Abstract
In recent decades, theories have been presented to explain the nature of dyslexia, but the causes of dyslexia remained unclear. Although the investigation of the causes of dyslexia presupposes a clear understanding of the concept of cause, such an understanding is missing. The present paper proposes the absence of at least one necessary condition or the absence of all sufficient conditions as causes for impaired reading. The causes of impaired reading include: an incorrect fixation location, too short a fixation time, the attempt to recognize too many letters simultaneously, too large saccade amplitudes, and too short verbal reaction times. It is assumed that a longer required fixation time in dyslexic readers results from a functional impairment of areas V1, V2, and V3 that require more time to complete temporal summation. These areas and areas that receive input from them, such as the fusiform gyrus, are assumed to be impaired in their ability to simultaneously process a string of letters. When these impairments are compensated by a new reading strategy, reading ability improves immediately.
1. Introduction
Since the German ophthalmologist Oswald Berkhan [1] first described the symptoms of dyslexia in 1881 and Rudolf Berlin introduced the term ”dyslexia“ [2], numerous theories have been proposed about its causes and treatments, [3,4] (for review). The magnocellular theory of dyslexia [5,6,7,8,9], the theory of unusual foveal and parafoveal processing of letters including an unusual crowding effect [10,11,12,13,14,15,16,17,18,19], and the temporal summation theory [20,21,22,23] regard developmental dyslexia (DD) as a visual perceptual disorder. Other theories assume that DD results from an impaired ability to process auditory stimuli [24,25,26,27] or is caused by impaired control of reading eye movements [6,7,8,9,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43].
Although the phonological awareness theory of DD has the most followers, it cannot adequately explain what causes DD. This theory includes different abilities, such as identifying phonemes; rhyming; naming letters, objects, and colors, and splitting words into syllables. It is assumed that an impairment in these abilities causes DD and that DD is due to an impaired ability to associate letter sequences with sound sequences [44,45,46,47,48,49,50,51,52,53,54,55,56]. Such impairments may coexist with DD, but a causal relationship between these impairments and DD has never been proven. Studies on the correlation between various performance deficits and dyslexia assume that deficits in the phonological domain are most frequent in readers with dyslexia, whereas deficits in the visual domain seldom occur [57]. However, this result is predetermined by the study design because visual influences, such as fixation location in the word, fixation time, direction and amplitudes of reading saccades, and extent of the visual field of attention, have not been examined (e.g., [57]). Assessing the impact of these visual influences proved them to be necessary conditions for reading, and their absence caused impaired reading [20,21,22].
Noncausal correlations between impaired reading performance and other performance impairments must be clearly distinguished from causal relationships. A causal relationship can only be stated when the criteria for a causal relationship have been clarified. A major weakness of theories concerning DD is that research in DD has hitherto ignored the longstanding discussions in the philosophy of science attempting to specify these criteria, presumably because previous mathematically formulated approaches [58,59,60,61,62,63,64,65] are difficult to transfer to experimental research. Therefore, the present study aims to formulate simple experimental criteria for a causal relationship as opposed to correlations without a causal relationship. An impairment can only be considered a cause of poor reading ability if there is a clear and logically consistent concept of ‘cause’ which can be applied to the search for causes of dyslexia. Since the concept of cause is based on the concepts of necessary and sufficient conditions [66,67,68,69,70,71,72,73], these concepts must be defined in terms of experimental methods. The definition provided here does not use a mathematical approach but offers a description of the notion of causality based on the experimental practice. The use of this approach can identify necessary and sufficient conditions in the study of dyslexia.
2. What Are Causes of Dyslexia?
2.1. Necessary Conditions, Sufficient Conditions, and Causes
A condition is considered necessary if it is indispensable for correct reading even if at least one sufficient condition is fulfilled. Suppose it can be demonstrated in a statistically sufficient number of experimental trials that pseudowords made up of five letters can be read correctly if the fixation time is at least 500 ms and that they cannot be read correctly if the fixation time is less than 500 ms. Then a fixation time of at least 500 ms is a necessary condition for five-letter pseudowords to be read correctly, provided all other experimental conditions remain constant.
A condition is considered sufficient for correct reading if it is dispensable when at least one other sufficient condition and all necessary conditions are fulfilled. Suppose it can be demonstrated in a statistically sufficient number of experimental trials that pseudowords can be read correctly if they are made up of four letters and if the fixation time is at least 500 ms. Let us also assume that pseudowords cannot be read correctly if they are composed of more than four letters or if the fixation interval is less than 500 ms. This implies that a word length not exceeding four letters is a sufficient (but not necessary) condition for pseudowords to be read correctly, and a fixation time of at least 500 ms is a sufficient (but not necessary) condition for pseudowords to be read correctly. To read words correctly, only one of these two conditions needs to be met, and each condition can be swapped with the other.
Def. 1: Let D be the set of all conditions under which an event E occurs (e.g., a person P can read flawlessly). Elements of D may exist at the same time as E or may have existed before E. Let N be a subset of D that contains only the conditions N1, …, Nk, and let H be a different subset of D that contains only the conditions H1, …, Hq. Then an element of N is a necessary condition for E (flawless reading) if and only if E is no longer present (flawless reading is no longer possible) if at least one element of N is missing (or has been missing) and at least one element of H is (or was) present.
An element (condition) of H is a sufficient condition for an event E (flawless reading) if and only if E is present (flawless reading is possible) if this element of H or other elements of H are present (or were present) and all elements of N (which are different from the conditions that are elements of H) are (or were) present. E is not present (a flawless reading is no longer possible) if all conditions that are elements of H are missing even if all elements of N are (or were) present.
In addition to the elements of sets N and H, another set of conditions (C) must be fulfilled. Only if conditions that are elements of C are fulfilled, conditions that are elements of the set N become necessary, and conditions that are elements of the set H become sufficient. For example, in the normally developed brain, the fibers from the nasal halves of the retinae cross in the optic chiasm so that the information reaches the contralateral cerebral hemisphere. In contrast, the fibers from the temporal halves of the retinae reach the ipsilateral cerebral hemisphere. Thus, stimuli on the right halves of the retinae are processed in the right cerebral hemisphere. These anatomical conditions (conditions which are elements of set C) are the presuppositions that a functional right occipital lobe is a necessary condition for visual stimuli to be detected when they are projected onto the right halves of the retinae (i.e., when they appear in the left visual hemifield). However, if unusual neuronal connections have developed after surgical removal of the right hemisphere in early childhood, connecting the entire retina with the remaining left hemisphere, the existence of a functional right occipital lobe is no longer a necessary condition for the processing of visual stimuli in the left visual hemifield.
There are many necessary and sufficient conditions for a person to be able to read correctly, including anatomical, physiological, biochemical, and psychological conditions. These conditions cannot all be explicitly formulated and are only partially known to the examiner. According to Definition 1, if a person is able to read, all the necessary conditions and at least one sufficient condition for reading are fulfilled, although we do not know them all. Nevertheless, we are able to find out which necessary condition is missing or if no sufficient condition is fulfilled. Since there are many necessary and sufficient conditions, more than one necessary condition may be missing, many sufficient conditions may be missing and may not have been replaced by other sufficient conditions, or even all sufficient conditions may be missing. This means that many necessary conditions and/or many unreplaced sufficient conditions may be missing. Therefore, dyslexia may not always have a single cause, but may have many causes. To recognize the necessary and sufficient conditions for better reading, reading performance must be tested in the alternating presence and absence of conditions suspected to be necessary or sufficient for reading. This means that we need to examine the conditions under which readers make reading errors and the conditions under which the same readers read correctly. To demonstrate the influence of conditions on reading performance, it is necessary to compare a group of subjects whose reading performance is tested in the presence and absence of these conditions (elements of D) with a control group. The reading performance of the control group is tested under unchanged conditions.
If it is demonstrated that reading performance is reduced or even impossible because at least one or more necessary conditions are lacking and/or because no sufficient condition is present, these are causes of reduced reading performance or the inability to read. Concerning the concepts of causation specified here and earlier [58,59,60,61,62,63,64,65], impairments that have been demonstrated to occur together with DD (e.g., [13,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,74,75,76,77,78,79,80,81,82,83,84,85,86,87]) turn out to be only concomitant impairments that do not fulfill the requirement for a causal relationship.
It may be that conditions that appear to be necessary or sufficient conditions are composed of several features, but only one feature may be a necessary or sufficient for reading ability whereas other features may have no influence. In this case, not all features that appear to constitute the necessary conditions can be regarded as necessary, and not all features that constitute the sufficient conditions can be regarded as sufficient. Then it must be investigated experimentally which features are necessary or sufficient and which features are irrelevant. If one finds for example that it is a necessary condition for a person to be able to read words when they are presented with a luminance of 4 cd/m2 on a 68 cd/m2 background, then only the difference in luminance is a necessary condition for reading. The fact that the words are presented in dark blue or in black is an irrelavant feature. The color is not part of the necessary condition.
Poor reading ability can result from many different impairments. The problem is to rule out all possible causes of dyslexia other than those being investigated. Many possible causes are evident because they can be easily identified and ruled out. Examples include eye diseases that prevent a clear image of the word to be projected onto the retina, visual field defects, or amblyopia. They are easy to diagnose. Other possible influences, such as inappropriate eye movements, incorrect fixation of the word to be read, too short a fixation time, insufficient focus of attention, the number of letters that can be recognized simultaneously (simultaneous recognition), and the verbal reaction time that a reader needs to retrieve sound sequences from memory when reading aloud, are not usually assessed in routine reading tests. The mere finding that these possible influences are associated with reading difficulties does not allow us to conclude that they have a causal influence on reading performance.
If it can be proven that normal readers and dyslexic readers only differ in one feature F1 which is present in good readers and absent in poor readers, it may be concluded that the absence of feature F1 is the cause of dyslexia. Thus, it is assumed that the presence of F1 is a necessary condition for good reading and its absence is a cause of dyslexia. This conclusion is correct only if all other possible causes have been ruled out and if it has been demonstrated that the presence of F1 is indeed a necessary condition for good reading. To test whether a possible influence (F1) is a necessary condition for normal reading ability, the only way to do this is to test whether reading normalizes when F1 is present and whether reading deteriorates when F1 is absent. However, this is not possible in many cases. A blind or amblyopic area of the retina cannot be made to disappear or reappear at will. In this case, knowledge of the visual system may lead to the conclusion that reading is not possible if the foveal and perifoveal areas of the retina are blind or severely amblyopic. According to Def. 1, such a conclusion must be based on the assumption that visual function would return if the damaged areas of the visual system recovered and regained their function. This hypothesis is supported by the observation that visual function can recover in previously blind areas of the retina after the function of neural networks in an affected region of the visual system has been restored.
