Learned Irrelevance, Perseveration, and Cognitive Aging: A Cross-Sectional Study of Cognitively Unimpaired Older Adults
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Outcome Measures
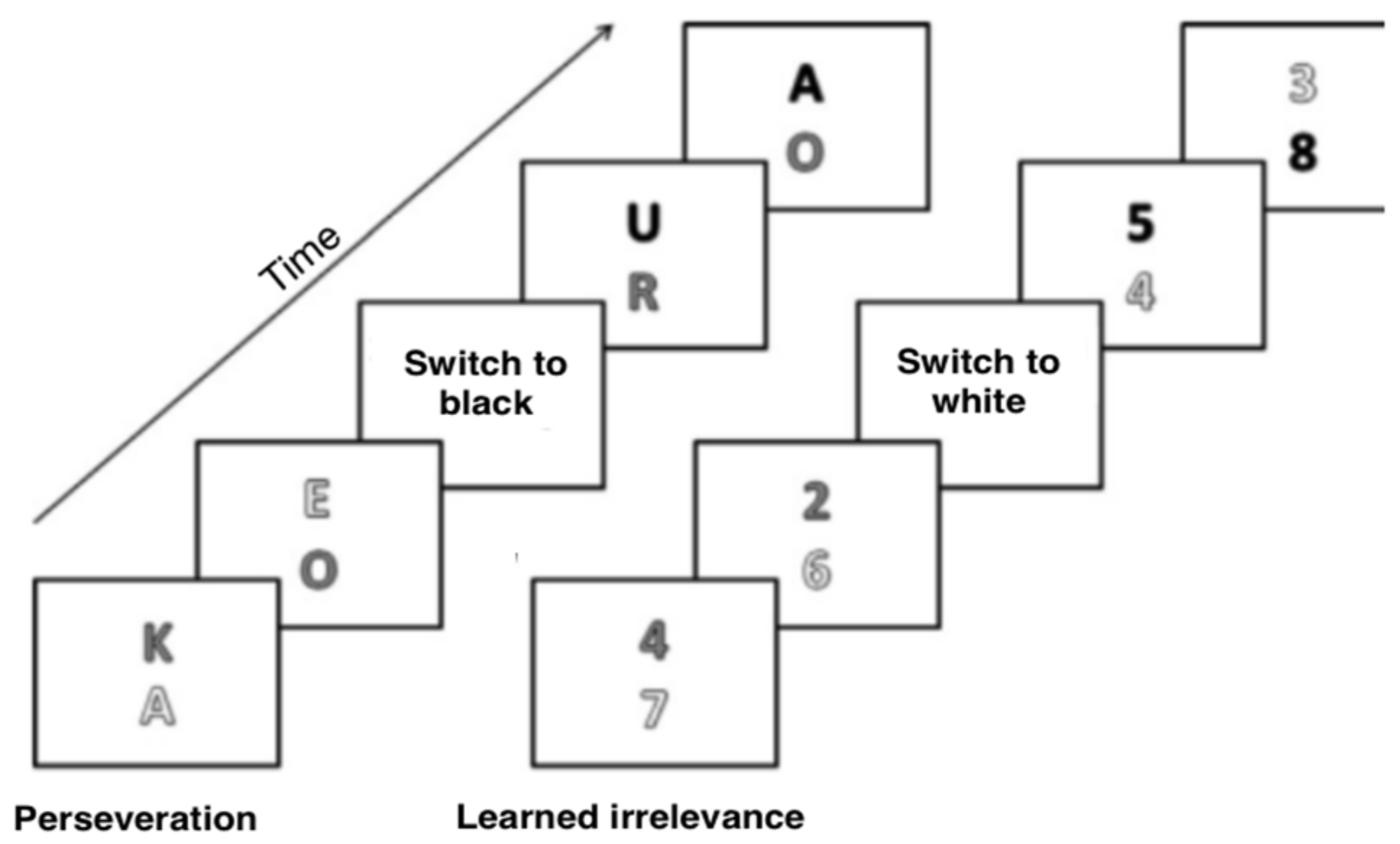
2.3. Materials and Procedure
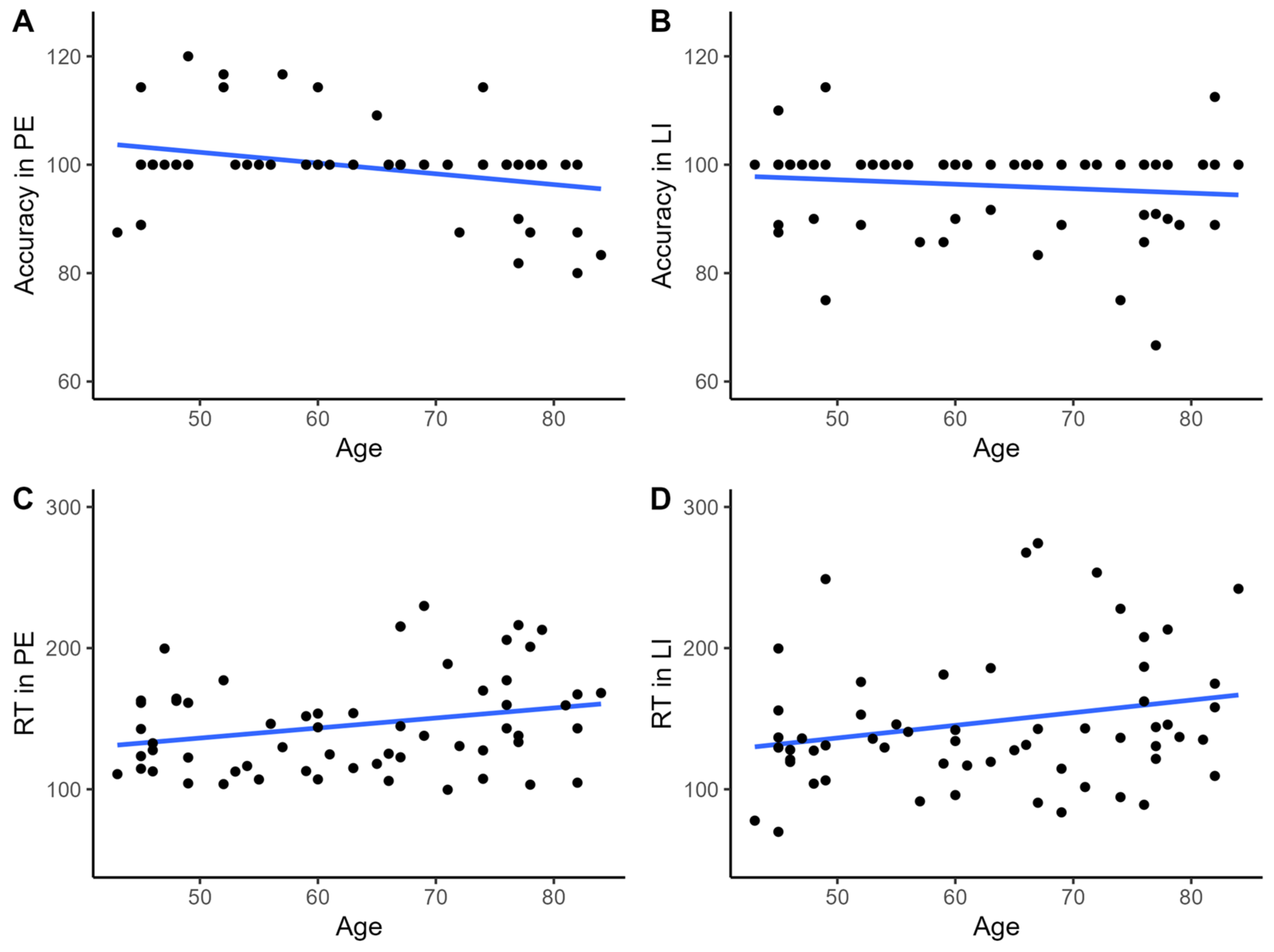
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Owen, A.M.; Roberts, A.C.; Hodges, J.R.; Summers, B.A.; Polkey, C.E.; Robbins, T.W. Contrasting Mechanisms of Impaired Attentional Set-Shifting in Patients with Frontal Lobe Damage or Parkinson’s Disease. Brain J. Neurol. 1993, 116 Pt 5, 1159–1175. [Google Scholar] [CrossRef] [PubMed]
- Fallon, S.J.; Hampshire, A.; Barker, R.A.; Owen, A.M. Learning to Be Inflexible: Enhanced Attentional Biases in Parkinson’s Disease. Cortex J. Devoted Study Nerv. Syst. Behav. 2016, 82, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Gruszka, A.; Hampshire, A.; Owen, A. Learned Irrelevance Revisited: Pathology-Based Individual Differences, Normal Variation and Neural Correlates. In Handbook of Individual Differences in Cognition; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Maes, J.H.R.; Vich, J.; Eling, P.a.T.M. Learned Irrelevance and Response Perseveration in a Total Change Dimensional Shift Task. Brain Cogn. 2006, 62, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Slabosz, A.; Lewis, S.J.G.; Smigasiewicz, K.; Szymura, B.; Barker, R.A.; Owen, A.M. The Role of Learned Irrelevance in Attentional Set-Shifting Impairments in Parkinson’s Disease. Neuropsychology 2006, 20, 578–588. [Google Scholar] [CrossRef]
- Hughes, L.E.; Altena, E.; Barker, R.A.; Rowe, J.B. Perseveration and Choice in Parkinson’s Disease: The Impact of Progressive Frontostriatal Dysfunction on Action Decisions. Cereb. Cortex 2013, 23, 1572–1581. [Google Scholar] [CrossRef]
- Sandson, J.; Albert, M.L. Perseveration in Behavioral Neurology. Neurology 1987, 37, 1736. [Google Scholar] [CrossRef]
- Daigneault, S.; Braun, C.M.J.; Whitaker, H.A. Early Effects of Normal Aging on Perseverative and Non-perseverative Prefrontal Measures. Dev. Neuropsychol. 1992, 8, 99–114. [Google Scholar] [CrossRef]
- Foldi, N.S.; Helm-Estabrooks, N.; Redfield, J.; Nickel, D.G. Perseveration in Normal Aging: A Comparison of Perseveration Rates on Design Fluency and Verbal Generative Tasks. Aging Neuropsychol. Cogn. 2003, 10, 268–280. [Google Scholar] [CrossRef]
- Ridderinkhof, K.R.; Span, M.M.; van der Molen, M.W. Perseverative Behavior and Adaptive Control in Older Adults: Performance Monitoring, Rule Induction, and Set Shifting. Brain Cogn. 2002, 49, 382–401. [Google Scholar] [CrossRef]
