Cognitive Processes and Resting-State Functional Neuroimaging Findings in High Schizotypal Individuals and Schizotypal Personality Disorder Patients: A Systematic Review
Abstract
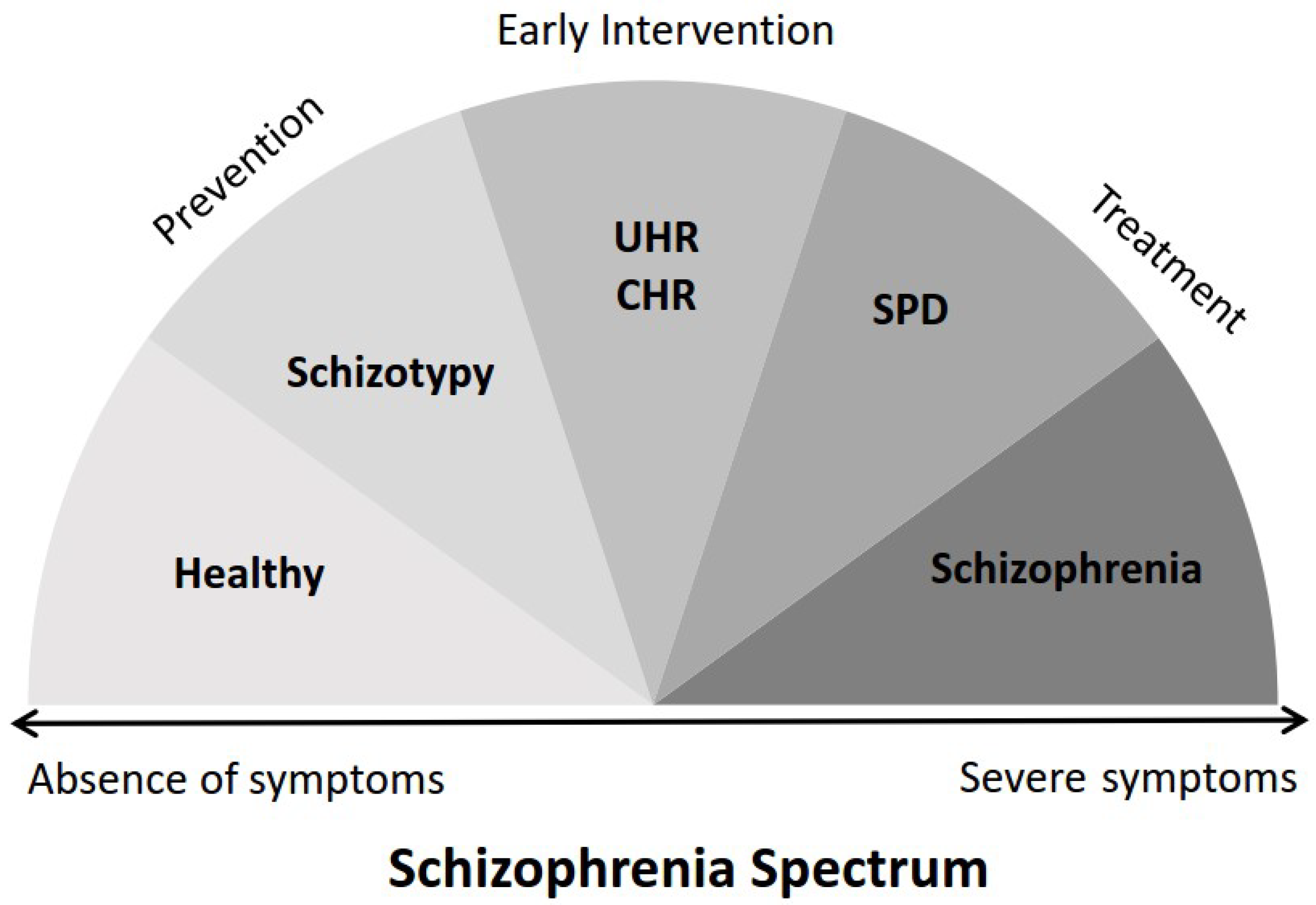
1. Introduction
2. Materials and Methods
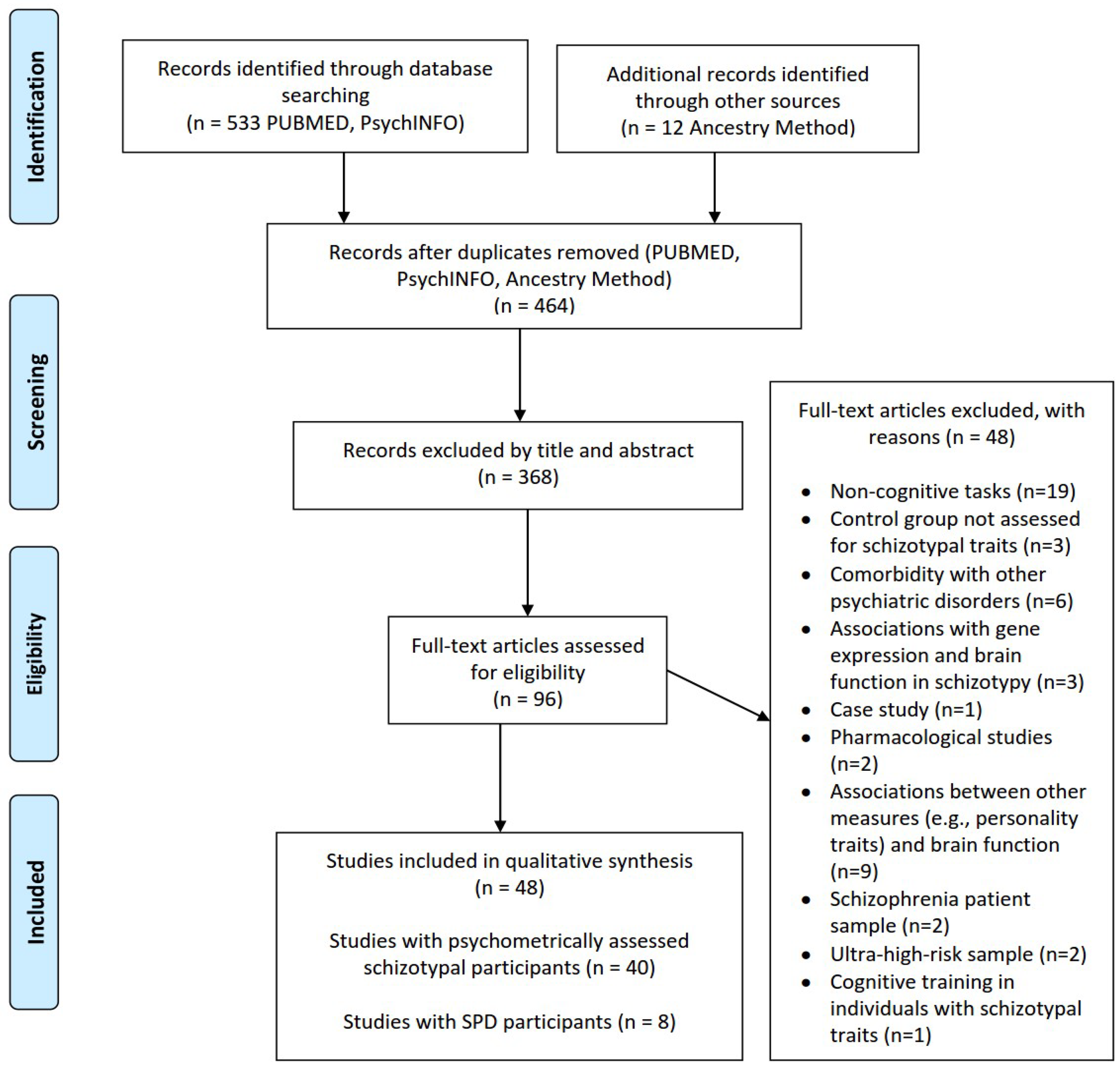
2.1. Review Question and Literature Search/Information Sources
2.2. Eligibility Criteria
2.3. Data Collection and Extraction
2.4. Quality Assessment of Studies
3. Results
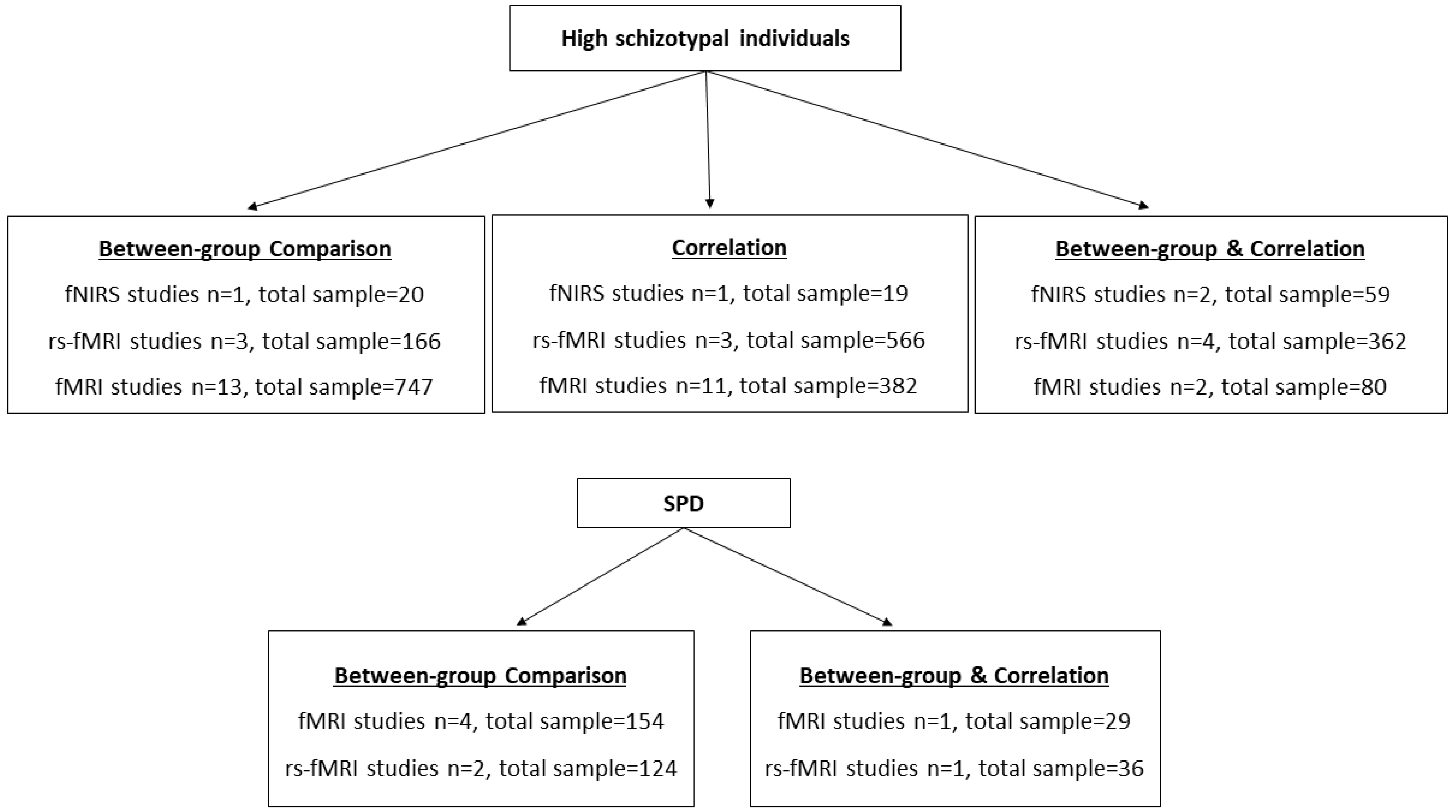
3.1. Characteristics of the Included Studies
3.2. Results of Quality Assessment
3.3. Functional Near-Infrared Spectroscopy (fNIRS) Studies
Cognitive Correlates of Neuroanatomical Features of High Schizotypal Individuals
3.4. Resting-State fMRI Findings
3.4.1. Neuroanatomical Features of High Schizotypal Individuals
Total Schizotypy
Negative Schizotypy
Positive Schizotypy
Disorganized Schizotypy
3.4.2. Neuroanatomical Characteristics of SPD Patients
3.5. fMRI Studies
3.5.1. Cognitive Correlates of Neuroanatomical Features of Schizotypal Individuals
Social Cognition
Memory and Learning
Response Inhibition and Decision Making
Creativity
3.5.2. Cognitive Correlates of Neuroanatomical Features of SPD Patients
Social Cognition
Working Memory
4. Discussion
4.1. fNIRS Studies
4.2. rsFMRI Studies
4.3. fMRI Studies
4.3.1. Social Cognition
4.3.2. Memory and Learning
4.3.3. Response Inhibition and Decision Making
4.3.4. Creativity
5. Conclusions
Supplementary Materials
Funding
Data Availability Statement
Conflicts of Interest
References
- DeRosse, P.; Karlsgodt, K.H. Examining the Psychosis Continuum. Curr. Behav. Neurosci. Rep. 2015, 2, 80–89. [Google Scholar] [CrossRef]
- Keshavan, M.S.; Collin, G.; Guimond, S.; Kelly, S.; Prasad, K.M.; Lizano, P. Neuroimaging in Schizophrenia. Neuroimaging Clin. N. Am. 2020, 30, 73–83. [Google Scholar] [CrossRef]
- Rosell, D.R.; Futterman, S.E.; McMaster, A.; Siever, L.J. Schizotypal personality disorder: A current review. Curr. Psychiatry Rep. 2014, 16, 452. [Google Scholar] [CrossRef] [PubMed]
- Kirchner, S.K.; Roeh, A.; Nolden, J.; Hasan, A. Diagnosis and treatment of schizotypal personality disorder: Evidence from a systematic review. NPJ Schizophr. 2018, 4, 20. [Google Scholar] [CrossRef]
- Kwapil, T.R.; Barrantes-Vidal, N. Schizotypy: Looking back and moving forward. Schizophr. Bull. 2015, 41 (Suppl. S2), S366–S373. [Google Scholar] [CrossRef] [PubMed]
- Linscott, R.J.; van Os, J. An updated and conservative systematic review and meta-analysis of epidemiological evidence on psychotic experiences in children and adults: On the pathway from proneness to persistence to dimensional expression across mental disorders. Psychol. Med. 2013, 43, 1133–1149. [Google Scholar] [CrossRef] [PubMed]
- Grant, P. Is Schizotypy per se a Suitable Endophenotype of Schizophrenia?—Do Not Forget to Distinguish Positive from Negative Facets. Front. Psychiatry 2015, 6, 143. [Google Scholar] [CrossRef]
- Lenzenweger, M.F. Thinking clearly about schizotypy: Hewing to the schizophrenia liability core, considering interesting tangents, and avoiding conceptual quicksand. Schizophr. Bull. 2015, 41 (Suppl. S2), S483–S491. [Google Scholar] [CrossRef]
- Chapman, L.J.; Chapman, J.P.; Raulin, M.L. Scales for physical and social anhedonia. J. Abnorm. Psychol. 1976, 85, 374–382. [Google Scholar] [CrossRef]
- Mason, O.; Claridge, G. The Oxford-Liverpool Inventory of Feelings and Experiences (O-LIFE): Further description and extended norms. Schizophr. Res. 2006, 82, 203–211. [Google Scholar] [CrossRef]
- Raine, A. The SPQ: A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr. Bull. 1991, 17, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Wuthrich, V.M.; Bates, T.C. Confirmatory factor analysis of the three-factor structure of the schizotypal personality questionnaire and Chapman schizotypy scales. J. Pers. Assess. 2006, 87, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Baysan Arabaci, L. Effect of age and gender on schizotypal personality traits in the normal population. Psychiatry Clin. Neurosci. 2009, 63, 663–669. [Google Scholar] [CrossRef]
- Fossati, A.; Raine, A.; Carretta, I.; Leonardi, B.; Maffei, C. The three-factor model of schizotypal personality: Invariance across age and gender. Pers. Individ. Differ. 2003, 35, 1007–1019. [Google Scholar] [CrossRef]
- Raine, A. Schizotypal personality: Neurodevelopmental and psychosocial trajectories. Annu. Rev. Clin. Psychol. 2006, 2, 291–326. [Google Scholar] [CrossRef]
- Compton, M.T.; Goulding, S.M.; Bakeman, R.; McClure-Tone, E.B. Confirmation of a four-factor structure of the Schizotypal Personality Questionnaire among undergraduate students. Schizophr. Res. 2009, 111, 46–52. [Google Scholar] [CrossRef]
- Stefanis, N.C.; Smyrnis, N.; Avramopoulos, D.; Evdokimidis, I.; Ntzoufras, I.; Stefanis, C.N. Factorial composition of self-rated schizotypal traits among young males undergoing military training. Schizophr. Bull. 2004, 30, 335–350. [Google Scholar] [CrossRef]
- Tsaousis, I.; Zouraraki, C.; Karamaouna, P.; Karagiannopoulou, L.; Giakoumaki, S.G. The validity of the Schizotypal Personality Questionnaire in a Greek sample: Tests of measurement invariance and latent mean differences. Compr. Psychiatry 2015, 62, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, U.; Meyhöfer, I.; Steffens, M.; Wagner, M.; Koutsouleris, N. Genetics, cognition, and neurobiology of schizotypal personality: A review of the overlap with schizophrenia. Front. Psychiatry 2014, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.T.; Seal, M.L.; Pantelis, C.; Phillips, L.J. Evidence of a dimensional relationship between schizotypy and schizophrenia: A systematic review. Neurosci. Biobehav. Rev. 2013, 37, 317–327. [Google Scholar] [CrossRef]