As mentioned above, the question of whether fixation duration is a necessary condition for reading can be easily tested by offering pseudowords with different presentation times. However, prolonging fixation time alone may not improve the ability to recognize pseudowords. Using pseudowords instead of natural words has the advantage that pseudowords can only be read correctly if every letter is recognized. A natural word can be correctly guessed if only a few letters and the shape of the word are recognized. The ability to recognize pseudowords depends on the length of the fixation time and the number of letters that make up a pseudoword. It is therefore necessary to manipulate both simultaneously in order to test whether both are sufficient conditions for pseudoword recognition. There are significant differences between readers in the number of letters that can be recognized simultaneously. Repeated studies [20,21,22] have shown that good readers can often recognize at least six letters simultaneously in less than 250 ms, whereas some poor readers require a fixation time of at least 500 ms to recognize three letters. As fixation time increases, an increased number of letters can be recognized simultaneously. Reading errors occur when children try to recognize more letters at the same time than they can. Letters are then omitted, replaced by other letters, moved to the wrong place in the word, or letters are added to the word that are not in the word being read [20,21,22].
Even if the number of letters in the pseudowords is limited (e.g., to four letters) and the fixation time is sufficiently prolonged (e.g., up to 500 ms), many subjects may still not be able to recognize all the pseudowords correctly. It has been shown that subjects are only able to do this if the verbal reaction time during reading aloud is sufficiently prolonged (on average to approximately 1500 ms). This was achieved by offering a sound after the presentation of the pseudoword and instructing the subjects to begin pronouncing the pseudoword to be read only after the sound. The time between the pseudoword presentation and the start of the correct pronunciation of the pseudoword was measured using a computer. Under appropriate conditions, even children with severe dyslexia were able to correctly read at least 95% of the pseudowords [20,21,22].
2.2. Dyslexia Is Not Always Due to an Impaired Visual Attention Span, to Lateral Masking, or a Phononological Impairment
The visual attention span hypothesis assumes that the ability to process multiple letters that make up a word is impaired in children with dyslexia and that this is due to a reduced visual attention span [88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108]. Whether this impairment is due to a reduced attention span or any other visual deficit depends on what is understood by ”attention span“. Bosse et al. [90] (Abstract) defined ”… the visual attentional span is the amount of distinct visual elements which can be processed in parallel in a multi-element array“. The question is whether this definition is appropriate and how visual attention can be distinguished from other visual performances.
Def. 2: Visual attention is directed to a location in the visual field under given environmental conditions:
- (1)
- If the processing of stimuli improves when a person voluntarily attempts to process visual stimuli optimally at that location;
- (2)
- and/or if the processing of stimuli improves when stimuli are (consciously) expected at that location;
- (3)
- and/or if the processing of stimuli improves when environmental conditions indicate the location where stimuli will appear;
- (4)
- and/or when the visual system attempts to process stimuli optimally at that location even if the person does not voluntarily try to process stimuli optimally at that location.
The finding that dyslexic readers perform worse than normal readers on visual attention tasks [90,91,92,93,94,95,96,97,98,101,102,103,104,105,106,107,108] does not allow us to conclude that this reduced visual attention causes dyslexia. Poor visual attention revealed by attention tests may only accompany dyslexia but not cause it. The question remains whether the poorer performance of dyslexic readers is due to an attentional or a sensory deficit [99,100]. Confirming a causal relationship between poor attention and dyslexia requires normalizing attention in dyslexic readers and testing their reading performance under normal and impaired attention capacities. If this is not possible, the role of attention in reading must be inferred from readers’ performances on reading tests. Dyslexic readers have longer verbal reaction times than normal readers [101,102,103] and perform worse than normal readers when reading five-letter strings presented for 200 ms [90]. They also need longer exposure times than normal readers when reading single letters [107]. Dyslexic children performed worse than normal-reading children when requested to pick a string of symbols that had been previously shown for 100 ms from two symbol strings. The authors concluded that dyslexic readers had poorer visual sensitivity than normal readers and that there is an “… hitherto unrecognized visual component in children’s reading difficulties” [108].
When examining a person´s ability to read a three-letter pseudoword as well as s/he can, a mark can be presented on a monitor, and the person can be asked to fixate on this mark. A pseudoword to be read can then be displayed on the monitor such that the middle of the pseudoword matches the location of the fixation point. The pseudoword to be read is then located in the fovea, the location with the highest visual acuity. Simultaneously, all distracting stimuli must be eliminated. It has been demonstrated that many children with dyslexia are even unable to recognize three letters within a fixation time of 250 ms under these experimental conditions. When the fixation time was prolonged up to 500 ms, all children (n = 200) were able to recognize three letters simultaneously [20,21,22]. This shows that the children were able to focus their attention on the words when given sufficient time. The result can be interpreted in terms of attention as items (1)–(3) of Def. 2 are fulfilled. The number of letters in pseudowords that could be read without error also depended on the fixation time for four-letter, five-letter, and six-letter pseudowords. According to the definition provided by Bosse et al. [90], this results from a reduced attention span. However, this was not the case. If the children were unable to recognize pseudowords that were presented for 250 ms after the children had focused their attention for several seconds on the fixation point and a pseudoword was subsequently presented, the children focused their attention for some seconds plus 250 ms on the location where the pseudoword appeared. This fixation time was not sufficient to recognize the pseudoword. However, if pseudowords were always correctly recognized at a presentation time of, e.g., 500 ms, the few seconds in which attention was focused on the fixation point plus a pseudoword fixation time of 500 ms was sufficient to correctly recognize almost all letters in the pseudowords. The difference in 250 ms fixation time was decisive. The time interval in which the children focused their attention on the fixation point before a pseudoword appeared varied from trial to trial. However, the inability to recognize a pseudoword presented for 250 ms was independent of the time children focused their attention on the fixation point before a pseudoword appeared. This means that the time given to the children to focus their attention on the location where the pseudoword appeared did not influence their ability to recognize the pseudoword. Recognition of the pseudoword was determined solely by the time interval during which the pseudoword appeared. This means that the children did not need more time to focus their attention on the location where a pseudoword appeared. The children were also able to extend their attention to the entire pseudoword because at a longer presentation time, all pseudowords could be recognized. According to Def. 2, an impaired ability to recognize a given number of letters that make up a pseudoword within a sufficiently long period of fixation cannot result from a reduced attention span.
Vidyasagar and Pammer [109] tried to explain dyslexia as a deficit in visuo-spatial attention due to poor control of the dorsal visual pathway. The authors assume that “… letters are recognized sequentially with only one or a few letters being processed at a time by the object recognition system and this temporal sequence preserves the special sequence of letters” ([109] p. 58). They assumed that single letters or small groups of letters are scanned sequentially from left to right at a speed of less than 45 ms per item during fixation of a word. This shift in attention is believed to be controlled by the mainly magnocellular pathway of the dorsal stream including the posterior parietal cortex. Neurons in the dorsal stream are assumed to provide information about the location of items to be selected for detailed sequential analysis and recognition by neurons of the ventral stream. Dyslexia is believed to be caused by a dysfunction of magnocellular neurons in the dorsal pathway. According to this theory, a dyslexic reader who can recognize only two or three letters at a time attempts to read a six-letter word moving his/her focus of attention in steps of only two or three letters from left to right over the word, reading a sequence of only two or three letters at a time. Thus, dyslexic readers should be able to read words of any length that are in the area of sufficient visual acuity. However, this is not the case. If the theory were correct, a child who can read three letters at a time should always be able to recognize the first three letters of a word correctly, regardless of its length. However, dyslexic readers who can read three-letter words perfectly often misread the first three letters of five- or six-letter words and are unable to recognize letters at any position in the word [20,21,22]. The recognition of letters at the beginning of the word is also affected by the length of the whole word. This would not be the case if a reader were to read by recognizing a sequence of segments, one after another, each consisting of only two or three letters. The theory also fails to explain why the frequency of reading errors increases from the first letter at the beginning of the word to the third and remains approximately constant for the fourth, fifth, and sixth letters in words longer than three letters [20,21,22]. The theory implies that the ability to read two or three letters at a time decreases from left to right. This also remains unexplained.
Dyslexia can be regarded as the result of the longer fixation time needed to complete temporal summation. This is in agreement with the finding that detection and recognition of visual stimuli and visual acuity improve with an increase in the fixation interval, i.e., with increasing temporal summation [110,111,112,113,114,115,116,117,118,119,120,121]. These results are also in agreement with the finding that responses of the visual cortex increase monotonically but sub-linearly with increased duration of the stimulus [119,120,121,122,123,124]. An increase in visual fixation time results in an increase in the time interval during which temporal summation is completed. Temporal summation has been demonstrated predominantly in areas V1, V2, and V3 and to a lesser extent in areas V4, the anteriorly adjacent area VO, the occipitotemporal cortex corresponding to area MT, and the intraparietal sulcus [119,125]. The finding that the ability to read pseudowords immediately improved to the extent that all dyslexic readers could correctly recognize at least 95% of pseudowords of a given length when the fixation time was increased [20,21,22] shows that there is not only a correlation but also a causal relationship between reduced reading performance and the time available for temporal summation and recognition. Children with DD need more time for temporal summation and visual recognition.