- Head, D.; Kennedy, K.M.; Rodrigue, K.M.; Raz, N. Age-Differences in Perseveration: Cognitive and Neuroanatomical Mediators of Performance on the Wisconsin Card Sorting Test. Neuropsychologia 2009, 47, 1200–1203. [Google Scholar] [CrossRef]
- Lemaire, P.; Brun, F. Adults’ Age-Related Differences in Strategy Perseveration Are Modulated by Response-Stimulus Intervals and Problem Features. Q. J. Exp. Psychol. 2014, 67, 1863–1870. [Google Scholar] [CrossRef]
- Mecklinger, A.D.; von Cramon, D.Y.; Springer, A.; Matthes-von Cramon, G. Executive Control Functions in Task Switching: Evidence from Brain Injured Patients. J. Clin. Exp. Neuropsychol. 1999, 21, 606–619. [Google Scholar] [CrossRef]
- Cinan, S.; Tanör, O.O. An Attempt to Discriminate Different Types of Executive Functions in the Wisconsin Card Sorting Test. Mem. Hove Engl. 2002, 10, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Monsell, S. Costs of a Predictible Switch between Simple Cognitive Tasks. J. Exp. Psychol. Gen. 1995, 124, 207–231. [Google Scholar] [CrossRef]
- Glosser, G.; Goodglass, H. Disorders in Executive Control Functions among Aphasic and Other Brain-Damaged Patients. J. Clin. Exp. Neuropsychol. 1990, 12, 485–501. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, N. Stimulus Selection: Learning to Ignore Stimuli That Predict No Change in Reinforcement. In Constraints on Learning: Limitations and Predispositions; Academic Press: Cambridge, MA, USA, 1973. [Google Scholar]
- Aron, A.R. Progress in Executive-Function Research: From Tasks to Functions to Regions to Networks. Curr. Dir. Psychol. Sci. 2008, 17, 124–129. [Google Scholar] [CrossRef]
- Gauntlett-Gilbert, J.; Roberts, R.C.; Brown, V.J. Mechanisms Underlying Attentional Set-Shifting InParkinsons Disease. Neuropsychologia 1999, 37, 605–616. [Google Scholar] [CrossRef]
- Lewis, S.J.G.; Slabosz, A.; Robbins, T.W.; Barker, R.A.; Owen, A.M. Dopaminergic Basis for Deficits in Working Memory but Not Attentional Set-Shifting in Parkinson’s Disease. Neuropsychologia 2005, 43, 823–832. [Google Scholar] [CrossRef]
- Young, M.E. The Problem with Categorical Thinking by Psychologists. Behav. Process 2016, 123, 43–53. [Google Scholar] [CrossRef]
- Preacher, K.J. Extreme Groups Designs. In The Encyclopedia of Clinical Psychology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1–4. ISBN 978-1-118-62539-2. [Google Scholar]
