105, 1561–1566. [Google Scholar] [CrossRef]
- Schober, A.; Bernhagen, J.; Weber, C. Chemokine-like functions of MIF in atherosclerosis. J. Mol. Med. 2008, 86, 761–770. [Google Scholar] [CrossRef]
- Rammos, C.; Hendgen-Cotta, U.B.; Sobierajski, J.; Adamczyk, S.; Hetzel, G.R.; Kleophas, W.; Dellanna, F.; Kelm, M.; Rassaf, T. Macrophage migration inhibitory factor is associated with vascular dysfunction in patients with end-stage renal disease. Int. J. Cardiol. 2013, 168, 5249–5256. [Google Scholar] [CrossRef]
- Nam, K.W.; Kwon, H.M.; Lee, Y.S. Distinct association between cerebral arterial pulsatility and subtypes of cerebral small vessel disease. PLoS ONE 2020, 15, e0236049. [Google Scholar] [CrossRef] [PubMed]
- Sumaiya, K.; Langford, D.; Natarajaseenivasan, K.; Shanmughapriya, S. Macrophage migration inhibitory factor (MIF): A multifaceted cytokine regulated by genetic and physiological strategies. Pharmacol. Ther. 2022, 233, 108024. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.M.; Kithcart, A.P.; Pitt, D.; Guan, Z.; Alexander, J.; Williams, J.L.; Shawler, T.; Dagia, N.M.; Popovich, P.G.; Satoskar, A.R.; et al. Macrophage migration inhibitory factor potentiates autoimmune-mediated neuroinflammation. J. Immunol. 2013, 191, 1043–1054. [Google Scholar] [CrossRef]
- Jiang, L.; Cai, X.; Yao, D.; Jing, J.; Mei, L.; Yang, Y.; Li, S.; Jin, A.; Meng, X.; Li, H.; et al. Association of inflammatory markers with cerebral small vessel disease in community-based population. J. Neuroinflamm. 2022, 19, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.R.; Chuang, Y.C.; Chao, C.H.; Yeh, T.M. Macrophage migration inhibitory factor induces vascular leakage via autophagy. Biol. Open 2015, 4, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Walsh, J.; Tozer, D.J.; Sari, H.; Hong, Y.T.; Drazyk, A.; Williams, G.; Shah, N.J.; O’Brien, J.T.; Aigbirhio, F.I.; Rosenberg, G.; et al. Microglial activation and blood-brain barrier permeability in cerebral small vessel disease. Brain 2021, 144, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Zakzanis, K.K.; Mraz, R.; Graham, S.J. An fMRI study of the Trail Making Test. Neuropsychologia 2005, 43, 1878–1886. [Google Scholar] [CrossRef] [PubMed]