- Barrantes-Vidal, N.; Grant, P.; Kwapil, T.R. The role of schizotypy in the study of the etiology of schizophrenia spectrum disorders. Schizophr. Bull. 2015, 41 (Suppl. S2), S408–S416. [Google Scholar] [CrossRef] [PubMed]
- Chemerinski, E.; Triebwasser, J.; Roussos, P.; Siever, L.J. Schizotypal personality disorder. J. Pers. Disord. 2013, 27, 652–679. [Google Scholar] [CrossRef] [PubMed]
- Mohr, C.; Ettinger, U. An overview of the association between schizotypy and dopamine. Front. Psychiatry 2014, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Walter, E.E.; Fernandez, F.; Snelling, M.; Barkus, E. Genetic consideration of schizotypal traits: A review. Front. Psychol. 2016, 7, 1769. [Google Scholar] [CrossRef]
- Attademo, L.; Bernardini, F.; Verdolini, N. Neural correlates of schizotypal personality disorder: A systematic review of neuroimaging and EEG studies. Curr. Med. Imaging 2017, 17, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Kirschner, M.; Hodzic-Santor, B.; Antoniades, M.; Nenadic, I.; Kircher, T.; Krug, A.; Meller, T.; Grotegerd, D.; Fornito, A.; Arnatkeviciute, A.; et al. Cortical and subcortical neuroanatomical signatures of schizotypy in 3004 individuals assessed in a worldwide ENIGMA study. Mol. Psychiatry 2022, 27, 1167–1176. [Google Scholar] [CrossRef]
- Kraguljac, N.V.; McDonald, W.M.; Widge, A.S.; Rodriguez, C.I.; Tohen, M.; Nemeroff, C.B. Neuroimaging biomarkers in schizophrenia. Am. J. Psychiatry 2021, 178, 509–521. [Google Scholar] [CrossRef]
- Tonini, E.; Quidé, Y.; Kaur, M.; Whitford, T.J.; Green, M.J. Structural and functional neural correlates of schizotypy: A systematic review. Psychol. Bull. 2021, 147, 828–866. [Google Scholar] [CrossRef]
- Ettinger, U.; Mohr, C.; Gooding, D.C.; Cohen, A.S.; Rapp, A.; Haenschel, C.; Park, S. Cognition and brain function in schizotypy: A selective review. Schizophr. Bull. 2015, 41 (Suppl. S2), S417–S426. [Google Scholar] [CrossRef]
- McCleery, A.; Nuechterlein, K.H. Cognitive impairment in psychotic illness: Prevalence, profile of impairment, developmental course, and treatment considerations. Dialogues Clin. Neurosci. 2019, 21, 239–248. [Google Scholar] [CrossRef]
- Mitropoulou, V.; Harvey, P.D.; Maldari, L.A.; Moriarty, P.J.; New, A.S.; Silverman, J.M.; Siever, L.J. Neuropsychological performance in schizotypal personality disorder: Evidence regarding diagnostic specificity. Biol. Psychiatry 2002, 52, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Siddi, S.; Petretto, D.R.; Preti, A. Neuropsychological correlates of schizotypy: A systematic review and meta-analysis of cross-sectional studies. Cogn. Neuropsychiatry 2017, 22, 186–212. [Google Scholar] [CrossRef] [PubMed]
- Green, M.F.; Horan, W.P.; Lee, J. Social cognition in schizophrenia. Nat. Rev. Neurosci. 2015, 16, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Zouraraki, C.; Karamaouna, P.; Giakoumaki, S.G. Facial emotion recognition and schizotypal traits: A systematic review of behavioural studies. Early Interv. Psychiatry 2023, 17, 121–140. [Google Scholar] [CrossRef] [PubMed]
- Mwansisya, T.E.; Hu, A.; Li, Y.; Chen, X.; Wu, G.; Huang, X.; Lv, D.; Li, Z.; Liu, C.; Xue, Z.; et al. Task and resting-state fMRI studies in first-episode schizophrenia: A systematic review. Schizophr. Res. 2017, 189, 9–18. [Google Scholar] [CrossRef]
- Sheffield, J.M.; Barch, D.M. Cognition and resting-state functional connectivity in schizophrenia. Neurosci. Biobehav. Rev. 2016, 61, 108–120. [Google Scholar] [CrossRef]
- Mehta, U.M.; Ibrahim, F.A.; Sharma, M.S.; Venkatasubramanian, G.; Thirthalli, J.; Bharath, R.D.; Bolo, N.R.; Gangadhar, B.N.; Keshavan, M.S. Resting-state functional connectivity predictors of treatment response in schizophrenia—A systematic review and meta-analysis. Schizophr. Res. 2021, 237, 153–165. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Gohel, S.; Vaccaro, D.H.; Chu, K.W.; Tang, C.Y.; Goldstein, K.E.; New, A.S.; Siever, L.J.; McClure, M.; Perez-Rodriguez, M.M.; et al. Frontotemporal thalamic connectivity in schizophrenia and schizotypal personality disorder. Psychiatry Res. Neuroimaging 2022, 322, 111463. [Google Scholar] [CrossRef]
- Chou, P.H.; Huang, C.J.; Sun, C.W. The potential role of functional near-infrared spectroscopy as clinical biomarkers in schizophrenia. Curr. Pharm. Des. 2020, 26, 201–217. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Löhönen, J.; Isohanni, M.; Nieminen, P.; Miettunen, J. Coverage of the bibliographic databases in mental health research. Nord. J. Psychiatry 2010, 64, 181–188. [Google Scholar] [CrossRef]
- Fervaha, G.; Remington, G. Neuroimaging findings in schizotypal personality disorder: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 96–107. [Google Scholar] [CrossRef]
- Kozhuharova, P.; Saviola, F.; Ettinger, U.; Allen, P. Neural correlates of social cognition in populations at risk of psychosis: A systematic review. Neurosci. Biobehav. Rev. 2020, 108, 94–111. [Google Scholar] [CrossRef]
- O’Connor, D.; Green, S.; Higgins, J.P.T. Chapter 5: Defining the review question and developing criteria for including studies. In Cochrane Handbook of Systematic Reviews of Interventions; Higgins, J.P.T., Green, S., Eds.; John Wiley & Sons: Chichester, UK, 2008. [Google Scholar]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 16 February 2023).