- Roberts, A.; De Salvia, M.; Wilkinson, L.; Collins, P.; Muir, J.; Everitt, B.; Robbins, T. 6-Hydroxydopamine Lesions of the Prefrontal Cortex in Monkeys Enhance Performance on an Analog of the Wisconsin Card Sort Test: Possible Interactions with Subcortical Dopamine. J. Neurosci. 1994, 14, 2531–2544. [Google Scholar] [CrossRef]
- Lubow, R.E. Latent Inhibition as a Measure of Learned Inattention: Some Problems and Solutions. Behav. Brain Res. 1997, 88, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kafkas, A.; Montaldi, D. How Do Memory Systems Detect and Respond to Novelty? Neurosci. Lett. 2018, 680, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Yamaguchi, S.; Kobayashi, S. Impaired Novelty Detection and Frontal Lobe Dysfunction in Parkinson’s Disease. Neuropsychologia 2000, 38, 645–654. [Google Scholar] [CrossRef] [PubMed]
- Hampshire, A.; Owen, A.M. Fractionating Attentional Control Using Event-Related FMRI. Cereb. Cortex 2006, 16, 1679–1689. [Google Scholar] [CrossRef]
- Hampshire, A.; Gruszka, A.; Fallon, S.J.; Owen, A.M. Inefficiency in Self-Organized Attentional Switching in the Normal Aging Population Is Associated with Decreased Activity in the Ventrolateral Prefrontal Cortex. J. Cogn. Neurosci. 2008, 20, 1670–1686. [Google Scholar] [CrossRef]
- Schmitter-Edgecombe, M.; Langill, M. Costs of a Predictable Switch Between Simple Cognitive Tasks Following Severe Closed-Head Injury. Neuropsychology 2006, 20, 675–684. [Google Scholar] [CrossRef]
- Baddeley, A.; Chincotta, D.; Adlam, A. Working Memory and the Control of Action: Evidence from Task Switching. J. Exp. Psychol. Gen. 2001, 130, 641–657. [Google Scholar] [CrossRef]
- Dreisbach, G.; Goschke, T. How Positive Affect Modulates Cognitive Control: Reduced Perseveration at the Cost of Increased Distractibility. J. Exp. Psychol. Learn. Mem. Cogn. 2004, 30, 343–353. [Google Scholar] [CrossRef]
- Monsell, S. Task Switching. Trends Cogn. Sci. 2003, 7, 134–140. [Google Scholar] [CrossRef]
- Dreisbach, G.; Müller, J.; Goschke, T.; Strobel, A.; Schulze, K.; Lesch, K.-P.; Brocke, B. Dopamine and Cognitive Control: The Influence of Spontaneous Eyeblink Rate and Dopamine Gene Polymorphisms on Perseveration and Distractibility. Behav. Neurosci. 2005, 119, 483–490. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Z. Positive Affect and Cognitive Control: Approach-Motivation Intensity Influences the Balance Between Cognitive Flexibility and Stability. Psychol. Sci. 2014, 25, 1116–1123. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.; Dreisbach, G.; Brocke, B.; Lesch, K.