Many dyslexic readers are unable to recognize all the letters in a pseudoword consisting of more than three or four letters, even if the subjects´ gaze is directed to the center of the word in the absence of distracting stimuli, when the subject is given sufficient time to focus his/her attention on the word and when the subject makes every effort to do so. These readers’ reading performance immediately improved when the number of letters to be recognized simultaneously was reduced [20,21,22]. The assumption that impaired simultaneous recognition in reading is due to early cortical processing is supported by the finding that the numbers of objects that are visually processed at a time without counting them is a basic feature of the early stages of processing in the visual system. Event-related potentials (ERP) have shown that neural responses approximately 90 ms after stimulus onset are sensitive to the number of items to be registered simultaneously [126]. The intensity of the BOLD signal in functional MRI increased in areas V1, V2, and V3 when the number of items to be registered in a visual array increased [127]. Longer words activated the medial and superior lingual gyrus, fusiform gyrus, and medial cuneus [126,127,128,129]. These results indicate that DD is due to an impairment of the visual system that requires longer fixation times and that has a decreased ability to recognize multiple letters at a time [20,21,22].
An inability to recognize several items at a time (simultaneous agnosia) has already been reported in brain damaged patients [130,131,132,133,134,135,136,137,138,139,140]. These patients were unable to overview a set of objects in an otherwise unimpaired visual field. They could only detect one object among several ones at a time, although there was no visual field defect when the visual field was assessed with a single stimulus. Impaired simultaneous recognition in reading may be regarded as a mild form of simultaneous agnosia that only becomes apparent in tasks such as reading. An area in which only a limited number of letters can be recognized at a time is different from a visual field of attention. The visual field of attention is an area in the retina where the detection and recognition of visual stimuli is improved and which expands according to how much attention is focused on this area [141,142,143,144,145]. In contrast, an area in which only a limited number of letters can be recognized cannot be expanded nor can more letters be recognized even if all attention is focused on that area.
It has also been assumed that dyslexia may be due to an unusual masking of letters by flanking letters (crowding effect) [10,11,12,13,14,15,16,17,18,19]. In this case, letters to the left and to the right of a letter decrease its recognition. Recent studies have shown that crowding plays no decisive role in the recognition of a letter string consisting of up to six letters. Letters at the end of pseudowords flanked by a letter on only one side were misread as often as letters flanked by letters on both sides [20,21,22]. The results of these studies demonstrate that the rate of incorrectly read letters in three-, four-, and five-letter pseudowords increases from the first letter up to the third letter but not from third to fifth letter. In six-letter pseudowords, the rate of misread letters increased only from the first to the fourth letter and remained constant from the fourth to the sixth letter. The fixation point was at the third letter in all trials. All dyslexic readers were able to read at least 95% of the pseudowords correctly when they fixated them in the middle, when the length of the pseudowords was adjusted to the children’s ability to recognize a string of letters at a time, and when the fixation time and the time from the beginning of the presentation of the word to the start of pronouncing the word was sufficiently prolonged. Whenever a word was read incorrectly, it was subsequently also spelled incorrectly [20,21,22]. This demonstrates that words were misread because the letter strings were not visually recognized correctly and that the crowding effect played no important role.
The finding that dyslexic readers were able to recognize and pronounce 95% of the pseudowords correctly when sufficient fixation time and enough vocal reaction time were provided demonstrates that all dyslexic children were able to associate the correct sequence of sounds with the sequence of letters [20,21,22]. Therefore, DD cannot generally be attributed to an impaired ability to associate letter sequences with sound sequences, as hypothesized by the phonological awareness theory [44,45,46,47,48,49,50,51,52,53,54,55,56].
When reading a text, a sufficiently long fixation period, a limit on the number of letters a subject tries to read at a time, and a sufficiently long verbal reaction time when reading aloud may not yet significantly improve reading performance. This raises the question of what role eye movements play in reading, whether normal reading eye movements are a necessary condition for normal reading, and whether unusual eye movements are a cause of reading difficulties. Findings on the role of fixation times, the number of letters a reader can recognize simultaneously, and the role of verbal reaction time already show that reading difficulties cannot be attributed solely to unusual eye movements during reading. The role of reading eye movements in dyslexia needs to be assessed in relation to each reader’s ability to recognize a string of letters simultaneously within a given fixation interval and in relation to the verbal reaction time required by each reader.
2.3. The Role of Inappropriate Reading Eye Movements in Dyslexia
The question of whether reading eye movements that deviate from the norm can cause a ‘‘reading disorder’’ has been controversial [6,7,8,9,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. It has been argued that irregular eye movements often found in subjects with a ‘‘reading disorder’’ can also occur in good readers and that some poor readers also demonstrate normal eye movements.
Reading requires that the word or word segment to be read is displayed in the area of the retina that has a sufficiently high visual acuity: the fovea and perifoveal area. This is true for all languages. To make the best possible use of the highest visual acuity area, the center of the word or word segment to be read should be located at about the center of the fovea. When the reader directs his/her gaze toward the beginning of a word or word segment to be read, the word or word segment to be read is shifted to the right half of the fovea and perifoveal area, and letters at the right end of the word or word segment to be read may be outside the range of sufficiently high visual acuity, and they cannot be recognized. When the reader directs the gaze toward the end of the word or word segment, the word or word segment is shifted to the left half of the fovea and perifoveal area. The letters at the beginning of the word or word segment may then be outside the range of sufficiently high visual acuity and cannot be recognized. This means that the position of the gaze in the word is a necessary condition for simultaneously recognizing as many letters as possible.
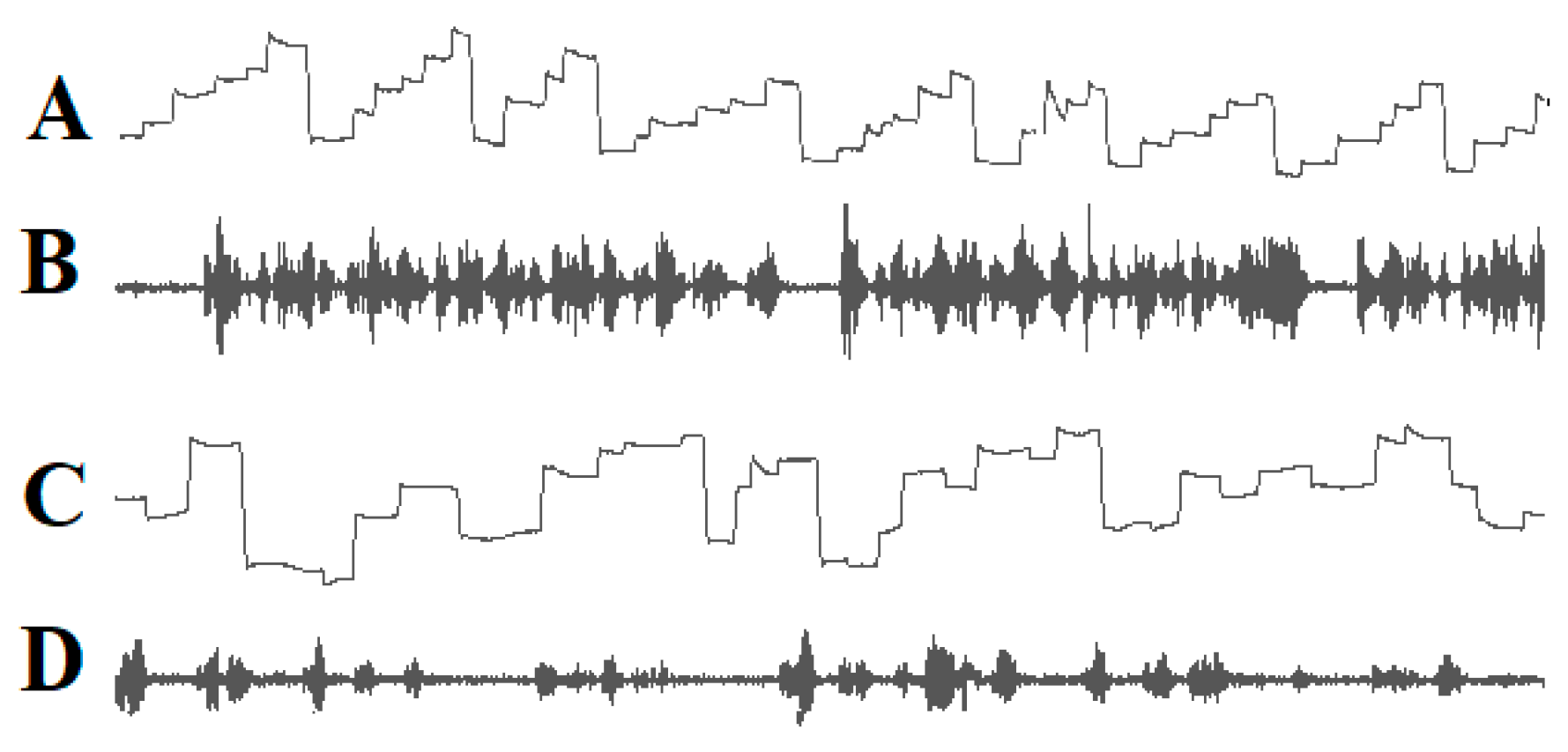
Since the words or word segments to be read must be shifted into the fovea and perifoveal area, the eyes must move in the reading direction, and the saccade amplitude must be adjusted so that after each saccade, the next word to be read is projected onto the fovea and perifoveal region. Good readers complete a succession of staircase-like reading saccades in the reading direction whereas many poor readers execute irregular eye movements that are often directed opposite to the reading direction (Figure 1).
Figure 1.
Eye movements (A) and speech spectrogram (B) of a good reader. Ascending lines: eye movements to the right; descending lines: eye movements to the left. The reader exerts a sequence of staircase-like eye movements in the reading direction. The speech spectrogram shows fluent reading without interruptions. Irregular eye movements (C) and the speech spectrogram (D) of a dyslexic child. The child exerts many eye movements opposite to the reading direction. The speech spectrogram demonstrates that the child reads haltingly with many pauses.
If we compare good readers with poor readers and find that poor readers make more irregular eye movements than good readers, we may assume that there is a causal relationship between irregular eye movements and poor reading performance. The assumption of a causal relationship implies that poor reading performance disappears when eye movements are normalized. If reading performance does not improve after eye movements have normalized, a non-causal correlation must be assumed. This means that it must be examined whether abnormal eye movements are a necessary or sufficient condition for poor reading performance. The normalization of eye movements can be achieved by guiding reading eye movements using a computer. It can be tested whether saccades of appropriate amplitudes, a sufficiently long fixation interval, a sufficiently long verbal reaction time, and an attempt to recognize no more letters than a reader can, lead to a drastic reduction in reading errors.