| NC Group (n = 52) | IC Group (n = 65) | χ2/t/u | p | |
|---|---|---|---|---|
| Male proportion (%) | 55.77% | 52.31% | 0.139 | 0.852 |
| Age (years) | 60.3 ± 8.8 | 61.6 ± 9.0 | −0.820 | 0.414 |
| BMI (Kg/m2) | 24.81 (23.21, 27.47) | 24.90 (22.82, 26.15) | −1.039 | 0.300 |
| Education (years) | 6.50 (5, 9) | 8 (3.5, 9) | −0.480 | 0.634 |
| Hypertension, n (%) | 59.62% | 78.46% | 4.894 | 0.041 |
| Diabetes, n (%) | 25.00% | 24.62% | 0.002 | 1.000 |
| CHD, n (%) | 9.62% | 7.69% | 0.137 | 0.749 |
| Stroke, n (%) | 32.69% | 29.23% | 0.049 | 0.844 |
| Smoke, n (%) | 32.69% | 32.70% | 0.163 | 0.693 |
| Alcohol, n (%) | 26.92% | 13.85% | 3.128 | 0.102 |
| TC (mmol/L) | 4.25 (3.52, 5.26) | 4.31 (3.40, 5.16) | −0.236 | 0.815 |
| TG (mmol/L) | 1.26 (0.91, 2.11) | 1.25 (0.86, 1.74) | −0.771 | 0.443 |
| HDL (mmol/L) | 1.18 (1.06, 1.41) | 1.13 (1.02, 1.28) | −1.405 | 0.161 |
| LDL (mmol/L) | 2.44 (1.79, 3.05) | 2.38 (1.83, 3.06) | −0.167 | 0.869 |
| FBG (mmol/L) | 5.19 (4.76, 5.87) | 4.88 (4.47, 5.84) | −1.171 | 0.243 |
| Scr (µmol/L) | 60.45 (52.43, 67.68) | 64.10 (51.10, 70.40) | −0.570 | 0.571 |
| UA (µmol/L) | 261.50 (214.75, 305.00) | 266.00 (216.50, 322.50) | −0.225 | 0.824 |
| Hcy (µmol/L) | 13.96 (11.07, 20.73) | 14.27 (11.35, 19.64) | −0.154 | 0.879 |
| NC Group (n = 52) | IC Group (n = 65) | χ2/t/u | p | |
|---|---|---|---|---|
| MIF (pg/mL) | 139.50 (114.25, 180.75) | 177.00 (141.50, 218.50) | −2.992 | 0.003 |
| Total Fazekas score | 3.00 (1.25, 3.00) | 3.10 (2.00, 4.00) | −2.998 | 0.003 |
| periventricular WMH | 2.00 (1.00, 2.00) | 2.10 (1.00, 2.00) | −2.476 | 0.013 |
| deep WMH | 1.00 (0.25, 1.00) | 1.10 (1.00, 2.00) | −3.045 | 0.002 |
| Stroop D-Time | 21.00 (18.25, 28.00) | 27.00 (20.00, 33.50) | −2.444 | 0.014 |
| Stroop W-Time | 30.50 (21.25, 38.75) | 34.00 (29.00, 41.00) | −2.517 | 0.012 |
| Stroop C-Time | 38.00 (34.00, 52.50) | 52.00 (39.00, 77.50) | −3.141 | 0.002 |