-P.; Strobel, A.; Goschke, T. Dopamine and Cognitive Control: The Influence of Spontaneous Eyeblink Rate, DRD4 Exon III Polymorphism and Gender on Flexibility in Set-Shifting. Brain Res. 2007, 1131, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Tharp, I.J.; Pickering, A.D. Individual Differences in Cognitive-Flexibility: The Influence of Spontaneous Eyeblink Rate, Trait Psychoticism and Working Memory on Attentional Set-Shifting. Brain Cogn. 2011, 75, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wecker, N.S.; Kramer, J.H.; Hallam, B.J.; Delis, D.C. Mental Flexibility: Age Effects on Switching. Neuropsychology 2005, 19, 345–352. [Google Scholar] [CrossRef]
- Choynowski, M. Test Znajomości Słów; Pracownia Psychometryczna PAN: Warsaw, Poland, 1967. [Google Scholar]
- Łucki, W. Zestaw Prób Do Badania Procesów Poznawczych u Pacjentów z Uszkodzeniami Mózgu; Pracownia Testów Psychologicznych PTP: Warsaw, Poland, 1995. [Google Scholar]
- De Carolis, L.; Galli, S.; Bianchini, E.; Rinaldi, D.; Raju, M.; Caliò, B.; Alborghetti, M.; Pontieri, F.E. Age at Onset Influences Progression of Motor and Non-Motor Symptoms during the Early Stage of Parkinson’s Disease: A Monocentric Retrospective Study. Brain Sci. 2023, 13, 157. [Google Scholar] [CrossRef]
- Klotzbier, T.J.; Schott, N.; Almeida, Q.J. Profiles of Motor-Cognitive Interference in Parkinson’s Disease—The Trail-Walking-Test to Discriminate between Motor Phenotypes. Brain Sci. 2022, 12, 1217. [Google Scholar] [CrossRef]
- Salthouse, T.A.; Toth, J.; Daniels, K.; Parks, C.; Pak, R.; Wolbrette, M.; Hocking, K.J. Effects of Aging on Efficiency of Task Switching in a Variant of the Trail Making Test. Neuropsychology 2000, 14, 102–111. [Google Scholar] [CrossRef]
- Wecker, N.S.; Kramer, J.H.; Wisniewski, A.; Delis, D.C.; Kaplan, E. Age Effects on Executive Ability. Neuropsychology 2000, 14, 409–414. [Google Scholar] [CrossRef]
- Casagrande, M.; Agostini, F.; Favieri, F.; Forte, G.; Giovannoli, J.; Guarino, A.; Marotta, A.; Doricchi, F.; Martella, D. Age-Related Changes in Hemispherical Specialization for Attentional Networks. Brain Sci. 2021, 11, 1115. [Google Scholar] [CrossRef]
- Commodari, E.; Guarnera, M. Attention and Aging. Aging Clin. Exp. Res. 2008, 20, 578–584. [Google Scholar] [CrossRef]