In our experiments [20,21,22,146,147]:
- (1)
- A yellow cursor indicated the location in the word where the gaze should be directed;
- (2)
- Green cursors to the left and right of the yellow cursor indicated how many letters to the left and right of the fixation point the subject should try to recognize together with the letter at the fixation point;
- (3)
- The onset of the movement of the yellow and green cursors in the reading direction indicated when fixation on a word or word segment should be terminated;
- (4)
- The amplitude of the movement of the cursors in the reading direction indicated the direction and amplitude of the saccade to be made in order to read the next word or word segment;
- (5)
- A sound signal indicated when the subject should begin to pronounce the word or word segment to be read.
Five independent studies in which 350 children with dyslexia participated [20,21,22,146,147] have shown that reading errors decrease immediately with an effect size of up to Hedges g = 2.65 if:
- (1)
- Fixation times are long enough to recognize a string of letters;
- (2)
- Readers do not try to recognize more letters in a word at the same time than they can;
- (3)
- Eye movement amplitudes in the reading direction do not exceed the number of letters that the reader can simultaneously recognize;
- (4)
- The reader does not start pronouncing the words or word segments to be read too early.
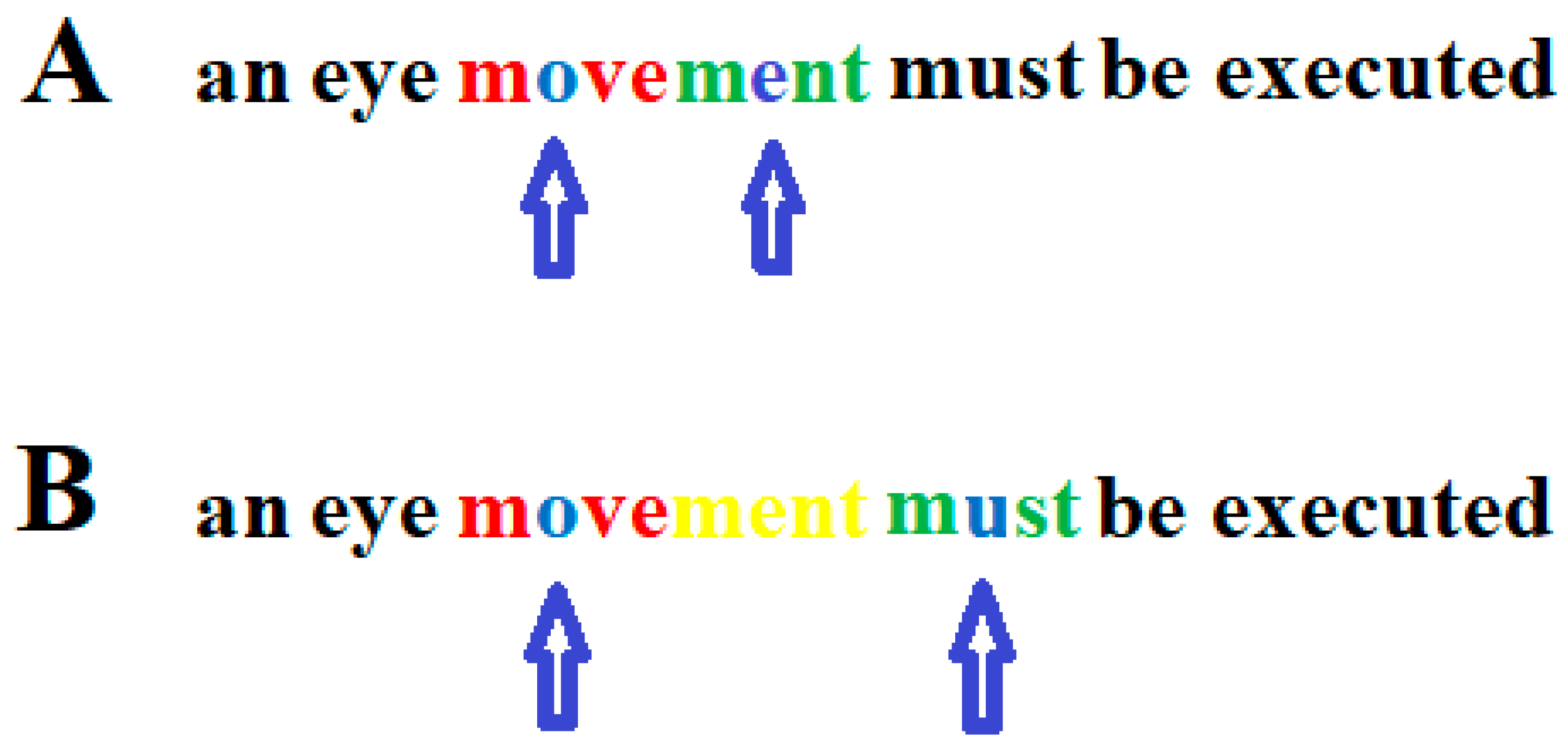
Eye movements were recorded to check whether the readers´ eyes followed the curser. If the reader’s eye movements did not follow the cursor exactly, the text to the left and right of the word being read was erased. In this way, staircase-like eye movements can be induced, such as those seen in good readers. However, inducing staircase eye movements in dyslexic readers, such as those seen in good readers, is not sufficient to improve the reading ability in dyslexic readers. Whether reading eye movements are appropriate does not depend on whether the sequence of saccades matches that of good readers. Rather, it depends on the individual’s ability to simultaneously recognize a string of letters and the duration of the fixation interval required to detect a given string of letters that make up a word [20,21,22,146,147]. Therefore, eye movements cannot be considered a cause of dyslexia if they are unusual and deviate from the norm represented by typical readers. After a word or word segment has been read, a saccade is initiated to the next word or word segment to be read. This saccade should be aimed at approximately the middle of the word or word segment to be read subsequently. The amplitude of the saccade must not exceed the number of letters that can be recognized simultaneously so that the word segment to be read next follows the previous word segment without a gap between the word segments (Figure 2).
Figure 2.
Correct (A) and too large (B) saccade during reading. In the upper graph, the arrows indicate on which letter within a word segment a person who can only recognize four letters simultaneously must focus his/her gaze. One letter to the left and two letters to the right (red) of the focused letter (blue) must be recognized simultaneously together with the focused letter. After the first word segment (blue and red) has been read, the gaze only moves so far in the reading direction that the next word segment to be read (blue and green) follows without a gap between the two word segments. (B): After the first word segment (blue and red) was read, the eyes jumped too far to the next word segment (blue and green) so that the letters “ment” (yellow) were overlooked. Arrows indicate the letter on which the gaze is focused.
When a five-letter word segment has been read with the gaze directed to the middle letter and a saccade over seven letters to the next word segment has been executed, the child can recognize the fixated letter and two letters to the left and two letters to the right of the fixated middle letter of the word segment to be read. If the child can only recognize five letters at a time and such a large saccade is executed, two letters are overlooked between the two word segments. This demonstrates that the amplitudes of reading saccades that exceed the number of letters that the reader can recognize simultaneously will cause reading errors. Thus, whether the amplitude of a reading eye movement is appropriate depends on the number of letters a reader can simultaneously recognize. As the duration of the fixation interval also determines whether a word can be correctly recognized, the time at which a saccade is initiated is critical for flawless reading.
To execute adequate eye movements that match the number of letters that can be recognized simultaneously, the reader must be able to control reading eye movements. It has been demonstrated that even the eyes of children with severe dyslexia can be guided by a computer so that they can learn to execute appropriate reading eye movements within fewer than 30 min [20,21,22,146,147]. This shows that the children were able to control their eye movements but did not do so spontaneously and had to control them voluntarily. This means that reading disorders do not occur because eye movements deviate from the norm but because the amplitudes of many eye movements are too large and do not correspond to the length of simultaneously recognized words, which causes letters to be overlooked (Figure 2).
When children meet the requirements for flawless reading, appropriate saccades may be interspersed with eye movements opposite to the reading direction or with searching eye movements in both directions. As long as there is a sequence of correct saccades, unusual saccades that occur between the correct saccades do not make flawless reading impossible. As long as readers fixate the words to be read for a sufficiently long time, do not try to recognize more letters than they can at a time, do not start pronouncing the words to be read too early, and make appropriate saccades, eye movement impairment does not result in reading impairment.
If a reader´s fixation time is too short and/or if a reader tries to recognize more letters simultaneously than s/he can, many words will not be recognized. Readers realize that the words they attempted to recognize were incorrectly recognized and that a sequence of words does not make sense. Then, readers often make eye movements opposite to the reading direction to refixate on words that have already been read or make searching eye movements in and opposite to the reading direction [21,40]. In this case, unusual eye movements are the result of reading impairments such as too short fixation times and/or the inability to recognize as many letters simultaneously as the reader tries to recognize. Thus, unusual eye movements can be a cause as well as a consequence of poor reading performance.
2.4. DD Is Not Caused by a Phonological Impairment
Reading requires knowing which sounds are associated with which letters or sequences of letters. In languages such as Spanish, German, and Italian, where there is a high grapheme–phoneme correspondence, it is easier to learn the association between sounds and letters than in languages such as English or French in which the pronunciation of the same letter can vary in different words and a sound is often represented by a sequence of letters. The ability to learn the grapheme–phoneme association of a language is not equally developed among all individuals. Even some children with German as their native language have great difficulty learning the grapheme–phoneme correspondence of certain letters, such as “m/n, b/d, or p/q”. Therefore, it is not surprising that children whose native languages have ambiguous grapheme–phoneme correspondence have even greater difficulties. These problems can be easily eliminated in the case of native German speakers by testing whether the grapheme–phoneme connection is mastered for all letters and letter sequences and excluding those children who do not know the phonemes associated with certain letters. The causes of reading problems can then be examined independently of the inability to store the association between some letters and the corresponding sounds in memory. In languages where grapheme–phoneme correspondence is variable and sophisticated, readers must retrieve pronunciation from memory when reading real words or pseudowords. This problem is present in all reading tests with native English and French speakers. Even in three-letter words (e.g., the pronunciation of the letter “a” in the words ‘tar’, ‘raw’, ‘tan’ or the pronunciation of the letter “e” in words like “sea”, “set”, “sew”), the pronunciation of letters can vary. In languages such as Spanish, German, or Italian, the problem of variable pronunciation of letters is almost non-existent. In these languages, the causes of reading problems can be investigated independent of the impaired ability to master the grapheme–phoneme correspondence. It has been demonstrated that children may have severe DD even if there is no impairment in their knowledge of grapheme–phoneme correspondence. Studies with native German speakers in whom impaired knowledge of grapheme–phoneme correspondence was excluded [20,21,22,146,147] demonstrated that impaired knowledge of grapheme–phoneme correspondence plays only a minor role in dyslexia. The causes of DD revealed in native German speakers will also be present in dyslexic English or French readers because the necessary conditions for reading German are the same as for reading English or French. If native English or French speakers have reading problems due to impaired knowledge of grapheme–phoneme correspondence, they may not be dyslexic in a language with high grapheme–phoneme correspondence. Since dyslexia can have causes other than impairment in finding the correct sequence of sounds for a sequence of letters, other possible causes must be examined independently of a phonological impairment. From the definition of “cause” given above, it follows that any study that aims to find causes of dyslexia must be a study involving therapy because it investigates whether the presence or absence of a condition improves or worsens the reading ability of a dyslexic reader.