- Herzog, R.; Álvarez-Pasquin, M.J.; Díaz, C.; Del Barrio, J.L.; Estrada, J.M.; Gil, Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health 2013, 13, 154. [Google Scholar] [CrossRef]
- Friston, K. Ten ironic rules for non-statistical reviewers. NeuroImage 2012, 61, 1300–1310. [Google Scholar] [CrossRef]
- Peng, M.M.; Xing, J.; Tang, X.; Wu, Q.; Wei, D.; Ran, M.S. Disease-Related Risk Factors for Caregiver Burden among Family Caregivers of Persons with Schizophrenia: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 1862. [Google Scholar] [CrossRef]
- Folley, B.S.; Park, S. Verbal creativity and schizotypal personality in relation to prefrontal hemispheric laterality: A behavioral and near-infrared optical imaging study. Schizophr. Res. 2005, 80, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Ozeki, Y.; Terada, S.; Kunugi, H. Functional near-infrared spectroscopy reveals altered hemispheric laterality in relation to schizotypy during verbal fluency task. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1944–1951. [Google Scholar] [CrossRef]
- Hori, H.; Nagamine, M.; Soshi, T.; Okabe, S.; Kim, Y.; Kunugi, H. Schizotypal traits in healthy women predict prefrontal activation patterns during a verbal fluency task: A near-infrared spectroscopy study. Neuropsychobiology 2008, 57, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Iwama, Y.; Nishimaru, H.; Matsumoto, J.; Setogawa, T.; Ono, T.; Nishijo, H. Examination of the Prefrontal Cortex Hemodynamic Responses to the Fist-Edge-Palm Task in Naïve Subjects Using Functional Near-Infrared Spectroscopy. Front. Hum. Neurosci. 2021, 15, 617626. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yan, C.; Yin, D.Z.; Fan, M.X.; Cheung, E.F.; Pantelis, C.; Chan, R.C. Neurobiological changes of schizotypy: Evidence from both volume-based morphometric analysis and resting-state functional connectivity. Schizophr. Bull. 2015, 41 (Suppl. S2), S444–S454. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.H.; Li, Z.; Wei, X.H.; Jiang, X.Q.; Geng, F.L.; Zou, L.Q.; Lui, S.S.; Cheung, E.F.; Pantelis, C.; et al. Altered corticostriatal functional connectivity in individuals with high social anhedonia. Psychol. Med. 2016, 46, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ettinger, U.; Meindl, T.; Chan, R. Association of schizotypy with striatocortical functional connectivity and its asymmetry in healthy adults. Hum. Brain Mapp. 2018, 39, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Sabaroedin, K.; Tiego, J.; Parkes, L.; Sforazzini, F.; Finlay, A.; Johnson, B.; Pinar, A.; Cropley, V.; Harrison, B.J.; Zalesky, A.; et al. Functional Connectivity of Corticostriatal Circuitry and Psychosis-like Experiences in the General Community. Biol. Psychiatry 2019, 86, 16–24. [Google Scholar] [CrossRef]
- Waltmann, M.; O’Daly, O.; Egerton, A.; McMullen, K.; Kumari, V.; Barker, G.J.; Williams, S.; Modinos, G. Multi-echo fMRI, resting-state connectivity, and high psychometric schizotypy. NeuroImage Clin. 2019, 21, 101603. [Google Scholar] [CrossRef]
- Wang, Y.M.; Cai, X.L.; Zhou, H.Y.; Zhang, R.T.; Zhang, Y.J.; Wang, Y.Y.; Cheung, E.; Chan, R. Altered default mode network functional connectivity in individuals with co-occurrence of schizotypy and obsessive-compulsive traits. Psychiatry Res. Neuroimaging. 2020, 305, 111170. [Google Scholar] [CrossRef]
- Zhang, R.T.; Yang, Z.Y.; Wang, Y.M.; Wang, Y.; Yang, T.X.; Cheung, E.; Martin, E.A.; Chan, R. Affective forecasting in individuals with social anhedonia: The role of social components in anticipated emotion, prospection and neural activation. Schizophr. Res. 2020, 215, 322–329. [Google Scholar] [CrossRef]
- Kozhuharova, P.; Saviola, F.; Diaconescu, A.; Allen, P. High schizotypy traits are associated with reduced hippocampal resting state functional connectivity. Psychiatry Res. Neuroimaging 2021, 307, 111215. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Wang, Y.M.; Zhang, R.T.; Cheung, E.; Pantelis, C.; Chan, R. Neural correlates of audiovisual temporal binding window in individuals with schizotypal and autistic traits: Evidence from resting-state functional connectivity. Autism Res. 2021, 14, 668–680. [Google Scholar] [CrossRef]
- Wang, Y.M.; Cai, X.L.; Zhang, R.T.; Zhang, Y.J.; Zhou, H.Y.; Wang, Y.; Wang, Y.; Huang, J.; Wang, Y.Y.; Cheung, E.; et al. Altered brain structural and functional connectivity in schizotypy. Psychol. Med. 2022, 52, 834–843. [Google Scholar] [CrossRef]
- Mohanty, A.; Herrington, J.D.; Koven, N.S.; Fisher, J.E.; Wenzel, E.A.; Webb, A.G.; Heller, W.; Banich, M.T.; Miller, G.A. Neural mechanisms of affective interference in schizotypy. J. Abnorm. Psychol. 2005, 114, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.O.; Pruessner, J.; Czechowska, Y.; Lepage, M. Individual differences in trait anhedonia: A structural and functional magnetic resonance imaging study in non-clinical subjects. Mol. Psychiatry 2007, 12, 703–775. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.O.; Armony, J.; Malla, A.; Lepage, M. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. J. Psychiatr. Res. 2010, 44, 707–716. [Google Scholar] [CrossRef]
- Modinos, G.; Renken, R.; Shamay-Tsoory, S.G.; Ormel, J.; Aleman, A. Neurobiological correlates of theory of mind in psychosis proneness. Neuropsychologia 2010, 48, 3715–3724. [Google Scholar] [CrossRef]
- Rapp, A.M.; Mutschler, D.E.; Wild, B.; Erb, M.; Lengsfeld, I.; Saur, R.; Grodd, W. Neural correlates of irony comprehension: The role of schizotypal personality traits. Brain Lang. 2010, 113, 1–12. [Google Scholar] [CrossRef]
- Germine, L.T.; Garrido, L.; Bruce, L.; Hooker, C. Social anhedonia is associated with neural abnormalities during face emotion processing. NeuroImage 2011, 58, 935–945. [Google Scholar] [CrossRef] [PubMed]
- Corlett, P.R.; Fletcher, P.C. The neurobiology of schizotypy: Fronto-striatal prediction error signal correlates with delusion-like beliefs in healthy people. Neuropsychologia 2012, 50, 3612–3620. [Google Scholar] [CrossRef]
- Ettinger, U.; Corr, P.J.; Mofidi, A.; Williams, S.C.; Kumari, V. Dopaminergic basis of the psychosis-prone personality investigated with functional magnetic resonance imaging of procedural learning. Front. Hum. Neurosci. 2013, 7, 130. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Y.; Jin, Z.; Di, X.; Yang, T.; Gur, R.C.; Gur, R.E.; Shum, D.H.; Cheung, E.F.; Chan, R.C. Happy facial expression processing with different social interaction cues: An fMRI study of individuals with schizotypal personality traits. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 44, 108–117. [Google Scholar] [CrossRef]
- van der Meer, L.; Groenewold, N.A.; Pijnenborg, M.; Aleman, A. Psychosis-proneness and neural correlates of self-inhibition in theory of mind. PLoS ONE 2013, 8, e67774. [Google Scholar] [CrossRef]
- Fink, A.; Weber, B.; Koschutnig, K.; Benedek, M.; Reishofer, G.; Ebner, F.; Papousek, I.; Weiss, E.M. Creativity and schizotypy from the neuroscience perspective. Cogn. Affect. Behav. Neurosci. 2014, 14, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Hooker, C.I.; Benson, T.L.; Gyurak, A.; Yin, H.; Tully, L.M.; Lincoln, S.H. Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia. J. Abnorm. Psychol. 2014, 123, 190–204. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, T.X.; Su, L.; Yan, C.; Wang, Y.; Huang, J.; Fan, M.X.; Yin, D.Z.; Jin, Z.; Zeng, Y.W.; et al. Neural correlates of prospective memory in individuals with schizotypal personality features. Neuropsychology 2014, 28, 373–381. [Google Scholar] [CrossRef]
- Park, H.R.; Kirk, I.J.; Waldie, K.E. Neural correlates of creative thinking and schizotypy. Neuropsychologia 2015, 73, 94–107. [Google Scholar] [CrossRef]
- Simon, J.J.; Cordeiro, S.A.; Weber, M.A.; Friederich, H.C.; Wolf, R.C.; Weisbrod, M.; Kaiser, S. Reward System Dysfunction as a Neural Substrate of Symptom Expression Across the General Population and Patients With Schizophrenia. Schizophr. Bull. 2015, 41, 1370–1378. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.H.; Li, Z.; Wei, X.H.; Jiang, X.Q.; Neumann, D.L.; Shum, D.H.; Cheung, E.F.; Chan, R.C. Dimensional schizotypy and social cognition: An fMRI imaging study. Front. Behav. Neurosci. 2015, 9, 133. [Google Scholar] [CrossRef]
- Yin, H.; Tully, L.M.; Lincoln, S.H.; Hooker, C.I. Adults with high social anhedonia have altered neural connectivity with ventral lateral prefrontal cortex when processing positive social signals. Front. Hum. Neurosci. 2015, 9, 469. [Google Scholar] [CrossRef]
- Chan, R.C.; Li, Z.; Li, K.; Zeng, Y.W.; Xie, W.Z.; Yan, C.; Cheung, E.F.; Jin, Z. Distinct processing of social and monetary rewards in late adolescents with trait anhedonia. Neuropsychology 2016, 30, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Wang, Y.; Su, L.; Xu, T.; Yin, D.Z.; Fan, M.X.; Deng, C.P.; Wang, Z.X.; Lui, S.S.; Cheung, E.F.; et al. Differential mesolimbic and prefrontal alterations during reward anticipation and consummation in positive and negative schizotypy. Psychiatry Res. Neuroimaging 2016, 254, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Modinos, G.; McLaughlin, A.; Egerton, A.; McMullen, K.; Kumari, V.; Barker, G.J.; Keysers, C.; Williams, S.C. Corticolimbic hyper-response to emotion and glutamatergic function in people with high schizotypy: A multimodal fMRI-MRS study. Transl. Psychiatry 2017, 7, e1083. [Google Scholar] [CrossRef]
- Günther, V.; Zimmer, J.; Kersting, A.; Hoffmann, K.T.; Lobsien, D.; Suslow, T. Automatic processing of emotional facial expressions as a function of social anhedonia. Psychiatry Res. Neuroimaging 2017, 270, 46–53. [Google Scholar] [CrossRef]