- Kray, J.; Lindenberger, U. Adult Age Differences in Task Switching. Psychol. Aging 2000, 15, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Isobe, M.; Vaghi, M.; Fineberg, N.A.; Apergis-Schoute, A.; Bullmore, E.T.; Sahakian, B.J.; Robbins, T.W.; Chamberlain, S.R. Set-Shifting Related Basal Ganglia Deformation as a Novel Familial Marker of Obsessive-Compulsive Disorder. Br. J. Psychiatry J. Ment. Sci. 2022, 220, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.D.; Andrews, T.C.; Grasby, P.M.; Brooks, D.J.; Robbins, T.W. Contrasting Cortical and Subcortical Activations Produced by Attentional-Set Shifting and Reversal Learning in Humans. J. Cogn. Neurosci. 2000, 12, 142–162. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, D.; Manktelow, A.E.; Sahakian, B.J.; Menon, D.K.; Stamatakis, E.A. Cognitive Flexibility: A Default Network and Basal Ganglia Connectivity Perspective. Brain Connect. 2016, 6, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Florio, T.M.; Scarnati, E.; Rosa, I.; Di Censo, D.; Ranieri, B.; Cimini, A.; Galante, A.; Alecci, M. The Basal Ganglia: More than Just a Switching Device. CNS Neurosci. Ther. 2018, 24, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Woods, A.J.; Clark, L.A.; Criss, C.R.; Shadmehr, R.; Grooms, D.R. The Aging Brain & the Dorsal Basal Ganglia: Implications for Age-Related Limitations of Mobility. Adv. Geriatr. Med. Res. 2019, 1, e190008. [Google Scholar] [CrossRef]
- Hubble, J.P. Aging and the Basal Ganglia. Neurol. Clin. 1998, 16, 649–657. [Google Scholar] [CrossRef]
- Severson, J.A.; Marcusson, J.; Winblad, B.; Finch, C.E. Age-Correlated Loss of Dopaminergic Binding Sites in Human Basal Ganglia. J. Neurochem. 1982, 39, 1623–1631. [Google Scholar] [CrossRef]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Neuropsychological and Clinical Heterogeneity of Cognitive Impairment and Dementia in Patients with Parkinson’s Disease. Lancet Neurol. 2010, 9, 1200–1213. [Google Scholar] [CrossRef]
- Siokas, V.; Liampas, I.; Lyketsos, C.G.; Dardiotis, E. Association between Motor Signs and Cognitive Performance in Cognitively Unimpaired Older Adults: A Cross-Sectional Study Using the NACC Database. Brain Sci. 2022, 12, 1365. [Google Scholar] [CrossRef]
- Horn, J.L.; Cattell, R.B. Age Differences in Fluid and Crystallized Intelligence. Acta Psychol. 1967, 26, 107–129. [Google Scholar] [CrossRef]
- Stankov, L. Aging, Attention, and Intelligence. Psychol. Aging 1988, 3, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Bolla, K.I.; Lindgren, K.N.; Bonaccorsy, C.; Bleecker, M.L. Predictors of Verbal Fluency (FAS) in the Healthy Elderly. J. Clin. Psychol. 1990, 46, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Crawford, J.; Bryan, J.; Luszcz, M.; Obonsawin, M.; Stewart, L. The Executive Decline Hypothesis of Cognitive Aging: Do Executive Deficits Qualify as Differential Deficits and Do They Mediate Age-Related Memory Decline? Aging Neuropsychol. Cogn. 2000, 7, 9–31. [Google Scholar] [CrossRef]