3. Is Dyslexia Due to an Impaired Learning Capacity?
It may be assumed that dyslexia develops because children cannot learn to read due to a reduced learning capacity. This assumption is supported by studies which have found that dyslexic readers perform worse than normal readers on tasks such as the digit span test [148]. Children with dyslexia also showed significantly poorer performance than normal-reading children on word learning tasks [149]. According to the authors, the results indicate that children with dyslexia have word learning difficulties beyond their phonological impairments. However, children with dyslexia were only impaired in the phonological aspects of word learning tests [150]. Other studies found that children with dyslexia performed worse than normal readers on phonemic awareness tasks and, to some extent, on rhyme awareness tasks, but they had only small impairments on verbal short-term memory tasks [151] (for review). Kimel et al. [152] found only a small difference between the performance of readers with DD and normal readers on tasks that required the children to learn repeated sequences of syllables. The authors conclude that if there is an impairment in serial order learning in children with dyslexia, it is mild and cannot be considered a core deficit. In agreement with these results, Debska et al. [153] found that the predominant deficit in children with DD was phonological (51%). A total of 26% of the children with dyslexia showed no cognitive deficit at all, and only 14% of them demonstrated an implicit learning impairment in a reaction time task. Children with dyslexia performed even better than normal readers on verbal learning tasks [154]. Lazzaro et al. [155] compared short-term and long-term memory in children with DD and normal readers. Children with DD performed worse than normal-reading, age-matched children but as well as normal-reading children at the same reading level on verbal, visual-object, and visual-spatial short-term and long-term memory tasks. The authors concluded that memory may not have a causal effect on reading performance.
Children with dyslexia improve when they receive a training that practices phonological awareness, rapid naming, phonemic decoding, word reading, spelling, and reading, e.g., [151,156,157,158,159,160]. Torgesen et al. [156] found that first graders with poor letter-sound knowledge who received an 8-month reading intervention in which the children practiced phonological awareness, rapid naming, phonemic decoding, word reading, spelling, and reading comprehension outperformed controls but only on the phonemic decoding, rapid naming, and spelling tasks. Reading accuracy and efficiency as well as spelling skills also improved when children with dyslexia received reading therapy [158]. Children with dyslexia improved their reading performance after taking part in a 16-week remediation program that included reading syllable lists as quickly as possible, phonological awareness exercises, memorizing syllables or words after they disappeared from the screen, and choosing real words from real and invented words [159]. After 6 weeks of computer-based training, in which children were taught to read single letters, letter combinations, and words of different lengths, reading ability improved only in boys but not in girls [160].
It is not surprising that phonological awareness, rapid naming, phonemic decoding, word reading, spelling, and reading comprehension improve after systematic training in these skills. When good readers read correctly, they learn which graphemes correspond to which phonemes, and each reading session can reinforce their knowledge of the grapheme–phoneme correspondence. Good reading requires a continuous learning process with reading practice. Poor readers, who read haltingly with many reading mistakes, do not have this constant reading experience. They realize that the words and sentences they are trying to read do not make sense and that their reading is therefore incorrect. They find reading unpleasant, so they avoid it. The ability to retrieve the correct sound sequences that correspond to letter sequences is a necessary condition for flawless reading. However, this is not the only necessary condition for flawless reading. Other necessary conditions include directing the gaze for a given time interval approximately to the middle of the word or word segment to be read, recognizing a string of letters that make up a word at a time, not starting the pronunciation too early, and performing eye movements which match the number of letters that can be recognized at a time. First graders learn letter–sound correspondences and how to replace letter-by-letter reading with reading a few letters simultaneously. These reading abilities are learned through the teacher’s reading instructions. Establishing other necessary conditions, such as where and how long to fixate in a word, how many letters to read simultaneously, and the appropriate eye movements, must be learned without teacher instruction through repeated reading in a self-generated learning process. Dyslexia develops when a child fails to generate at least one of these necessary conditions. Dyslexia can therefore be seen as an impaired ability to individually create all the necessary conditions for error-free reading. However, dyslexic children are not incapable of learning to create all the necessary and sufficient conditions for error-free reading. Repeated studies [20,21,146,147] have shown that children with dyslexia are able to learn to establish all the necessary and sufficient conditions in fewer than 30 min using computer-based reading therapy.
4. What Functional MRI and Cerebral Lesions Reveal about the Neurobiological Basis of Dyslexia
The question of whether an impairment is a cause of DD or whether it is merely an accompanying deficit with no causal significance is also fundamental to the assessment of neural dysfunctions that may occur together with impaired abilities such as dyslexia [161,162]. Theories regarding the neural basis of DD mainly rely on functional magnetic resonance imaging (fMRI) studies [125,126,127,128,129,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187,188,189,190]. An increase in the blood-oxygen-level-dependent (BOLD) signal in an area of the brain shows that this area is receiving sensory afferents or input from other brain areas [191,192]. If the BOLD signal is smaller in dyslexic readers than in good readers, it does not mean that the activation of this brain area, such as the ventral occipitotemporal cortex including the word form area [163,165,167,169,179,181], is a necessary or sufficient condition for flawless reading. Underactivation of a brain area in dyslexic readers may be present in dyslexic readers without impacting the reading process. To conclude that an underactivated brain region contributes to reading impairment, a dysfunction in another brain area concomitant with the underactivated area must be excluded. If a brain region that plays a role in the reading process is functionally impaired, other brain regions may compensate for this impairment. It has been demonstrated that loss of one or even both occipital lobes in early development can be compensated to a considerable extent [193,194] and that reading is even possible in the absence of the left ventral occipito-temporal cortex [195]. It can also be assumed that not all functional impairments of neural networks are reflected in functional MRI [196].
Reading impairment may not be due to the underactivated but compensated region detected in functional MRI. It may be due to functional impairment of another region not sufficiently compensated. The results of many studies [163,197,198,199,200,201,202,203,204,205,206,207,208,209,210,211,212,213,214,215,216,217,218,219,220] indicate that brain regions of dyslexic readers that were less activated than those of normal readers contain the fusiform gyrus, superior temporal gyrus, precuneus, inferior parietal cortex, including the angular gyrus, and the inferior frontal gyrus of the left cerebral hemisphere. If at least one of these brain structures is underactivated in readers who are regarded as dyslexic according to commonly used reading tests, we may conclude that this underactivation is the cause of the reading disorder. However, this is not entirely correct. It has been repeatedly demonstrated [20,21,22,146,147] that readers with dyslexia need longer fixation intervals and longer verbal response times and that their reading saccades are unsuitable for fluent reading (Figure 1 and Figure 2). Repeated studies have also demonstrated that even children with severe dyslexia dramatically reduce the rate of reading mistakes within 30 min of practice when they adopt a new reading strategy tailored to their impaired abilities [20,21,22,146,147]. Children with dyslexia can learn (1) to fixate on the words or word segments to be read approximately in the middle, (2) to increase their fixation time, (3) not to attempt to recognize more letters simultaneously than they can recognize at a time, (4) to match the amplitudes of the saccades to the number of letters that they can read simultaneously, and (5) to increase the time from the onset of fixation of the words or word segments to be read to the onset of pronunciation. When dyslexic children used such a new reading strategy tailored to their abilities, the rate of reading mistakes was reduced to approximately one-third of the error rate before acquiring the new reading strategy. Reading was slowed down due to the necessary increase in fixation time and verbal response time, but the error rate was dramatically reduced. This means that the underactivation of cerebral regions observed in dyslexic readers causes impaired reading abilities only when the location of fixation, duration of the fixation interval, simultaneous recognition, saccades of eye movements, and verbal reaction times are not adapted to the reader. Activity in areas V1, V2, and V3, where the temporal summation is predominantly achieved and which is also present to a lesser extent in areas V4, the anteriorly adjacent area VO, area MT, and the intraparietal sulcus [119,125], precedes activity in the fusiform gyrus, superior temporal gyrus, precuneus, and inferior parietal cortex, including the angular gyrus and the inferior frontal gyrus of the left cerebral hemisphere. If areas V1, V2, and V3 need longer fixation times to complete temporal summation, insufficient temporal summation due to too short fixation times may cause lower activation in areas that receive input from areas V1, V2, and V3. This is in agreement with the finding that the fMRI signal of a cerebral region is mainly generated by input to this region [191,192]. It is also possible that a developmental dysfunction that affected the visual cortex also affected regions of the parietal and temporal lobe, whereby the latter dysfunctions did not substantially impair reading abilities.
It has been demonstrated [205,221] that surgical removal of the Visual Word Form Area in the left fusiform cortex results in an impaired ability to simultaneously recognize a string of letters. If only one specific cerebral damage occurred, if reading performance was normal before the cerebral damage and deteriorated after the cerebral damage, as was the case in these studies, this indicates that a normal function of the surgically removed brain structure is a necessary condition for normal reading performance. Therefore, one can conclude that the function of the Visual Word Form Area is also a necessary condition for the ability to simultaneously recognize a string of letters.