- Papanastasiou, E.; Mouchlianitis, E.; Joyce, D.W.; McGuire, P.; Banaschewski, T.; Bokde, A.; Bromberg, U.; Büchel, C.; Quinlan, E.B.; Desrivières, S.; et al. Examination of the Neural Basis of Psychoticlike Experiences in Adolescence During Reward Processing. JAMA Psychiatry 2018, 75, 1043–1051. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Liu, W.H.; Wei, X.H.; Jiang, X.Q.; Lui, S.; Ho-Wai So, S.; Cheung, E.; Debbane, M.; Chan, R. Negative Schizotypy and Altered Functional Connectivity During Facial Emotion Processing. Schizophr. Bull. 2018, 44 (Suppl. S2), S491–S500. [Google Scholar] [CrossRef]
- Schmidt, S.N.L.; Fenske, S.C.; Kirsch, P.; Mier, D. Nucleus accumbens activation is linked to salience in social decision making. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Olano, M.A.; Elizalde Acevedo, B.; Chambeaud, N.; Acuña, A.; Marcó, M.; Kochen, S.; Alba-Ferrara, L. Emotional salience enhances intelligibility in adverse acoustic conditions. Neuropsychologia 2020, 147, 107580. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Schmidt, S.; Frank, J.; Witt, S.H.; Hass, J.; Kirsch, P.; Mier, D. Hyperfunctioning of the right posterior superior temporal sulcus in response to neutral facial expressions presents an endophenotype of schizophrenia. Neuropsychopharmacology 2020, 45, 1346–1352. [Google Scholar] [CrossRef]
- Koenigsberg, H.W.; Buchsbaum, M.S.; Buchsbaum, B.R.; Schneiderman, J.S.; Tang, C.Y.; New, A.; Goodman, M.; Siever, L.J. Functional MRI of visuospatial working memory in schizotypal personality disorder: A region-of-interest analysis. Psychol. Med. 2005, 35, 1019–1030. [Google Scholar] [CrossRef]
- Dickey, C.C.; Morocz, I.A.; Minney, D.; Niznikiewicz, M.A.; Voglmaier, M.M.; Panych, L.P.; Khan, U.; Zacks, R.; Terry, D.P.; Shenton, M.E.; et al. Factors in sensory processing of prosody in schizotypal personality disorder: An fMRI experiment. Schizophr. Res. 2010, 121, 75–89. [Google Scholar] [CrossRef]
- Hazlett, E.A.; Zhang, J.; New, A.S.; Zelmanova, Y.; Goldstein, K.E.; Haznedar, M.M.; Meyerson, D.; Goodman, M.; Siever, L.J.; Chu, K.W. Potentiated amygdala response to repeated emotional pictures in borderline personality disorder. Biol. Psychiatry 2012, 72, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Vu, M.A.; Thermenos, H.W.; Terry, D.P.; Wolfe, D.J.; Voglmaier, M.M.; Niznikiewicz, M.A.; McCarley, R.W.; Seidman, L.J.; Dickey, C.C. Working memory in schizotypal personality disorder: fMRI activation and deactivation differences. Schizophr. Res. 2013, 151, 113–123. [Google Scholar] [CrossRef]
- Stanfield, A.C.; Philip, R.; Whalley, H.; Romaniuk, L.; Hall, J.; Johnstone, E.C.; Lawrie, S.M. Dissociation of Brain Activation in Autism and Schizotypal Personality Disorder during Social Judgments. Schizophr. Bull. 2017, 43, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shen, J.; Wu, J.; Yu, X.; Lou, W.; Fan, H.; Shi, L.; Wang, D. Altered default mode network functional connectivity in schizotypal personality disorder. Schizophr. Res. 2014, 160, 51–56. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, Y.; Zhang, T.; Li, H.; Tang, Y.; Li, C.; Luo, X.; He, Y.; Lu, Z.; Wang, J. Reduced functional connectivity between bilateral precuneus and contralateral parahippocampus in schizotypal personality disorder. BMC Psychiatry 2017, 17, 48. [Google Scholar] [CrossRef] [PubMed]
- Ueno, M.; Takano, K.; Asai, T.; Tanno, Y. Reliability and validity of the Japanese version of the Oxford schizotypal personality scale. Jpn. J. Pers. 2010, 18, 161–164. [Google Scholar] [CrossRef]
- Chan, R.C.; Wang, Y.; Yan, C.; Zhao, Q.; McGrath, J.; Hsi, X.; Stone, W.S. A study of trait anhedonia in non-clinical Chinese samples: Evidence from the Chapman Scales for Physical and Social Anhedonia. PLoS ONE 2012, 7, e34275. [Google Scholar] [CrossRef] [PubMed]
- Eckblad, M.L.; Chapman, L.J.; Chapman, J.P.; Mishlove, M. The Revised Social Anhedonia Scale. 1982. Unpublished Test. Unpublished Test. 1982. [Google Scholar]
- Eckblad, M.; Chapman, L.J. Magical ideation as an indicator of schizotypy. J. Consult. Clin. Psychol. 1983, 51, 215–225. [Google Scholar] [CrossRef]
- Chapman, L.J.; Chapman, J.P.; Raulin, M.L. Body-image aberration in Schizophrenia. J. Abnorm. Psychol. 1978, 87, 399–407. [Google Scholar] [CrossRef]
- Peters, E.; Joseph, S.; Day, S.; Garety, P. Measuring delusional ideation: The 21-item Peters et al. Delusions Inventory (PDI). Schizophr. Bull. 2004, 30, 1005–1022. [Google Scholar] [CrossRef]
- Stefanis, N.C.; Hanssen, M.; Smirnis, N.K.; Avramopoulos, D.A.; Evdokimidis, I.K.; Stefanis, C.N.; Verdoux, H.; Van Os, J. Evidence that three dimensions of psychosis have a distribution in the general population. Psychol. Med. 2002, 32, 347–358. [Google Scholar] [CrossRef]
- Konings, M.; Bak, M.; Hanssen, M.; van Os, J.; Krabbendam, L. Validity and reliability of the CAPE: A self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr. Scand. 2006, 114, 55–61. [Google Scholar] [CrossRef]
- Peters, E.R.; Joseph, S.A.; Garety, P.A. Measurement of delusional ideation in the normal population: Introducing the PDI (Peters et al. Delusions Inventory). Schizophr. Bull. 1999, 25, 553–576. [Google Scholar] [CrossRef]
- Eysenck, H.J.; Eysenck, S.B.G. Eysenck Personality Questionnaire—Revised (EPQ-R); Hodder and Stoughton: London, UK, 1991. [Google Scholar]
- Claridge, G.; Broks, P. Schizotypy and hemisphere function: I. Theoretical considerations and the measurement of schizotypy. Pers. Individ. Diff. 1984, 5, 633–648. [Google Scholar] [CrossRef]
- Dandash, O.; Fornito, A.; Lee, J.; Keefe, R.S.; Chee, M.W.; Adcock, R.A.; Pantelis, C.; Wood, S.J.; Harrison, B.J. Altered striatal functional connectivity in subjects with an at-risk mental state for psychosis. Schizophr. Bull. 2014, 40, 904–913. [Google Scholar] [CrossRef]
- Fornito, A.; Harrison, B.J.; Goodby, E.; Dean, A.; Ooi, C.; Nathan, P.J.; Lennox, B.R.; Jones, P.B.; Suckling, J.; Bullmore, E.T. Functional dysconnectivity of corticostriatal circuitry as a risk phenotype for psychosis. JAMA Psychiatry 2013, 70, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Jing, R.X.; Zhao, R.J.; Shi, L.; Sun, H.Q.; Ding, Z.; Lin, X.; Lu, L.; Fan, Y. Association between functional and structural connectivity of the corticostriatal network in people with schizophrenia and unaffected first-degree relatives. J. Psychiatry Neurosci. 2020, 45, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Pani, S.M.; Sabaroedin, K.; Tiego, J.; Bellgrove, M.A.; Fornito, A. A multivariate analysis of the association between corticostriatal functional connectivity and psychosis-like experiences in the general community. Psychiatry Res. Neuroimaging 2021, 307, 111202. [Google Scholar] [CrossRef] [PubMed]
- Sarpal, D.K.; Robinson, D.G.; Fales, C.; Lencz, T.; Argyelan, M.; Karlsgodt, K.H.; Gallego, J.A.; John, M.; Kane, J.M.; Szeszko, P.R.; et al. Relationship between Duration of Untreated Psychosis and Intrinsic Corticostriatal Connectivity in Patients with Early Phase Schizophrenia. Neuropsychopharmacology 2017, 42, 2214–2221. [Google Scholar] [CrossRef]
- Ribolsi, M.; Daskalakis, Z.J.; Siracusano, A.; Koch, G. Abnormal asymmetry of brain connectivity in schizophrenia. Front. Hum. Neurosci. 2014, 8, 1010. [Google Scholar] [CrossRef]
- Umetsu, A.; Okuda, J.; Fujii, T.; Tsukiura, T.; Nagasaka, T.; Yanagawa, I.; Sugiura, M.; Inoue, K.; Kawashima, R.; Suzuki, K.; et al. Brain activation during the fist-edge-palm test: A functional MRI study. NeuroImage 2002, 17, 385–392. [Google Scholar] [CrossRef]
- Karcher, N.R.; Rogers, B.P.; Woodward, N.D. Functional Connectivity of the Striatum in Schizophrenia and Psychotic Bipolar Disorder. Biol. Psychiatry. Cogn. Neurosci. Neuroimaging 2019, 4, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Sarpal, D.K.; Robinson, D.G.; Lencz, T.; Argyelan, M.; Ikuta, T.; Karlsgodt, K.; Gallego, J.A.; Kane, J.M.; Szeszko, P.R.; Malhotra, A.K. Antipsychotic treatment and functional connectivity of the striatum in first-episode schizophrenia. JAMA Psychiatry 2015, 72, 5–13. [Google Scholar] [CrossRef]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional neuroanatomy of the basal ganglia. Cold Spring Harb. Perspect. Μed. 2012, 2, a009621. [Google Scholar] [CrossRef] [PubMed]
- Cools, R. Dopaminergic control of the striatum for high-level cognition. Curr. Opin. Neurobiol. 2011, 21, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Voorn, P.; Vanderschuren, L.J.; Groenewegen, H.J.; Robbins, T.W.; Pennartz, C.M. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004, 27, 468–474. [Google Scholar] [CrossRef] [PubMed]
- McCutcheon, R.A.; Abi-Dargham, A.; Howes, O.D. Schizophrenia, Dopamine and the Striatum: From Biology to Symptoms. Trends Neurosci. 2019, 42, 205–220. [Google Scholar] [CrossRef]
- Simpson, E.H.; Kellendonk, C.; Kandel, E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron 2010, 65, 585–596. [Google Scholar] [CrossRef]
- Kaur, A.; Basavanagowda, D.M.; Rathod, B.; Mishra, N.; Fuad, S.; Nosher, S.; Alrashid, Z.A.; Mohan, D.; Heindl, S.E. Structural and Functional Alterations of the Temporal lobe in Schizophrenia: A Literature Review. Cureus 2020, 12, e11177. [Google Scholar] [CrossRef]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef]
- Del Fabro, L.; Schmidt, A.; Fortea, L.; Delvecchio, G.; D’Agostino, A.; Radua, J.; Borgwardt, S.; Brambilla, P. Functional brain network dysfunctions in subjects at high-risk for psychosis: A meta-analysis of resting-state functional connectivity. Neurosci. Biobehav. Rev. 2021, 128, 90–101. [Google Scholar] [CrossRef]
- Dong, D.; Wang, Y.; Chang, X.; Luo, C.; Yao, D. Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophr. Bull. 2018, 44, 168–181. [Google Scholar] [CrossRef]
- O’Neill, A.; Mechelli, A.; Bhattacharyya, S. Dysconnectivity of Large-Scale Functional Networks in Early Psychosis: A Meta-analysis. Schizophr. Bull. 2019, 45, 579–590. [Google Scholar] [CrossRef]