| Stroop D-Mistake | 0.00 (0.00, 0.00) | 0.10 (0.00, 0.00) | −2.397 | 0.015 |
| Stroop W-Mistake | 0.00 (0.00, 0.00) | 0.10 (0.00, 1.00) | −3.622 | <0.001 |
| Stroop C-Mistake | 1.50 (0.00, 2.00) | 3.00 (1.00, 4.00) | −2.605 | 0.009 |
| SIE-Time | 16.00 (10.00, 26.25) | 26.00 (15.50, 43.00) | −2.527 | 0.011 |
| SIE-Mistake | 1.00 (0.00, 2.00) | 2.00 (0.50, 4.00) | −2.178 | 0.029 |
| CTT A-Time | 74.00 (53.00, 108.75) | 105.00 (67.50, 168.50) | −2.957 | 0.003 |
| CTT B-Time | 155.50 (120.50, 269.25) | 266.00 (152.00, 428.00) | −3.475 | <0.001 |
| CIE (B-A) | 97.00 (49.25, 141.50) | 139.00 (90.00, 253.00) | −3.190 | 0.001 |
| CTT A-Mistake | 0.00 (0.00, 0.00) | 0.10 (0.00, 1.00) | −2.958 | 0.003 |
| CTT B-Mistake-Number | 0.00 (0.00, 0.00) | 0.10 (0.00, 1.00) | −2.184 | 0.028 |
| CTT B-Mistake-Color | 0.00 (0.00, 1.00) | 0.10 (0.00, 2.00) | −2.363 | 0.018 |
| BNT | 21.96 ± 3.71 | 18.88 ± 3.63 | 4.529 | <0.001 |
| IADL | 8.00 (8.00, 8.00) | 7.90 (7.00, 8.00) | −3.621 | <0.001 |
| β | SE | Wald χ2 | OR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Hypertension | 0.232 | 0.485 | 0.229 | 1.262 | 0.487~3.266 | 0.632 |
| Total Fazekas score | 0.352 | 0.168 | 4.401 | 1.422 | 1.023~1.976 | 0.036 |
| MIF (pg/mL) | 0.070 | 0.003 | 4.296 | 1.007 | 1.000~1.014 | 0.038 |
| r | p | |
|---|---|---|
| Total MoCA score | −0.252 | 0.006 |
| Stroop D-Time | 0.234 | 0.011 |
| Stroop W-Time | 0.264 | 0.004 |
| Stroop C-Time | 0.334 | <0.001 |
| Stroop D-Mistake | 0.070 | 0.454 |
| Stroop W-Mistake | 0.244 | 0.008 |
| Stroop C-Mistake | 0.295 | 0.001 |
| SIE-Time | 0.266 | 0.004 |
| SIE-Mistake | 0.325 | <0.001 |
| CTT A-Time | 0.225 | 0.015 |
| CTT B-Time | 0.344 | <0.001 |
| CIE (B-A) | 0.336 | <0.001 |
| CTT A-Mistake | 0.047 | 0.616 |
| CTT B-Mistake-Number | 0.153 | 0.100 |
| CTT B-Mistake-Color | 0.117 | 0.209 |
| BNT | −0.124 | 0.183 |
| IADL | −0.199 | 0.031 |
| r | p | |
|---|---|---|
| Total Fazekas score | 0.193 | 0.037 |
| Total MoCA score | −0.316 | 0.001 |
| Stroop D-Time | 0.133 | 0.154 |
| Stroop W-Time | 0.151 | 0.104 |
| Stroop C-Time | 0.238 | 0.010 |
| Stroop D-Mistake | −0.021 | 0.823 |