5. Conclusions
Whether there is a causal relationship between impairments that precede or coexist with dyslexia can only be assessed when based on a clear scientific concept of cause. A cause can be regarded as the absence of at least one necessary condition and/or the absence of all sufficient conditions. Whether a condition is necessary or sufficient can only be determined on the basis of experiments in which presumed necessary or sufficient conditions are alternately present or absent. Considering that correct reading requires the presence of all necessary conditions and at least one sufficient condition, dyslexia is present when at least one necessary condition or all sufficient conditions are missing. To test whether all the necessary and sufficient conditions for normal reading are met, it is necessary to find the conditions under which dyslexic children immediately and dramatically reduce their rate of reading errors without further training. This means that the search for necessary and sufficient conditions for reading includes a highly successful reading therapy. If one necessary condition or all sufficient conditions are lacking, this causes impaired reading performance.
The distribution of visual acuity in the retina requires eye movements to shift the word or word segment into the foveal region. The amplitudes of these eye movements must match the reader´s ability to simultaneously recognize a string of letters. To recognize a string of letters simultaneously, an individually required fixation time must be maintained to achieve the required temporal summation. Additionally, the individually required verbal reaction time must be maintained when reading aloud. When dyslexic readers learn a reading strategy in which all the necessary conditions are met, their reading performance improves immediately. This has enabled us to develop a powerful computer software that can improve the reading skills of children with severe dyslexia in a very short time. Repeated experiments with native German speakers have shown that phonological problems do not cause dyslexia in languages with high grapheme–phoneme correspondence.
Experiments to determine whether there is a causal relationship are often not feasible because it is impossible to set up experiments in which a necessary condition is alternately present or absent. The assumption of a causal relationship is then based on the finding that an impairment (e.g., poor reading skills) always occurs after or during an event (e.g., a brain lesion) and on what is already known about the function of a biological system (e.g., the brain).
Functional MRI and brain lesion studies have demonstrated that dyslexia results from uncompensated impairments of the primary and secondary visual cortex and areas that receive input from these early visual processing structures, such as the fusiform gyrus, superior temporal gyrus, precuneus, inferior parietal cortex, and left inferior frontal gyrus.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data have been created.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Berkhan, O. Über Störungen der Sprache und der Schriftsprache: Für Ärzte und Lehrer Dargestellt; August Hirschwald: Berlin, Germany, 1889. [Google Scholar]
- Berlin, R. Über Dyslexie. Arch. Psychiat. Nervenkrankh. 1884, 15, 276–278. [Google Scholar]
- Helland, T. Trends in dyslexia research during the period 1950 to 2020—Theories, definitions, and publications. Brain Sci. 2022, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Stein, J. Theories about developmental dyslexia. Brain Sci. 2023, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, M.S.; Rosen, G.D.; Drislane, F.W.; Galaburda, A.M. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Proc. Natl. Acad. Sci. USA 1991, 88, 7943–7947. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Walsh, V. To see but not to read; the magnocellular theory of dyslexia. Trends Neurosci. 1997, 20, 147–152. [Google Scholar] [CrossRef]
- Stein, J.; Walsh, V. Impaired neural timing in developmental dyslexia—The magnocellular hypothesis. Dyslexia 1999, 5, 59–77. [Google Scholar] [CrossRef]
- Stein, J. The magnocellular theory of dyslexia. In Developmental Dyslexia, Early Precursors, Neurobehavioral Markers, and Biological Substrates; Benasich, A.A., Fitch, R.H., Eds.; Brooks: Baltimore, MD, USA; London, UK; Sydney, Australia, 2012. [Google Scholar]
- Stein, J. The current status of the magnocellular theory of developmental dyslexia. Neuropsychologia 2019, 130, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Geiger, G.; Lettvin, J.Y. Peripheral vision in persons with dyslexia. N. Engl. J. Med. 1987, 316, 1238–1243. [Google Scholar] [CrossRef]
- Geiger, G.; Lettvin, J.Y.; Fahle, M. Dyslexic children learn a new visual strategy for reading: A controlled experiment. Vis. Res. 1993, 34, 1223–1233. [Google Scholar] [CrossRef]
- Spinelli, D.; De Luca, M.; Judica, A.; Zoccolotti, P. Crowding effects on word identification in developmental dyslexia. Cortex 2002, 38, 179–200. [Google Scholar] [CrossRef]
- Lorusso, M.L.; Facoetti, A.; Pesenti, S.; Cattaneo, C.; Molteni, M.; Geiger, G. Wider recognition in peripheral vision common to different subtypes of dyslexia. Vis. Res. 2004, 44, 2413–2424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Whitney, D.; Levi, D.M. Visual crowding: A fundamental limit on conscious perception and object recognition. Trends. Cogn. Sci. 2011, 15, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Zorzi, M.; Barbiero, C.; Facoetti, A.; Lonciari, I.; Carrozzi, M.; Montico, M.; Bravar, L.; George, F.; Pech-Georgel, C.; Ziegler, J.C. Extra-large letter spacing improves reading in dyslexia. Proc. Natl. Acad. Sci. USA 2012, 109, 11455–11459. [Google Scholar] [CrossRef] [PubMed]
- Gori, S.; Facoetti, A. How the visual aspects can be crucial in reading acquisition? The intriguing case of crowding and developmental dyslexia. J. Vis. 2015, 14, 15.1.8. [Google Scholar] [CrossRef]
- Strasburger, H. Seven Myths on Crowding and Peripheral Vision. Iperception 2020, 11, 2041669520913052. [Google Scholar] [CrossRef]
- Kirkby, J.A.; Barrington, R.S.; Drieghe, D.; Liversedge, S.P. Parafoveal processing and transposed-letter effects in dyslexic reading. Dyslexia 2022, 28, 359–374. [Google Scholar] [CrossRef]
- Kewan-Khalayly, B.; Migó, M.; Yashar, A. Transient attention equally reduces visual crowding in radial and tangential axes. J. Vis. 2022, 22, 3. [Google Scholar] [CrossRef]
- Werth, R. Rapid improvement of reading performance in children with dyslexia by altering the reading strategy: A novel approach to diagnoses und therapy of reading deficiencies. Restor. Neurol. Neurosci. 2018, 36, 679–691. [Google Scholar] [CrossRef]
- Werth, R. What causes Dyslexia? Identifying the causes and effective compensatory therapy. Restor. Neurol. Neurosci. 2019, 37, 591–608. [Google Scholar] [CrossRef]
- Werth, R. Dyslexic readers improve without training when using a computer-guided reading strategy. Brain Sci. 2021, 11, 526. [Google Scholar] [CrossRef]
- Werth, R. Is developmental dyslexia due to a visual and not a phonological impairment? Brain Sci. 2021, 11, 1313. [Google Scholar] [CrossRef] [PubMed]
- Tallal, P. Auditory temporal perception, phonics, and reading disabilities in children. Brain Lang. 1980, 9, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Tallal, P.; Miller, S.; Fitch, R.H. Neurobiological basis of speech: A case for the pre-eminence of temporal processing. Ann. N. Y. Acad. Sci. 1993, 682, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Merzenich, M.M.; Jenkins, W.M.; Johnston, P.; Schreiner, C.; Miller, S.L.; Tallal, P. Temporal processing deficits of language learning impaired children ameliorated by training. Science 1996, 271, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, S.; Mahnke, H.; Salz, T.; Tallal, P.; Roberts, T.; Merzenich, M.M. Cortical auditory signal processing in poor readers. Proc. Natl. Acad. Sci. USA 1999, 96, 6483–6488. [Google Scholar] [CrossRef]
- Pavlidis, G.T. Do eye movements hold the key to dyslexia? Neuropsychologia 1981, 19, 57–64. [Google Scholar] [CrossRef]
- Rayner, K.; Pollatsek, A. Eye movement control during reading: Evidence for direct control. Q. J. Exp. Psychol. Sect. A 1982, 33, 351–373. [Google Scholar] [CrossRef]
- Pavlidis, G.T. Eye movements in dyslexia: Their diagnostic significance. J. Learn. Disabil. 1985, 18, 42–50. [Google Scholar] [CrossRef]
- Rayner, K. Do faulty eye movements cause dyslexia? Dev. Neuropsychol. 1985, 1, 3–15. [Google Scholar] [CrossRef]
- Rainer, K. Eye movements and the perceptual span in beginning and skilled readers. J. Exp. Child Psychol. 1986, 41, 211–236. [Google Scholar] [CrossRef]
- Fischer, B.; Biscaldi, M.; Otto, P. Saccadic eye movements of dyslexic adult subjects. Neuropsychologia 1993, 31, 887–906. [Google Scholar] [CrossRef] [PubMed]
- Eden, G.F.F.; Stein, J.F.F.; Wood, H.M.M.; Wood, F.B.B. Differences in eye movements and reading problems in dyslexic and normal children. Vis. Res. 1994, 34, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Hyönä, J.; Olson, R.K. Eye fixation patterns among dyslexic and normal readers: Effect of word length and word frequency. J. Exp. Psychol. Learn. Mem. Cogn. 1995, 21, 1430–1440. [Google Scholar] [CrossRef] [PubMed]