- Friston, K.J.; Frith, C.D. Schizophrenia: A disconnection syndrome? Clin. Neurosci. 1995, 3, 89–97. [Google Scholar]
- Friston, K.; Brown, H.R.; Siemerkus, J.; Stephan, K.E. The dysconnection hypothesis (2016). Schizophr. Res. 2016, 176, 83–94. [Google Scholar] [CrossRef]
- Pettersson-Yeo, W.; Allen, P.; Benetti, S.; McGuire, P.; Mechelli, A. Dysconnectivity in schizophrenia: Where are we now? Neurosci. Biobehav. Rev. 2011, 35, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.F.; Kang, J.; Brege, I.S.; Tso, I.F.; Hosanagar, A.; Johnson, T.D. Meta-analysis of functional neuroimaging studies of emotion perception and experience in schizophrenia. Biol. Psychiatry 2012, 71, 136–145. [Google Scholar] [CrossRef]
- Adams, R.; David, A.S. Patterns of anterior cingulate activation in schizophrenia: A selective review. Neuropsychiatr. Dis. Treat. 2007, 3, 87–101. [Google Scholar] [CrossRef]
- Jáni, M.; Kašpárek, T. Emotion recognition and theory of mind in schizophrenia: A meta-analysis of neuroimaging studies. World J. Biol. Psychiatry 2018, 19 (Suppl. S3), S86–S96. [Google Scholar] [CrossRef] [PubMed]
- Dandash, O.; Pantelis, C.; Fornito, A. Dopamine, fronto-striato-thalamic circuits and risk for psychosis. Schizophr. Res. 2017, 180, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sabaroedin, K.; Razi, A.; Chopra, S.; Tran, N.; Pozaruk, A.; Chen, Z.; Finlay, A.; Nelson, B.; Allott, K.; Alvarez-Jimenez, M.; et al. Frontostriatothalamic effective connectivity and dopaminergic function in the psychosis continuum. Brain 2023, 146, 372–386. [Google Scholar] [CrossRef]
- Chen, X.-j.; Wang, Y.; Wang, Y.; Yang, T.-x.; Zou, L.-q.; Huang, J.; Li, F.-h.; Chen, A.-t.; Wang, W.-h.; Zheng, H.-f.; et al. Neural correlates of prospective memory impairments in schizophrenia. Neuropsychology 2016, 30, 169–180. [Google Scholar] [CrossRef]
- Zhou, F.C.; Zheng, W.; Lu, L.; Wang, Y.Y.; Ng, C.H.; Ungvari, G.S.; Li, J.; Xiang, Y.T. Prospective memory in schizophrenia: A meta-analysis of comparative studies. Schizophr. Res. 2019, 212, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Forbes, N.F.; Carrick, L.A.; McIntosh, A.M.; Lawrie, S.M. Working memory in schizophrenia: A meta-analysis. Psychol. Med. 2009, 39, 889–905. [Google Scholar] [CrossRef]
- Kirrane, R.M.; Siever, L.J. New perspectives on schizotypal personality disorder. Curr. Psychiatry Rep. 2000, 2, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Chamberlain, S.R.; Monti, M.M.; Duncan, J.; Owen, A.M. The role of the right inferior frontal gyrus: Inhibition and attentional control. NeuroImage 2010, 50, 1313–1319. [Google Scholar] [CrossRef]
- Zeng, J.; Yan, J.; Cao, H.; Su, Y.; Song, Y.; Luo, Y.; Yang, X. Neural substrates of reward anticipation and outcome in schizophrenia: A meta-analysis of fMRI findings in the monetary incentive delay task. Transl. Psychiatry 2022, 12, 448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.L.; Xu, T.; Yang, W.J.; Li, Y.D.; Sun, J.Z.; Wang, K.C.; Beaty, R.E.; Zhang, Q.L.; Zuo, X.N.; Qiu, J. Individual differences in verbal creative thinking are reflected in the precuneus. Neuropsychologia 2015, 75, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network: Anatomy, function, and relevance to disease. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef]
- Szucs, D.; Ioannidis, J.P. Sample size evolution in neuroimaging research: An evaluation of highly-cited studies (1990–2012) and of latest practices (2017–2018) in high-impact journals. NeuroImage 2020, 221, 117164. [Google Scholar] [CrossRef]
| fNIRS Studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study (Year) | Participant N, Mean Age (SD), Gender (M:F) | Sample | Design | Schizotypy Assessment | Neuroimaging Modality, Task | System, Spectrometer | Findings between Group Comparisons | Findings Correlations/ Regressions |
| Folley and Park (2005) [49] | 10 high SCT, mean age = 23.3 (1.6), 5:5 10 CG, mean age = 36.4 (3.1), 6:4 | Community sample | Between-group comparisons | SPQ three-factor model SCT Mean SPQ Total Score = 41.5 (1.1) CG Mean SPQ Total Score = 19.3 (3.5) | fNIRS, “alternative uses” divergent thinking task (DTT) | Hitachi ETG-100, 24-channel spectrometer | SCT ↑ right PFC activation during DTT vs. CG (FDR corrected p < 0.002v) | |
| Hori, Ozeki et al., 2008 [50] | 16 high SCT, mean age = 41.1 (11.8), 2:14 16 low SCT, mean age = 40.2 (10.1), 8:8 | Healthy individuals | Between-group comparisons and correlation analyses | SPQ three-factor model Median split High SCT Total SPQ Score = 19.6 (7.3) Low SCT SPQ Total Score = 6.3 (2.6) | fNIRS, Verbal fluency Letter and Category task (VFT) | FOIRE-3000, 31-channel spectrometer | High SCT ↑ right and ↓ left PFC activation in VFT (p < 0.05) | Positive association SPQ total score-right PFC dominance in the letter and category VFT (all p values < 0.05) |
| Hori, Nagamine et al., 2008 [51] | 14 high SCT, mean age = 33.8 (13.6), 0:14 13 low SCT, mean age = 43.9 (10.2), 0:13 | Healthy individuals | Between-group comparisons and correlation analyses | SPQ three-factor model Median Split High SCT SPQ Total Score = 16.7 (7) Low SCT SPQ Total Score = 4.5 (2.8) | fNIRS, Verbal fluency task (VFT)-letter version | Hitachi ETG-100, 24-channel spectrometer | High SCT ↑ bilateral PFC activation Low SCT ↑ left PFC activation High SCT showed sustained PFC activation in the post-task period vs. low SCT (all p values < 0.05) | Positive correlation SPQ subscale unusual perceptual experiences-activation of the four right and four left PFC channels (both p values < 0.05) Positive correlations SPQ total score (p < 0.01), cognitive-perceptual (p < 0.01), disorganized (p < 0.05) and interpersonal (p < 0.05) factors and odd speech, social anxiety subscales (all p values < 0.05) with the average activation of the four right PFC channels |
| Kobayashi et al., 2021 [52] | 19 healthy participants, mean age = 23.00 (0.30), 9:10 | Healthy individuals | Correlation analyses | Oxford Schizotypal Personality Scale (STA) Mean STA score = 9.15 (1.43) | fNIRS, Fist-Edge-Palm (FEP) task and palm tapping task as a control task | Shimadzu Co., Ltd., OMM3000, five-channel spectrometer | non-significant findings (all p values > 0.17). | |
| Resting-state fMRI studies | ||||||||
| Study (Year) | Participant N, mean age (SD), gender (M:F) | Sample | Design | Schizotypy assessment | Scanner, Strength | Findings between group comparisons | Findings correlations/regressions | |
| Wang, Yan et al., 2015 [53] | 35 High SCT, mean age = 19.7 (1.1), 19:16 34 Low SCT, mean age = 20.1 (0.9), 15:19 | College students | Between-group comparisons and correlation analyses | SPQ three-factor model High SCT SPQ Total Score> 10th percentile Low SCT SPQ Total Score bottom 50% | Siemens Trio, 3T | High SCT (a) ↓ FC left insula-left putamen, (b) ↑ FC left declive of cerebellum-right medial frontal gyrus, vs. Low SCT (AlphaSim correction both p values < 0.05) | No-significant findings (all p values >0.05). | |
| Wang et al., 2016 [54] | 21 High SocAn, mean age = 19.3 (1.0), 10:11 30 Low SocAn, mean age = 19.3 (0.9), 15:15 | Participants recruited from university | Between-group comparisons | Social Anhedonia Scale Scores 0.5 SD above or below the gender mean High SocAn Mean Total score = 15.19 (3.28) Low SocAn Mean Total Score = 3.07 (1.59) | Siemens Verio 3 T | High SocAn group ↓ FC posterior cingulate cortex-bilateral nucleus accumbens vs. Low SocAn group (p = 0.001) High SocAn group ↑ FC medial frontal gyrus-bilateral nucleus accumbens, insula-ventral caudate, superior frontal gyrus-dorsorostral putamen (all p values < 0.001) | ||
| Wang, Ettinger et al., 2018 [55] | 111 participants, mean age = 26.91 (7.9), 55:56 | Healthy individuals | Correlation analyses | SPQ three-factor model Mean SPQ Total score = 7.81(6.72) | Siemens MAGNETOM Verio, 3T | Negative correlations SPQ total score—FC between (a) right dorsal caudate-bilateral posterior cingulate, (b) left ventral rostral putamen (VRP)-right superior frontal gyrus (all pFWE values < 0.05) Positive correlations SPQ total score-FC between (a) right VRP-superior frontal gyrus, (b) left VRP-cingulate (all pFWE values < 0.05) Positive correlations Cognitive-perceptual SPQ factor-FC between (a) right VRP-right middle frontal gyrus and inferior parietal lobe, (b) left VRP-right medial frontal gyrus (all pFWE values < 0.05) Negative correlations Disorganized SPQ factor –FC between (a) right dorsal caudate-posterior cingulate, (b) left dorsal caudal putamen-left cuneus, (c) right dorsal rostral putamen-middle temporal gyrus (all pFWE values < 0.05) Positive correlation between SPQ total score and asymmetry index of the right VRP (p < 0.001). | ||
| Sabaroedin et al., 2019 [56] | 353 participants, median age = 22, 155:198 | Community sample | Correlation analyses | Online battery of Psychosis-Like Experiences (PLE) measures: 1. Short-form Oxford–Liverpool Inventory of Feelings and Experiences 2. Peters Delusion Inventory 3. Community Assessment of Psychotic Experience 4. Chapman Scales magical ideation, perceptual aberration, social and physical anhedonia | 3T | Positive PLE Dimension Higher scores on the positive PLE dimension were associated with (a) ↓ FC dorsorostral putamen-right DLPFC (p = 0.002, threshold-free cluster enhancement-TFCE corrected) (b) ↓ coupling dorsal caudate-left dorsal anterior cingulate cortex (ACC) (p = 0.011, TFCE corrected) (c) ↓ coupling dorsocaudal putamen (DCP)-right primary motor cortex (p = 0.01, TFCE corrected) Negative PLEs Dimension Negative PLEs correlation with ↑ FC DCP seeds-right primary motor area (p < 0.001 TFCE corrected) | ||