| Stroop W-Mistake | 0.110 | 0.239 |
| Stroop C-Mistake | 0.091 | 0.328 |
| SIE-Time | 0.186 | 0.045 |
| SIE-Mistake | 0.093 | 0.321 |
| CTT A-Time | 0.129 | 0.165 |
| CTT B-Time | 0.258 | 0.005 |
| CIE (B-A) | 0.304 | 0.001 |
| CTT A-Mistake | 0.158 | 0.089 |
| CTT B-Mistake-N | 0.133 | 0.154 |
| CTT B-Mistake-C | 0.146 | 0.117 |
| BNT | −0.213 | 0.021 |
| IADL | −0.126 | 0.176 |
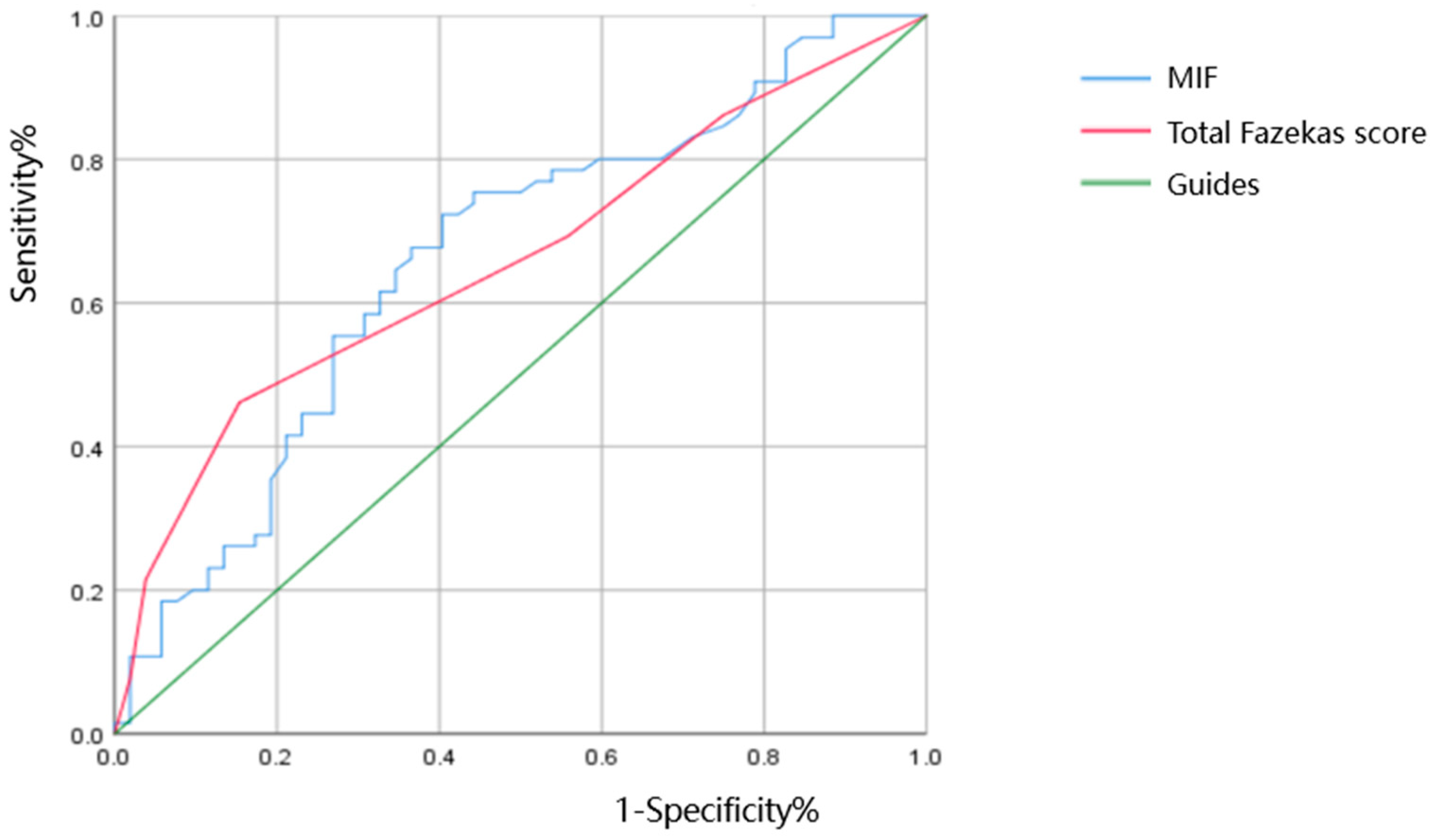
| AUC | Sensitivity | Specificity | 95% CI | p | |
|---|---|---|---|---|---|
| MIF (pg/mL) | 0.661 | 0.723 | 0.596 | 0.561~0.761 | 0.003 |
| Total Fazekas score | 0.658 | 0.462 | 0.846 | 0.559~0.756 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, J.; Wang, X.; Yu, M.; Zhang, S.; Li, Q.; Liu, H.; Zhang, J.; Cai, R.; Lu, C.; Li, S. The Relevance of Serum Macrophage Migration Inhibitory Factor Level and Executive Function in Patients with White Matter Hyperintensity in Cerebral Small Vessel Disease. Brain Sci. 2023, 13, 616. https://doi.org/10.3390/brainsci13040616
Zhao J, Wang X, Yu M, Zhang S, Li Q, Liu H, Zhang J, Cai R, Lu C, Li S. The Relevance of Serum Macrophage Migration Inhibitory Factor Level and Executive Function in Patients with White Matter Hyperintensity in Cerebral Small Vessel Disease. Brain Sciences. 2023; 13(4):616. https://doi.org/10.3390/brainsci13040616
Chicago/Turabian StyleZhao, Jianhua, Xiaoting Wang, Miao Yu, Shiyun Zhang, Qiong Li, Hao Liu, Jian Zhang, Ruiyan Cai, Chengbiao Lu, and Shaomin Li. 2023. "The Relevance of Serum Macrophage Migration Inhibitory Factor Level and Executive Function in Patients with White Matter Hyperintensity in Cerebral Small Vessel Disease" Brain Sciences 13, no. 4: 616. https://doi.org/10.3390/brainsci13040616
APA StyleZhao, J., Wang, X., Yu, M., Zhang, S., Li, Q., Liu, H., Zhang, J., Cai, R., Lu, C., & Li, S. (2023). The Relevance of Serum Macrophage Migration Inhibitory Factor Level and Executive Function in Patients with White Matter Hyperintensity in Cerebral Small Vessel Disease. Brain Sciences, 13(4), 616. https://doi.org/10.3390/brainsci13040616