- Biscaldi, M.; Gezeck, S.; Stuhr, V. Poor saccadic control correlates with dyslexia. Neuropsychologia 1998, 36, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- De Luca, M.; Di Pace, E.; Judica, A.; Spinelli, D.; Zoccolotti, P. Eye movement patterns in linguistic and non-linguistic tasks in developmental surface dyslexia. Neuropsychologia 1999, 37, 1407–1420. [Google Scholar] [CrossRef]
- Biscaldi, M.; Fischer, B.; Hartnegg, K. Voluntary saccadic control in dyslexia. Perception 2000, 29, 509–521. [Google Scholar] [CrossRef]
- Rayner, K.; Slattery, T.J.; Bélanger, N.N. Eye movements, the percepzual span, and reading speed. Bull. Rev. 2010, 17, 834–839. [Google Scholar] [CrossRef]
- Ward, L.M.; Kapoula, Z. Dyslexics’ Fragile Oculomotor Control Is Further Destabilized by Increased Text Difficulty. Brain Sci. 2021, 11, 990. [Google Scholar] [CrossRef]
- Temelturk, R.D.; Ozer, E. Binocular coordination of children with dyslexia and typically developing children in linguistic and non-linguistic tasks: Evidence from eye movements. Ann. Dyslexia 2022, 72, 426–444. [Google Scholar] [CrossRef] [PubMed]
- Premeti, A.; Bucci, M.P.; Isel, F. Evidence from ERP and eye movements as markers of language dysfunction in dyslexia. Brain Sci. 2022, 12, 73. [Google Scholar] [CrossRef]
- Caldani, S.; Acquaviva, E.; Moscoso, A.; Peyre, H.; Delorme, R.; Bucci, M.P. Reading performance in children with ADHD: An eye-tracking study. Ann. Dyslexia 2022, 72, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Wagner, R.K.; Torgesen, J.K. The nature of phonological processing and its causal role in the acquisition of reading skills. Psychol. Bull. 1987, 101, 192–212. [Google Scholar] [CrossRef]
- Bruce, D. An analysis of word sounds by young children. Br. J. Educ. Psychol. 1964, 34, 158–170. [Google Scholar] [CrossRef]
- Bryant, P.E.; MacLean, M.; Bradley, L.L.; Crossland, J. Rhyme and alliteration, phoneme detection, and learning to read. Dev. Psychol. 1990, 26, 429–438. [Google Scholar] [CrossRef]
- Torgesen, J.K.; Wagner, R.K.; Rashotte, C.A. Longitudinal studies of phonological processing and reading. J. Learn. Disabil. 1994, 27, 276–286. [Google Scholar] [CrossRef] [PubMed]
- McBride-Chang, C. What is phonological awareness? J. Educ. Psychol. 1995, 87, 179–192. [Google Scholar] [CrossRef]
- Yopp, H.K. A test for assessing phonemic awareness in young children. Read. Teach. 1995, 49, 20–29. [Google Scholar] [CrossRef]
- Torgesen, J.K.; Wagner, R.K.; Rashotte, C.A. Prevention and remediation of severe reading disabilities: Keeping the end in mind. Sci. Stud. Read. 1997, 1, 217–234. [Google Scholar] [CrossRef]
- Muter, V.; Hulme, C.; Snowling, M.J.; Taylor, S. Segmentation, not rhyming, predicts early progress in learning to read. J. Exp. Child. Psychol. 1998, 71, 3–27. [Google Scholar] [CrossRef]
- Ehri, L.C. Research on learning to read and spell: A personal historical perspective. Sci. Stud. Read. 1998, 2, 97–114. [Google Scholar] [CrossRef]
- Snowling, M. From language to reading and dyslexia. Dyslexia. Dyslexia 2001, 7, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Goswami, U. Phonology, reading development, and dyslexia: A cross-linguistic perspective. Ann. Dyslexia 2002, 52, 139–163. [Google Scholar] [CrossRef]
- Vellutino, F.R.; Fletcher, J.M.; Snowling, M.J. Scanlon, D.M. Specific reading disability (dyslexia): What have we learned in the past four decades. J. Child Psychol. Psychiat. 2004, 45, 2–40. [Google Scholar] [CrossRef] [PubMed]
- Goswami, U. Phonology, reading and reading difficulties. In Interdisciplinary Perspectives on Learning to Read. Culture, Cognition and Pedagogy; Hall, K., Goswami, U., Harrison, C., Ellis, S., Soler, Eds.; Routledge: London, UK, 2010; pp. 103–116. [Google Scholar]
- Ligges, C.; Lehmann, T. Multiple case study in German children with dyslexia: Characterization of phonological, auditory, visual, and cerebellar processing on the group and individual level. Brain Sci. 2022, 12, 1292. [Google Scholar] [CrossRef] [PubMed]
- Mackie, J.L. Causes and conditions. Am. Philos. Q. 1965, 2, 245–264. [Google Scholar]
- Spirtes, P.; Glymour, C.; Scheines, R. Causation, Prediction, and Search; MIT Press: Cambridge, UK, 1992. [Google Scholar]
- Lewis, D. Causation as influence. J. Philos. 2000, 97, 182–197. [Google Scholar] [CrossRef]
- Spohn, W. Causation: An alternative. Brit. J. Philos. Sci. 2006, 57, 93–119. [Google Scholar] [CrossRef]
- Pearl, J.; Glymour, M.; Jewell, N.P. Causal Inference in Statistics; Wiley: Chichester, UK, 2016. [Google Scholar]
- Pearl, J. Causality—Models, Reasoning and Inference; Cambridge University Press: Cambridge, UK; New York, NY, USA; Port Melbourne, Australia, 2018; pp. 316–320. [Google Scholar]
- Bressler, S.L.; Seth, A.K. Wiener-Granger causality: A well established methodology. Neuroimage 2011, 58, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Stokes, P.A.; Purdon, P.L. A study of problems encountered in Granger causality analysis from a neuroscience perspective. Proc. Natl. Acad. Sci. USA 2017, 114, E7063–E7072. [Google Scholar] [CrossRef]
- Ingthorson, R. The Logical vs. the Ontological Understanding of Conditions. Metaphysica 2008, 9, 129–137. [Google Scholar] [CrossRef][Green Version]
- Douven, I. The Evidential Support Theory of Conditionals. Synthese 2008, 164, 19–44. [Google Scholar] [CrossRef]
- Gomes, G. Are Necessary and Sufficient Conditions Converse Relations? Australas. J. Philos. 2009, 87, 375–387. [Google Scholar] [CrossRef]
- Gomes, G. Meaning Preserving Contraposition of Natural Language Conditionals. J. Prag. 2019, 152, 46–60. [Google Scholar] [CrossRef]
- Gomes, G. Concessive conditionals without Even if and non-concessive conditionals with Even if. Acta Analyt. 2020, 35, 1–21. [Google Scholar] [CrossRef]
- Crupi, V.; Iacona, A. The Evidential Conditional. Erkenntnis 2020, 87, 2897–2921. [Google Scholar] [CrossRef]
- Raidl, E.; Iacona, A.; Crupi, V. The Logic of the Evidential Conditional. Rev. Symb. Logic 2021, 15, 758–770. [Google Scholar] [CrossRef]
- Crupi, V. On the Logical Form of Concessive Conditionals. J. Philos. Logic 2022, 51, 633–651. [Google Scholar] [CrossRef]
- Farah, M.; Stowe, R.M.; Levinson, K.L. Phonological dyslexia. Loss of a reading specific component of cognitive architecture? Cogn. Neuropsychol. 1996, 13, 849–868. [Google Scholar] [CrossRef]
- Hari, R.; Renvall, H. Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn. Sci. 2001, 5, 525–532. [Google Scholar] [CrossRef]
- Roach, N.W.; Hogben, J.H. Attentional modulation of visual processing in adult dyslexia: A spatial cuing deficit. Psychol. Sci. 2004, 15, 650–654. [Google Scholar] [CrossRef]
- Buchholz, J.; Davis, A.A. Adults with dyslexia demonstrate space-based and object-based covert attention deficits: Shifting attention to the periphery and shifting attention between objects in the left visual field. Brain Cogn. 2005, 57, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Heim, S.; Tschierse, J.; Amunts, K.; Wilms, M.; Vossel, S.; Willmes, K.; Grabowska, A.; Huber, W. Cognitive subtypes of dyslexia. Acta Neurobiol. Exp. 2008, 68, 73–82. [Google Scholar]
- Daniel, A.; Abrams, D.A.; Nicol, T.; Zecker, S.; Kraus, N. Abnormal cortical processing of the syllable rate of speech in poor readers. J. Neurosci. 2009, 29, 7686–7693. [Google Scholar] [CrossRef]
- Menghini, D.; Finzi, A.; Benassi, M.; Bolzani, R.; Facoetti, A.; Giovagnoli, S.; Ruffino, M.; Vicari, S. Different underlying neurocognitive deficits in developmental dyslexia: A comparative study. Neuropsychologia 2010, 48, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Hornickel, J.; Kraus, N. Unstable representation of sound: A biological marker of dyslexia. J. Neurosci. 2013, 33, 3500–3504.67. [Google Scholar] [CrossRef]
- Lorusso, M.L.; Cantiani, C.; Molteni, M. Age, dyslexia subtype and comorbidity modulate rapid auditory processing in developmental dyslexia. Front. Hum. Neurosci. 2014, 8, 313. [Google Scholar] [CrossRef]
- van Bergen, E.; de Jong, P.F.; Maassen, B.; Krikhaar, E.; Plakas, A.; van der Leij, A. IQ of four-year-olds who go on to develop dyslexia. J. Learn. Disabil. 2014, 47, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Hendren, R.L.; Haft, S.L.; Black, J.M.; White, N.C.; Hoeft, F. Recognizing Psychiatric Comorbidity With Reading Disorders. Front. Psychiatry 2018, 9, 101. [Google Scholar] [CrossRef]
- Peters, L.; Bulthé, J.; Daniels, N.; Op de Beeck, H.; De Smedt, B. Dyscalculia and dyslexia: Different behavioral, yet similar brain activity profiles during arithmetic. Neuroimage Clin. 2018, 18, 663–674. [Google Scholar] [CrossRef]
- Moll, K.; Landerl, K.; Snowling, M.J.; Schulte-Körne, G. Understanding comorbidity of learning disorders: Task-dependent estimates of prevalence. J. Child. Psychol. Psychiat. 2019, 60, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Maziero, S.; Tallet, J.; Bellocchi, S.; Jover, M.; Chaix, Y.; Jucla, M. Influence of comorbidity on working memory profile in dyslexia and developmental coordination disorder. J. Clin. Exp. Neuropsychol. 2020, 42, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Facoetti, A.; Paganoni, P.; Turatto, M.; Marzola, V.; Mascetti, G.G. Visual spatial attention in developmental dyslexia. Cortex 2000, 36, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Carrasco, M.; Williams, P.E.; Yeshurun, Y. Covert attention increases spatial resolution with or without masks: Support for signal enhancement. J. Vision 2002, 2, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Bosse, M.L.; Tainturier, M.J.; Valdois, S. Developmental dyslexia: The visual attention span deficit hypothesis. Cognition 2007, 104, 198–230. [Google Scholar] [CrossRef]