| Waltmann et al., 2019 [57] | 19 High positive SCT, mean age = 26.37 (7.09), 10:9 20 Low positive SCT, mean age = 26.35 (5.47), 10:10 | Healthy individuals | Between-group comparisons and correlation analyses | O-LIFE Short Version-Unusual Experiences factor High SCT mean Unusual Experiences Score = 11.42 (4.31) Low SCT mean Unusual Experiences Score = 0.75 (0.97) | General Electric Discovery MR750, 3T | High positive schizotypy group vs. low positive schizotypy group (a) ↓ FC ventral striatum- bilateral gyrus rectus and right medial orbital gyrus (cluster wise pFWE = 0.037), (b) ↓ FC ventrorostral putamen-right medial orbital gyrus, left gyrus rectus and right ACC (cluster wise pFWE < 0.001), vs. low positive schizotypy group (c) ↓ FC dorsolateral putamen-right hippocampus (cluster wise pFWE < 0.001), left middle occipital gyrus (cluster wise pFWE = 0.005), calcarine sulcus (cluster wise pFWE < 0.001) (d) ↓ FC dorsocaudal putamen-right middle occipital gyrus/calcarine sulcus (cluster wise pFWE < 0.001), left hippocampus (cluster wise pFWE < 0.001), cerebellar areas (cluster wise pFWE = 0.038) | Non-significant findings (all p values > 0.061) | |
| Wang et al., 2020 [58] | 30 high SCT, mean age = 21.30 (1.44), 13:17 30 low SCT, mean age = 22.40 (2.06), 12:18 | College students | Between-group comparisons | SPQ High SCT Mean SPQ Total Score = 47.57 (4.07) top 10th percentile Low SCT Mean SPQ Total Score = 12.53 (7.49) | GE, 3T | High SCT ↑ FC between (a) Default mode network (DMN)- Salience network, (b) DMN-Executive control network, vs. Low SCT (all p values < 0.001 alpha-sim correction) | ||
| Zhang et al., 2020 [59] | 40 SocAn (26 with available neuroimaging data) Mean age of SocAn individuals = 20.70 (4.52), 15:25 46 CG (29 with available neuroimaging data), mean age οf CG = 21.87 (2.61), 11:35 | Participants from a large sample pool | Between-group comparisons | Chapman Social Anhedonia Scale High SocAn Total score > 1.5 SD above the mean Low SocAn Total Score < 10 (below sample mean) | General Electric, Tesla not reported Task-affective forecasting | SocAn ↑ FC vs. CG (a) retrosplenial cortex-bilateral insula, snf medial frontal gyrus, (b) parahippocampal cortex-medial frontal gyrus SocAn ↓ FC vs. CG Hippocampal formation-parahippocampal cortex (All p values < 0.05 FDR corrected) | ||
| Kozhuharova et al., 2021 [60] | 22 high SCT, mean age = 19.45 (1.05), 8:14 23 low SCT, mean age = 20.13 (2.10), 6:17 | Student population | Between-group comparisons and correlation analyses | SPQ three-factor model Bottom and Top 10% deciles of SPQ High SCT SPQ Total Score > 41 Low SCT SPQ Total Score < 12 | Siemens Magnetom TIM Trio, 3T | High SCT ↓ FC vs. low SCT hippocampus-left dorsocaudal putamen (pFWE = 0.02), right caudate-left thalamus (pFWE = 0.04) | Positive effect between the positive SPQ factor and FC hippocampus—caudate and hippocampus—thalamus (both pFWE values = 0.02). | |
| Zhou et al.,2021 [61] | 115 participants, mean age = 21.37 (2.53) 40% males (full set of neuroimaging data only for 102 participants) | Healthy individuals | Correlation analyses | SPQ three factor SPQ Mean Total Score = 34.46 (17.27) | GE 3T | non-significant findings (all p values > 0.21) | ||
| Wang et al., 2022 [62] | 87 high SCT, mean age = 21.17 (2.22), 38:49 122 CG, mean age = 21.62 (2.15), 38:84 | College students | Between-group comparisons and correlation analyses | SPQ three-factor model High SCT SPQ Total Score > 41 (top 10th percentile) CG SPQ Total Score < 41 | GE, 3T | High SCT ↓ FC in areas of interest involved in sensorimotor network, auditory network, visual network, DMN network, task control network vs. CG (all p values < 0.05 FDR corrected) High SCT ↑ FC between (a) left superior frontal gyrus (frontoparietal task network)-right rolandic operculum area (auditor network), (b) right superior frontal gyrus (DMN)-right medial superior frontal gyrus (DMN) (all p values < 0.05 FDR corrected) | Negative correlation Cognitive-perceptual SPQ factor-FC strength left middle occipital gyrus-left inferior parietal lobule in high SCT (p = 0.003 FDR corrected) | |
| fMRI studies | ||||||||
| Study (Year) | Participant N, mean age (SD), gender (M:F) | Sample | Design | Schizotypy assessment | Scanner, Strength, Task | Findings between group comparisons | Findings correlations/ regressions | |
| Mohanty et al., 2005 [63] | 17 High POS SCT, mean age = 19.1 (1.9), 12:5 17 CG, mean age = 20.5 (3.9), 7:10 | Undergraduate students | Between-group comparisons | PerAb and MagId Scales High POS SCT score 1.5 SD > mean on PerAb or MagId CG score 0.5 SD < mean on both PerAb and MagId | GE Signa, 1.5T, Emotional Stroop task | Negative vs. Neutral Condition High POS SCT ↑ right DLPFC, right inferior frontal gyrus, right hippocampus, parahippocampal gyrus, left putamen, left cerebellum vs. CG (all p values < 0.05 corrected per-voxel error rate) High POS SCT ↓ left DLPFC, left superior temporal gyrus, right inferior temporal gyrus, right middle occipital gyrus vs. CG (all p values < 0.05 corrected per-voxel error rate) | ||
| Harvey et al., 2007 [64] | 29 participants, mean age = 28.9 (7.9), 14:15 (available neuroimaging data only for 17 participants) | Community sample | Correlation analysis | Revised Physical Anhedonia Scale (PAS) Mean Total score = 12.2 (7.7) | 1.5 T Siemens Sonata scanner Emotion processing | Condition Positive vs. neutral information processing positive correlation PAS Total Score-Ventral Medial PFC, right middle temporal gyrus, left superior temporal gyrus, right insula, right superior parietal lobule, right occipital lobe (all p values < 0.001) negative correlation PAS Total Score- left inferior frontal gyrus (p < 0.001) Negative vs. neutral information processing positive correlation PAS Total Score-bilateral middle temporal gyri, right superior parietal lobule, left supramarginal gyrus, right cuneus (all p values < 0.001) | ||
| Harvey et al., 2010 [65] | 26 participants, mean age = 30.7 (9.8), 13:13 | Community sample | Correlation analysis | Revised Physical Anhedonia Scale Mean PAS score = 13.0 (8.5) | 1.5 T Siemens Sonata Emotion Processing (identification) | Positive vs. neutral information processing Negative correlation PAS Total Score-left medial PFC, left inferior and right middle temporal gyri, left cuneus, right superior parietal gyrus (all p values < 0.001) negative correlation PAS Total Score-right anterior cingulate (p = 0.03) | ||
| Modinos, Renken et al., 2010 [66] | 18 high SCT, mean age = 19.8 (1.9), 10:8 18 Low SCT, mean age = 21.00 (2.8), 10:8 | Undergraduate students | Between-group comparisons | Positive subscale of the Community Assessment of Psychic Experiences questionnaire (CAPE) High SCT > 75th percentile Mean CAPE positive score = 1.74 (0.13) Low SCT < 25th percentile Mean CAPE positive score = 1.12 (0.04) | Philips Intera, 3T, Theory of Mind task | Second Order Mentalizing Condition High SCT ↑ anterior PFC, lateral PFC bilaterally, dorsomedial PFC vs. Low SCT (all p values < 0.05, cluster level corrected for multiple comparisons) | ||
| Rapp et al., 2010 [67] | 15 participants, mean age = 28.1 (8.0), 0:15 | Community sample | Correlation analyses | SPQ Mean SPQ Total Score = 14.5 (13.2) | Siemens TRIO, 3T, Irony comprehension task | Irony comprehension condition Positive correlations SPQ total score-left inferior frontal gyrus (p < 0.001) SPQ INT-right precentral gyrus, left thalamus, right inferior occipital gyrus (all p values < 0.001) SPQ CP-right superior frontal gyrus (p < 0.001) Negative correlations SPQ total score-middle temporal gyrus bilaterally, right superior occipital gyrus (all p values < 0.001). SPQ CP-middle temporal gyrus bilaterally, right middle occipital gyrus (all p values < 0.001) Literal comprehension condition: Positive correlations SPQ total score-right medial frontal gyrus (p < 0.001) SPQ INT-right superior frontal gyrus, right thalamus, right inferior occipital gyrus, right middle temporal gyrus, left inferior frontal gyrus, left caudate nucleus (all p values < 0.001) SPQ CP-left anterior cingulate (p < 0.001). Negative correlations SPQ total score-right superior/inferior parietal lobule (p < 0.001) SPQ CP-right superior parietal lobule, the middle temporal gyrus (all p values < 0.001) SPQ INT-language lateralization in middle temporal lobe (p < 0.05) | ||
| Germine et al., 2011 [68] | 15 High SocAn, mean age = 31.5 (10.7) 7:8 15 Low SocAn mean age = 32.5 (12.5) 7:8 | Community sample and university students | Between-group comparisons | Revised Chapman Social Anhedonia Scale High SocAn Total Score > 16 for females, >19 for males (top 10%) Mean SocAn Total Score = 26.3 (6.6) Low SocAn Total Score < 7 for females and < 9 for males Mean SocAn Total Score = 3.7 (2.9) | 3.0 T Siemens Trio Face emotion processing (emotion and identity discrimination) | Condition Emotion discrimination vs. Identity discrimination Low SocAn vs. High SocAn ↑ right superior frontal gyrus (pFWE < 0.05) and right superior temporal gyrus (pFWE < 0.05) Condition Emotion discrimination vs. object discrimination Low SocAn vs. High SocAn ↑ left superior frontal gyrus (pFWE < 0.05) Condition Emotion discrimination vs. pattern discrimination Low SocAn vs. High SocAn ↑ right superior temporal gyrus (pFWE < 0.05) | ||
| Corlett and Fletcher 2012 [69] | 18 participants, 10:8 (mean age or age range not reported) | Community sample | Correlation analyses | Chapman scales (PAS, SocAn, PerAb, MagId, PDI) Mean PDI Score = 5 (3.1) Mean MagId Score = 4.6 (3.5) Mean PerAb Score = 3.8 (4.6) Mean PAS Score = 8.8 (4.2) Mean SocAn Score = 5.6 (4.0). | Siemens Trio, 3T, Blocking of causal learning task | Negative correlation MagId score-striatal prediction error (PE) magnitude to the blocked cue (p < 0.05 FDR corrected) Negative association PDI distress score -PE response to violation of blocking-induced expectation in the frontal cortex, striatum and midbrain (all p < 0.05 FDR corrected) Positive association PDI distress score-inappropriate DLPFC responses during blocking trials (p < 0.05 FDR corrected) | ||