- Miller, E. Verbal Fluency as a Function of a Measure of Verbal Intelligence and in Relation to Different Types of Cerebral Pathology. Br. J. Clin. Psychol. 1984, 23 Pt 1, 53–57. [Google Scholar] [CrossRef]
- Friedman, N.P.; Miyake, A.; Corley, R.P.; Young, S.E.; DeFries, J.C.; Hewitt, J.K. Not All Executive Functions Are Related to Intelligence. Psychol. Sci. 2006, 17, 172–179. [Google Scholar] [CrossRef]
- Henry, J.D.; Phillips, L.H. Covariates of Production and Perseveration on Tests of Phonemic, Semantic and Alternating Fluency in Normal Aging. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2006, 13, 529–551. [Google Scholar] [CrossRef]
- Troyer, A.K.; Moscovitch, M.; Winocur, G. Clustering and Switching as Two Components of Verbal Fluency: Evidence from Younger and Older Healthy Adults. Neuropsychology 1997, 11, 138–146. [Google Scholar] [CrossRef]
- Ramanan, S.; Narayanan, J.; D’Souza, T.P.; Malik, K.S.; Ratnavalli, E. Total Output and Switching in Category Fluency Successfully Discriminates Alzheimer’s Disease from Mild Cognitive Impairment, but Not from Frontotemporal Dementia. Dement. Neuropsychol. 2015, 9, 251–257. [Google Scholar] [CrossRef]
- Cahill, L. Why Sex Matters for Neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Seidman, L.J.; Horton, N.J.; Makris, N.; Kennedy, D.N.; Caviness, V.S.; Faraone, S.V.; Tsuang, M.T. Normal Sexual Dimorphism of the Adult Human Brain Assessed by in Vivo Magnetic Resonance Imaging. Cereb. Cortex 2001, 11, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Gur, R.C.; Turetsky, B.I.; Matsui, M.; Yan, M.; Bilker, W.; Hughett, P.; Gur, R.E. Sex Differences in Brain Gray and White Matter in Healthy Young Adults: Correlations with Cognitive Performance. J. Neurosci. Off. J. Soc. Neurosci. 1999, 19, 4065–4072. [Google Scholar] [CrossRef] [PubMed]
- Hamson, D.K.; Roes, M.M.; Galea, L.A.M. Sex Hormones and Cognition: Neuroendocrine Influences on Memory and Learning. Compr. Physiol. 2016, 6, 1295–1337. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M.; Gresack, J.E. Sex Differences in the Behavioral Response to Spatial and Object Novelty in Adult C57BL/6 Mice. Behav. Neurosci. 2003, 117, 1283–1291. [Google Scholar] [CrossRef]
- Mackintosh, N.J. A Theory of Attention: Variations in the Associability of Stimuli with Reinforcement. Psychol. Rev. 1975, 82, 276–298. [Google Scholar] [CrossRef]