| Ettinger et al., 2013 [70] | 26 participants, mean age = 33.62 (13.21), 13:13 | Community sample | Psychoticism scale of EPQ-R, STA EPQ Mean Psychoticism Score = 6.35 (3.63) Mean STA Score = 6.23 (3.64) | Correlation analyses | General Electric Signa, 1.5T, procedural learning task (PL, i.e., difference between the mean RTs to random and pattern trials) | Procedural Learning vs. Control Condition Positive correlations EPQ-R Psychoticism right transverse temporal gyrus extending to the putamen, caudate, thalamus and insula (cluster pFWE = 0.001) EPQ-R Psychoticism- inferior frontal and precentral gyri (cluster pFWE = 0.007) EPQ-R Psychoticism- middle frontal gyrus extending to the precentral gyrus and anterior cingulate (cluster pFWE = 0.001) STA scores-right middle temporal gyrus (pFWE = 0.005) | ||
| Huang et al., 2013 [71] | 14 High SCT, mean age = 22.3 (2.1) 7:7 14 Low SCT, mean age = 20.7 (0.46), 8:6 | Community sample | Between-group comparisons | SPQ High SCT SPQ Mean Total Score = 45.79 (1.84) (top 10th percentile) Low SCT SPQ Mean Total Score = 11.07 (1.25) (lowest 50th percentile) | Siemens Trio A Tim 3T Dynamic happy facial expression processing and social interaction task | Happiness Disappearing Condition High SCT ↑ right anterior cingulate cortex vs. Low SCT (p < 0.05, AlphaSim corrected) Blame Social Interaction Cues Condition High SCT ↓ left cingulate cortex (p = 0.01 AlphaSim corrected) and right superior temporal gyrus vs. Low SCT (p < 0.01 AlphaSim corrected) | ||
| van der Meer et al., 2013 [72] | 18 High SCT, mean age = 19.7 (1.9), 10:8 19 Low SCT, mean age = 21.6 (2.6), 10:9 | Undergraduate students | Between-group comparisons | Community Assessment of Psychic Experiences (CAPE) positive subscale High SCT score one SD above mean CAPE Positive Mean Score = 1.80 (0.15) Low SCT score below the sample mean CAPE Positive Mean Score = 1.12 (0.04) | Philips Intera, 3T ToM Stop Signal Task | Self-perspective inhibition condition High SCT ↑ left inferior frontal gyrus vs. Low SCT (p < 0.05 FDR corrected) | ||
| Fink et al., 2014 [73] | 21 High SCT, mean age = 23.29 (4.17), 9:12 20 Low SCT, mean age = 22.85 (3.42), 8:12 | University students | Between-group comparisons | SPQ three-factor model High SCT SPQ scores range = 132–179 Low SCT SPQ scores range = 3–77 | Siemens Tim Trio, 3T, Alternative-uses task for creative cognition (AU) | Alternative vs. common uses Low SCT ↑ left superior/middle frontal (pFWE = 0.006, ηp2 = 0.19), left inferior frontal (pFWE = 0.002, ηp2 = 0.25), left inferior parietal regions (pFWE = 0.0004, ηp2 = 0.30), anterior cingulate (pFWE = 0.001, ηp2 = 0.28) vs. High SCT. High SCT ↑ left superior temporal gyrus (pFWE = 0.0001, ηp2 = 0.35), right precuneus (pFWE = 0.04, ηp2 = 0.11) vs. Low SCT. | ||
| Hooker et al., 2014 [74] | 15 High SocAn, mean age = 32.00 (12.75), 7:8 15 Low SocAn, mean age = 30.27 (10.47), 5:10 | Healthy individuals | Between-group differences | Revised Social Anhedonia Scale High SocAn score 1.96 SDs above population mean Mean SocAn Total score = 24.6 (5.63) Low SocAn score equal or less than 1 SD above the population mean Mean SocAn Total score = 2.67 (2.53) | Siemens Tim Trio, 3T, social reward task | Positive vs. Neutral Expressions High SocAn ↓ventral lateral prefrontal cortex (VLPFC), posterior insula, superior temporal gyrus, superior frontal gyrus vs. Low SocAn (all p values < 0.001) Positive vs. Negative Expressions High SocAn ↓rostral anterior cingulate cortex, middle cingulate cortex, posterior insula vs. Low SocAn (all p values < 0.001) | ||
| Wang et al., 2014 [75] | 19 SCT, mean age = 19.37 (1.07), 10:9 22 CG, mean age = 19.68 (0.72), 12:10 | University students | Between-group comparisons | SPQ three-factor model SCT scores top 10% Mean SPQ Total Score = 39.53 (3.63) CG lowest 50% mean Mean SPQ Total Score = 16.64 (4.86) | Siemens Trio A Tim, 3T, Event-based prospective memory task | Prospective memory vs. baseline Condition SCT ↓ inferior frontal gyrus, medial frontal gyrus vs. CG (both p values < 0.001 AlphaSim corrected) | ||
| Park et al., 2015 [76] | 48 participants, mean age = 23.42 (4.50), 17:31 | Participants recruited through university and online research recruitment websites | Correlation analyses | O-LIFE | Siemens Magnetom Skyra, 3T, figure and verbal creativity–drawing task | Create condition Negative Correlations UnExp-left superior medial frontal gyrus (p = 0.017), left middle frontal gyrus (0.013), left inferior parietal lobule p = 0.027), right inferior temporal gyrus (p < 0.001) O-LIFE ImpNon-left middle frontal gyrus (p = 0.028), the right inferior temporal gyrus (p < 0.001) Positive association O-LIFE IntAn-signal difference in Create and Trace conditions right middle occipital gyrus (p = 0.041). | ||
| Simon et al., 2015 [77] | 11 High CAPE, mean age = 28 (9), 3:8 14 Average CAPE, mean age = 26.4 (5.3), 6:8 12 Low CAPE, mean age = 25.5 (8.5), 4:8 | Healthy individuals recruited using the internet | Between-group comparisons and correlation analyses | CAPE Low CAPE 1 SD < mean High CAPE 1 SD > mean Average CAPE a deviation of less than 1 SD from mean | 1.5-Tesla Siemens Magnetom Avanto Cued Reinforcement Reaction Time task (anticipation of monetary gains and losses) | Anticipation of €2 compared with €0 condition High CAPE ↓ ventral striatum vs. Low CAPE (pFWE = 0.04) | Anticipation of a Reward ventral striatal connectivity- medial PFC, right dorsal striatum, bilateral insula, left DLPFC (all p values < 0.05) | |
| Wang et al., 2015 [78] | 56 participants, mean age = 19.25 (0.88), 31:25 | College students | Correlation analyses | Chapman Psychosis-Proneness Scales (Revised Social Anhedonia, Physical Ahedonia, Magical Ideation and Perceptual Aberration scales) | Siemens Verio, 3T, Visual theory of mind and empathy task | Theory of mind condition Positive correlation SocAn Score-right cuneus, bilateral middle temporal gyrus, medial frontal gyrus and right temporo-parietal junction (all p values < 0.05) Positive correlation PAS Score-left middle temporal gyrus (p < 0.05) Empathy condition Positive correlation SocAn Score-right cuneus, middle temporal gyrus (all p values < 0.05) | ||
| Yin et al., 2015 [79] | 15 High SocAn, mean age = 32.00 (12.75), 7:8 15 Low SocAn, mean age = 30.27 (10.47), 5:10 | Community sample | Between-group comparisons | Revised Social Anhedonia Scale High SocAn Mean Total Score = 24.60 (5.63) (1.96 SD above the population mean) Low SocAn Mean Total Score = 2.67 (2.53) (equal to or less than 1 SD above the population mean) | 3T Siemens Tim Trio Facial emotion processing | Condition Positive vs. Neutral Emotions High SocAn ↓ FC left VLPFC and left inferior parietal cortex, left precentral gyrus/motor cortex, bilateral inferior temporal sulcus, right superior temporal sulcus vs. Low SocAn (all pFWE < 0.05) | ||
| Chan et al., 2016 [80] | 8 High Anhedonia, mean age = 18.88 (1.81) 8:0 20 CG, mean age = 19.2 (1.77) 11:9 | Community sample | Between-group comparisons | Chapman PAS and SocAnh scales High Anhedonia SocAn Mean Total Score = 10.13 (4.29), PAS Mean Total Score = 31 (6.02) CG SocAn Mean Total Score = 7.75 (4.08) and PAS Mean Total Score = 16.65 (3.66) | Magnetom Verio Siemens 3T monetary incentive delay task (MID) and affective delay task (AID) | AID task Positive vs. Neutral affective Cues High Anhedonia ↓ left thalamus, left thalamus/pulvinar, right insula vs. CG (pFWE < 0.005) | ||
| Yan et al., 2016 [81] | 18 High POS SCT, mean age = 19.28 (1.18), 9:9 15 High NEG SCT, mean age = 19.33 (1.23), 8:7 22 CG, mean age = 19.78 (0.80), 11:11 | College students | Between-group comparisons and correlation analyses | SPQ, PAS, SocAn High SCT SPQ total score > 10th percentile of sample CG SPQ total score < 50th percentile of sample | Siemens Trio, 3T, Monetary Incentive Delay task | Gain vs. non-gain consummation High NEG SCT ↓ right postcentral gyrus, left parahippocampus gyrus/amygdala, the left culmen vs. CG (all p values < 0.05, AlphaSim corrected) High NEG SCT ↓ left putamen vs. CG (p = 0.0109 AlphaSim corrected) High NEG SCT ↓ right postcentral gyrus, left culmen, left precuneus, bilateral precentral gyrus, left parahippocampal gyrus/amygdala vs. High POS SCT (all p values < 0.05, AlphaSim corrected) Gain vs. non-gain anticipation High POS SCT ↑ right VLPFC vs. CG (p < 0.05, AlphaSim corrected) High NEG SCT ↓ left ventral striatum, left middle temporal gyrus, cerebellar tonsil bilaterally vs. CG (p < 0.05, AlphaSim corrected) High NEG SCT ↓ right ventral striatum-ACC, medial PFC, left superior temporal gyrus, right lingual gyrus, right fusiform gyrus, left middle temporal gyrus vs. High POS SCT (p < 0.05, AlphaSim corrected) | Gain anticipation Positive correlation SocAn-right anterior insula in SCT participants (p < 0.001 multiple comparison corrected) | |
| Modinos et al., 2017 [82] | 22 High UEx SCT mean age = 27.36 (7.61), 11:11 21 Low UEx SCT mean age = 27.00 (5.64), 12:9 | Participants recruited through online advertisement | Between-group comparisons | O-LIFE short version-Unusual Perceptual Experiences Subscale High UEx score > 7 Mean UEx Score = 11.59 (4.93) Low UEx score <2 Mean UEx Score = 0.86 (1.01) | General Electric Discovery MR750, 3T, Emotion-processing task | Condition Emotional vs. Neutral Pictures High UEx ↑ caudate (pFWE = 0.023) and ACC vs. Low UEx (pFWE = 0.051) | ||
| Günther et al., 2017 [83] | 18 High SocAn, mean age = 22.50 (2.73), 0:18 19 low SocAn, mean age = 22.42 (2.46), 0:19 | Participants recruited through online advertisement and public notices | Between-group comparisons | Chapman Social Anhedonia Scale High SocAn Mean Total score = 10.89 (1.91) (above the 84th percentile) Low SocAn Mean Total score = 0.74 (0.45) (below the 22nd Percentile) | 3T Magnetom Trio, Siemens, Masked Face Processing Task | Condition masked sad vs. neutral faces High SocAn ↑ bilateral thalamus, left red Nucleus vs. Low SocAn (both pFWE < 0.005) | ||
| Papanastasiou et al., 2018 [84] | 149 High CAPE, mean age = 19.02 (0.76), 50:99 149 low CAPE, mean age = 18.98 (0.74), 84:65 | Healthy adolescents from the IMAGEN database | Between-group comparisons | CAPE-42 questionnaire Upper-lower deciles. High CAPE (upper decile) Mean Total Score = 111.64 (21.26) Low CAPE (lower decile) Mean Total Score = 9.54 (4.75) | 3T Siemens, Philips, General Electrics, Bruker Adapted monetary incentive delay task (reward, anticipation outcome) | Anticipation High CAPE ↓ right Caudate Head vs. Low CAPE (p = 0.01) | ||
| Wang, Li et al., 2018 [85] | 34 High Neg SCT, mean age = 19.21 (0.95), 17:17 30 Low Neg SCT, mean age = 19.23 (0.86), 13:17 | College students | Between-group comparisons | Chapman Psychosis-Proneness Scales (Revised Social Anhedonia, Physical Anhedonia scales) High Neg SCT Score) > 23 (sum of scores on physical and social anhedonia) Low Neg SCT Score < 23 | Siemens Verio, 3T, Facial emotional valence discrimination task | Neutral Faces Condition High Neg SCT ↓ medial PFC, bilateral amygdala vs. Low Neg SCT (both pFWE < 0.05) Fearful Faces Condition High Neg SCT ↓ left amygdala (pFWE < 0.049) and ↓ FC right amygdala-medial frontal gyrus vs. Low Neg SCT (pFWE < 0.05) Happy Faces Condition High Neg SCT ↓ FC right amygdala-dorsal anterior cingulate cortex vs. Low Neg SCT (pFWE < 0.05) | ||
| Schmidt et al., 2019 [86] | 47 participants, mean age = 23.4 (3.6), 18:29 | Healthy individuals | Correlation analyses | SPQ | Siemens Magnetom Trio, 3T, Social jumping-to-conclusion task | All last faces vs. all previous faces condition Negative correlation constricted affect SPQ subscale-NAc (p = 0.029) | ||
| Olano et al., 2020 [87] | 25 participants, mean age = 30.56 (10.25), 9:16 | University students and technicians | Correlation analyses | O-LIFE short version Mean Total Score = 10.93(4.69) | Siemens Trio, 3T, Auditory emotional task | Low Intelligibility (most degraded) Condition Positive correlation O-LIFE Total Score-right anterior cingulate cortex, right orbitofrontal cortex and left medial temporal gyrus (all p values < 0.05) Positive correlation O-LIFE Unusual experiences subscale-right anterior cingulate cortex and left medial temporal gyrus (all p values < 0.05) | ||
| Yan et al., 2020 [88] | 74 participants, mean age = 23.50 (3.83), 34:40 | Not reported | Correlation analyses | SPQ three-factor model SPQ Mean Total Score = 11.00(9.09) | Siemens Tim TRIO, 3T, Social-cognitive task | Neutral Face Condition Positive correlation SPQ DIS factor-right posterior superior temporal sulcus (pFWE = 0.018) Positive correlation SPQ DIS factor-right-left FC of posterior superior temporal sulcus pFWE = 0.038 | ||
| Study (Year) | Participants n, Mean Age (SD), Gender (M:F) | Sample | Diagnostic Criteria for SPD Participants | Design | Scanner, Strength, Task | Findings between Group Comparisons | Finding Correlations |
|---|---|---|---|---|---|---|---|
| Koenigsberg et al., 2005 [89] | Six SPD participants, mean age = 33.0 (10.7), 5:1 Five CG, mean age = 30.0 (4.9), 3:2 | Not reported | DSM-IV criteria for SPD; SADS, SID-p | Between-group comparisons | GE Signa LX 8.2.5, 1.5T, Visuospatial working memory task | Maintenance Period-Memory vs. Control Condition SPD ↓ left ventral prefrontal cortex (p = 0.034), left superior frontal gyrus (p = 0.042), left intraparietal cortex (p = 0.016), left posterior inferior frontal gyrus vs. CG (p = 0.048) Retention Period-Memory vs. Control Condition SPD ↓left superior temporal gyrus (p = 0.036), left posterior inferior frontal gyrus vs. CG (p = 0.048) | |
| Dickey et al., 2010 [90] | 16 SPD, mean age = 39.1 (11.0), 13:3 13 CG, mean age = 35.2 (12.3), 9:4 | Community participants | DSM-IV criteria for SPD; SCID | Between-group comparisons and correlation analyses | GE Signa, 3T, Prosody identification task | Whole-brain analysis across conditions SPD-frontal, temporal areas and parahippocampus (all p values = 0.001) CG-frontal, temporal, parietal, insular regions (all p values = 0.001) | Non-significant findings in SPD group (all p values > 0.05) |
| Hazlett et al., 2012 [91] | 28 SPD, mean age = 35.9 (11) 16:12 32 CG, mean age = 32.8 (9.7) 12:20 | Not reported | DSM-IV criteria for SPD | Between-group comparisons | Siemens Allegra 3T affective picture processing task | SPD ↑ peak response in amygdala following picture onset vs. CG (p < 0.05 corrected) SPD slowest peak latency during picture processing vs. CG (p = 0.012) SPD ↑ amygdala to novel pictures vs. CG (p = 0.009) | |
| Vu et al., 2013 [92] | 15 SPD, mean age = 38.9 (12.8), 13:2 16 CG, mean age = 32.0 (12.0), 11:5 | Participants recruited through advertisements on local public transit, print media and websites | SCID | Between-group comparisons | GE, 3T, 2-back visual working memory task and 0-back continuous performance task | 0back vs. rest Condition SPD ↓ left postcentral gyrus vs. CG (p < 0.05 corrected) 2back vs. 0back Condition SPD ↓ left posterior cingulate gyrus, left superior temporal gyrus (STG)/insula, left middle frontal gyrus vs. CG (all p values < 0.05 corrected) | |
| Stanfield et al., 2017 [93] | 20 SPD, mean age = 37.3 (9.4) 14:6 32 CG, mean age = 36.6 (9.5), 22:10 | SPD previously participated in the Edinburgh High Risk Study of schizophrenia and also recruited from clinical services. Controls were recruited from participant and investigator acquaintances and the Scottish Mental Health Network research register | DSM-IV criteria for SPD (SCID-II) | Between-group comparisons | GE Medical Systems Signa Scanner 1.5T Social Judgment task | Non-significant findings (all p values > 0.05) | |
| Resting-state fMRI studies | |||||||
| Study (Year) | Participant N, mean age (SD), gender (M:F) | Sample | Diagnostic criteria for SPD participants | Design | Scanner, Strength | Findings between group comparisons | Finding correlations/ regressions |
| Zhang et al., 2014 [94] | 18 SPD, mean age = 19.7 (0.9), 18:0 18 CG, mean age = 20.3 (0.9), 18:0 | University students | DSM-IV criteria for SPD; SID-p | Between-group comparisons | Siemens Verio, 3T | Anterior component of Default Mode Network SPD ↑ FC in bilateral superior temporal gyrus and sub-lobar (bilateral putamen and caudate vs. CG Controls ↑ FC in left superior frontal gyrus, left medial frontal gyrus and cerebellum anterior lobe vs. SPD All p values < 0.05 with FDR Correction Posterior component of Default Mode Network SPD ↑ FC in posterior cingulate gyrus vs. CG CG ↑ FC cerebellum posterior lobe, right transverse temporal gyrus, left middle temporal gyrus vs. SPD CG ↓ FC in posterior bilateral cingulate gyrus vs. SPD All p values < 0.05 with FDR correction | |
| Zhu et al., 2017 [95] | 19 SPD, mean age = 19.98 (0.82), 17:2 17 CG, mean age = 19.71 (0.71), 16:1 | Undergraduate students | DSM-IV criteria for SPD; SCID-II, SPQ three-factor model, Symptom Checklist-90 (SCL-90) Controls had a score at low 10% of SPQ total score | Between-group comparisons and correlation analyses | Siemens Trio, 3T | SPD ↓ FC between the (a) right precuneus and bilateral parahippocampus and right middle temporal gyrus and (b) the right parahippocampus and right superior temporal gyrus vs. CG SPD ↑ FC between right precuneus and right middle frontal gyrus vs. CG all p values < 0.05 Alphasim correction | Negative correlation SPQ total score-FC between right precuneus and left parahippocampus in SPD (p = 0.006) Positive correlation constricted affect SPQ subscale-FC between right precuneus and middle temporal gyrus in SPD (p = 0.003) |
| Szeszko et al., 2022 [38] | 45 SPD, mean age = 45.2 (10.9), 35:10 SPD group was also categorized into two subgroups based on upper lower terciles for Total SPQ score 43 CG, mean age = 43.1 (9.9), 32:11 | Controls and SPD were recruited from the community surrounding the university | DSM-IV criteria for SPD; SCID I and SIDP-IV SPQ Mean total SPQ for SPD group = 30.1 (14.9) | Between-group comparisons | Siemens Allegra 3T or Siemens Skyra 3T | SPD with low SPQ scores ↑ FC from mediodorsal nucleus of thalamus to the rostral middle frontal cortex vs. SPD with high SPQ scores (p = 0.031) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zouraraki, C.; Karamaouna, P.; Giakoumaki, S.G. Cognitive Processes and Resting-State Functional Neuroimaging Findings in High Schizotypal Individuals and Schizotypal Personality Disorder Patients: A Systematic Review. Brain Sci. 2023, 13, 615. https://doi.org/10.3390/brainsci13040615
Zouraraki C, Karamaouna P, Giakoumaki SG. Cognitive Processes and Resting-State Functional Neuroimaging Findings in High Schizotypal Individuals and Schizotypal Personality Disorder Patients: A Systematic Review. Brain Sciences. 2023; 13(4):615. https://doi.org/10.3390/brainsci13040615
Chicago/Turabian StyleZouraraki, Chrysoula, Penny Karamaouna, and Stella G. Giakoumaki. 2023. "Cognitive Processes and Resting-State Functional Neuroimaging Findings in High Schizotypal Individuals and Schizotypal Personality Disorder Patients: A Systematic Review" Brain Sciences 13, no. 4: 615. https://doi.org/10.3390/brainsci13040615
APA StyleZouraraki, C., Karamaouna, P., & Giakoumaki, S. G. (2023). Cognitive Processes and Resting-State Functional Neuroimaging Findings in High Schizotypal Individuals and Schizotypal Personality Disorder Patients: A Systematic Review. Brain Sciences, 13(4), 615. https://doi.org/10.3390/brainsci13040615