- Różańska, A.; Król, W.; Orzechowski, J.; Gruszka, A. The Two-Factor Structure of Cognitive Flexibility: Tempo of Switching and Overcoming of Prepotent Responses. Adv. Cogn. Psychol. 2023, 19, 1–12. [Google Scholar] [CrossRef]
- Lawson, R.A.; Yarnall, A.J.; Duncan, G.W.; Breen, D.P.; Khoo, T.K.; Williams-Gray, C.H.; Barker, R.A.; Collerton, D.; Taylor, J.-P.; Burn, D.J.; et al. Cognitive Decline and Quality of Life in Incident Parkinson’s Disease: The Role of Attention. Parkinsonism Relat. Disord. 2016, 27, 47–53. [Google Scholar] [CrossRef]
- Lawson, R.; Yarnall, A.; Johnston, F.; Duncan, G.; Khoo, T.; Collerton, D.; Taylor, J.; Burn, D. Cognitive Impairment in Parkinson’s Disease: Impact on Quality of Life of Carers. Int. J. Geriatr. Psychiatry 2017, 32, 1362–1370. [Google Scholar] [CrossRef]


| Variable | Mean (SD) or N, % |
|---|---|
| Age, years | 63.0 (12.6) |
| Sex, male | 16, 26.7% |
| Accuracy LI | 96.2 (8.37) |
| Accuracy PE | 99.7 (8.06) |
| Reaction time in LI | 148.0 (50.0) |
| Reaction time in PE | 146.0 (34.5) |
| Intelligence score | 31.1 (6.41) |
| Verbal fluency score | 16.1 (4.52) |
| Variable | Coefficient (±Standard Error) | |||
|---|---|---|---|---|
| Task Condition | Learned Irrelevance | p-Value | Perseveration | p-Value |
| Intercept | 80.30 (33.16) | 0.019 | 100.74 (22.34) | <0.001 |
| Age, years | 0.83 (0.50) | 0.105 | 0.71 (0.35) | 0.046 |
| Sex, male | 20.97 (14.24) | 0.147 | - | - |
| R-squared (95% CI) | 0.086 (0.02–0.24) | 0.067 (0.02–0.21) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fijałkiewicz, A.; Batko, K.; Gruszka, A. Learned Irrelevance, Perseveration, and Cognitive Aging: A Cross-Sectional Study of Cognitively Unimpaired Older Adults. Brain Sci. 2023, 13, 473. https://doi.org/10.3390/brainsci13030473
Fijałkiewicz A, Batko K, Gruszka A. Learned Irrelevance, Perseveration, and Cognitive Aging: A Cross-Sectional Study of Cognitively Unimpaired Older Adults. Brain Sciences. 2023; 13(3):473. https://doi.org/10.3390/brainsci13030473
Chicago/Turabian StyleFijałkiewicz, Aleksandra, Krzysztof Batko, and Aleksandra Gruszka. 2023. "Learned Irrelevance, Perseveration, and Cognitive Aging: A Cross-Sectional Study of Cognitively Unimpaired Older Adults" Brain Sciences 13, no. 3: 473. https://doi.org/10.3390/brainsci13030473
APA StyleFijałkiewicz, A., Batko, K., & Gruszka, A. (2023). Learned Irrelevance, Perseveration, and Cognitive Aging: A Cross-Sectional Study of Cognitively Unimpaired Older Adults. Brain Sciences, 13(3), 473. https://doi.org/10.3390/brainsci13030473