- Franceschini, S.; Gori, M.; Ruffino, K.; Pedrolli, A.; Facoetti, A. A causal link between visual spatial attention and reading acquisition. Curr. Biol. 2012, 22, 814–819. [Google Scholar] [CrossRef]
- Ruffino, M.; Gori, S.; Boccardi, D.; Molteni, M.; Facoetti, A. Spatial and temporal attention in developmental dyslexia. Front. in Human Neurosci. 2014, 8, 8–331. [Google Scholar] [CrossRef]
- Chen, C.; Schneps, M.H.; Masyn, K.E.; Thomson, J.M. The effects of visual attention span and phonological decoding in reading comprehension in dyslexia: A path analysis. Dyslexia 2016, 22, 322–344. [Google Scholar] [CrossRef]
- Facoetti, A.; Turatto, M.; Lorusso, M.L.; Mascetti, G.G. Orienting of visual attention in dyslexia: Evidence for asymmetric hemispheric control of attention. 2001, 138, 46–53. Exp. Brain Res. 2001, 138, 46–53. [Google Scholar] [CrossRef]
- Facoetti, A.; Lorusso, M.L.; Paganoni, P.; Cattaneo, C.; Galli, R.; Umiltà, C.; Mascetti, G.G. Auditory and visual automatic attention deficits in developmental dyslexia. Brain Res. Cogn. Brain Res. 2003, 16, 185–191. [Google Scholar] [CrossRef]
- Roach, N.W.; Hogben, J.H. Spatial cueing deficits in dyslexia reflect generalised difficulties with attentional selection. Vision Res. 2008, 48, 193–207. [Google Scholar] [CrossRef][Green Version]
- Facoetti, A.; Zorzi, M.; Cestnick, L.; Lorusso, M.L.; Molteni, L.M.; Paganoni, P.; Umilta, C.; Mascetti, G.G. The relationship between visuo-spatial attention and nonword reading in developmental dyslexia. Cogn. Neuropsych. 2006, 23, 841–855. [Google Scholar] [CrossRef] [PubMed]
- Pina Rodrigues, A.; Castelo-Branco, M.; van Asselen, M. Disrupted spatial organization of cued exogenous attention persists into adulthood in developmental dyslexia. Front. Psychol. 2021, 12, 769237. [Google Scholar] [CrossRef]
- Skottun, B.C.; Skoyles, J. Dyslexia: Sensory deficits or inattention? Perception 2007, 36, 1084–1088. [Google Scholar] [CrossRef] [PubMed]
- Skottun, B.C. On the use of cues to assess attention in dyslexia. Front. Hum. Neurosci. 2014, 8, 983. [Google Scholar] [CrossRef]
- De Luca, M.; Burani, C.; Paizi, D.; Spinelli, D.; Zoccolotti, P. Letter and letter-string processing in developmental dyslexia. Cortex 2010, 46, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Zoccolotti, P.; De Luca, M.; Spinelli, D. Discrete versus multiple word displays: A re-analysis of studies comparing dyslexic and typically developing children. Front. Psychol. 2015, 6, 1530. [Google Scholar] [CrossRef] [PubMed]
- Burani, C.; Marcolini, S.; Traficante, D.; Zoccolotti, P. Reading derived words by Italian children with and without dyslexia: The effect of root length. Front. Psychol. 2018, 9, 647. [Google Scholar] [CrossRef]
- Franceschini, S.; Bertoni, S.; Puccio, G.; Gori, S.; Termine, C.; Facoetti, A. Visuo-spatial attention deficit in children with reading difficulties. Sci. Rep. 2022, 12, 13930. [Google Scholar] [CrossRef]
- Valdois, S. The visual-attention span deficit in developmental dyslexia: Review of evidence for a visual-attention-based deficit. Dyslexia 2022, 28, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Perry, C.; Long, H. What is going on with visual attention in reading and dyslexia? A critical review of recent studies. Brain Sci. 2022, 12, 87. [Google Scholar] [CrossRef]
- Stenneken, P.; Egetemeir, J.; Schulte-Körne, G.; Müller, H.J.; Schneider, W.X.; Finke, K. Slow perceptual processing at the core of developmental dyslexia: A parameter-based assessment of visual attention. Neuropsychologia 2011, 49, 3454–3465. [Google Scholar] [CrossRef] [PubMed]
- Pammer, K.; Lavis, R.; Hansen, P.; Cornelissen, P.L. Symbol-string sensitivity and children’s reading. Brain Lang. 2004, 89, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Vidyasagar, R.R.; Pammer, K. Dyslexia: A deficit in visuo-spatial attention, not a phonological processing. Trends Cogn. Sci. 2010, 14, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.H.; Cook, C. Visual acuity as a function of intensity and exposure-time. Am. J. Psychol. 1937, 49, 654–661. [Google Scholar] [CrossRef]
- Barlow, H.B. Temporal and spatial summation in human vision at different background intensities. J. Physiol. Lond. 1958, 141, 337–350. [Google Scholar] [CrossRef]
- Niwa, K.; Tokoro, T. Measurement of temporal summation of visual acuity with use of modified tachistoscope. Jpn. J. Ophthalmol. 1997, 41, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, K.; Bibby, B.M.; Timmermans, B.; Cleeremans, A.; Overgaard, M. Measuring consciousness: Task accuracy and awareness as sigmoid functions of stimulus duration. Conscious. Cogn. 2011, 20, 1659–1675. [Google Scholar] [CrossRef]
- McAnany, J.J. The effect of exposure duration on visual acuity for letter optotypes and gratings. Vision Res. 2014, 105, 86–91. [Google Scholar] [CrossRef]
- Windey, B.; Vermeiren, A.; Atas, A.; Cleeremans, A. The graded and dichotomous nature of visual awareness. Philos. Trans. R.Soc. B. Biol. Sci. 2014, 369, 20130282. [Google Scholar] [CrossRef][Green Version]
- Mulholland, P.J.; Redmond, T.; Garway-Heath, D.F.; Zlatkova, M.B.; Anderson, R.S. The Effect of Age on the Temporal Summation of Achromatic Perimetric Stimuli. Invest. Ophthalmol. Vis. Sci. 2015, 56, 6467–6472. [Google Scholar] [CrossRef][Green Version]
- Holmes, R.; Victora, M.; Wang, R.F.; Kwiat, P.G. Measuring temporal summation in visual detection with a single-photon source. Vis. Res. 2017, 140, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Stigliani, A.; Jeska, B.; Grill-Spector, K. Encoding model of temporal processing in human visual cortex. Proc. Natl. Acad. Sc.i USA 2017, 114, E11047–E11056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Benson, N.C.; Kay, K.N. Winawer, J. Compressive temporal summation in human visual cortex. J. Neurosci. 2018, 38, 691–709. [Google Scholar] [CrossRef]
- Beauny, A.; de Heering, A.; Muñoz Moldes, S.; Martin, J.-R.; de Beir, A.; Cleeremans, A. Unconscious categorization of sub-millisecond complex images. PLoS One 2020, 15, e0236467. [Google Scholar] [CrossRef]
- Heinrich, S.P.; Blechenberg, T.; Reichel, C.; Bach, M. The "speed" of acuity in scotopic vs. photopic vision. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- Harvey, B.M.; Dumoulin, S.O.; Fracasso, A.; Paul, J.M. A Network of Topographic Maps in Human Association Cortex Hierarchically Transforms Visual Timing-Selective Responses. Curr. Biol. 2020, 30, 1424–1434.e6. [Google Scholar] [CrossRef] [PubMed]
- Hendrikx, E.; Paul, J.M.; van Ackooij, M.; van der Stoep, N.; Harvey, B.M. Visual timing-tuned responses in human association cortices and response dynamics in early visual cortex. Nat. Commun. 2022, 8, 3952. [Google Scholar] [CrossRef]
- Paul, J.M.; van Ackooij, M.; Ten Cate, T.C.; Harvey, B.M. Numerosity tuning in human association cortices and local image contrast representations in early visual cortex. Nat. Commun. 2022, 13, 1340. [Google Scholar] [CrossRef]
- Weiner, K.S.; Sayres, R.; Vinberg, J.; Grill-Spector, K. fMRI-adaptation and category selectivity in human ventral temporal cortex: Regional differences across time scales. J. Neurophysiol. 2010, 103, 3349–3365. [Google Scholar] [CrossRef]
- Fornaciai, M.; Brannon, E.M.; Woldorff, M.G.; Park, J. Numerosity processing in early visual cortex. Neuroimage 2017, 157, 429–438. [Google Scholar] [CrossRef]
- DeWind, N.K.; Park, J.; Woldorff, M.G.; Brannon, E.M. Numerical encoding in early visual cortex. Cortex 2019, 114, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, A.; Humphreys, G.W.; Mayall, K.; Olson, A.; Price, C.J. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc. Royal Soc. Lond. Series B. 2000, 267, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M.; Sturm, D.; Richlan, F.; Kronbichler, M.; Ladurner, G.; Wimmer, H. A dual-route perspective on brain activation in response to visual words: Evidence for a length by lexicality interaction in the visual word form area (VWFA). Neuroimage 2010, 49, 2649–2661. [Google Scholar] [CrossRef] [PubMed]
- Bálint, D. Seelenlähmung des “Schauens”, optische Ataxie, räumliche Störung der Aufmerksamkeit. Mschr. Psychiat. Neurol. 1909, 25, 51–81. [Google Scholar] [CrossRef]
- Poppelreuter, W. Die Psychischen Schädigungen durch Kopfschuß im Kriege 1914–1918. Bd. I. Die Störungen der Niederen und Höheren Sehleistungen durch Verletzungen des Okzipitalhirns; L. Voss: Leipzig, Germany, 1917. [Google Scholar]
- Luria, A.R. Disorders of “simultaneous perception” in a case of bilateral occipito-parietal brain injury. Brain 1959, 82, 437–449. [Google Scholar] [CrossRef]
- Baylis, G.C.; Driver, J.; Baylis, L.L.; Rafal, R.D. Reading of letters and words in a patient with Balint’s syndrome. Neuropsychologia 1994, 32, 1273–1286. [Google Scholar] [CrossRef] [PubMed]
- Rizzo., M.; Vecera, S.P. Psychoanatomical substrates of Bálint’s syndrome. J. Neurol. Neurosurg. Psychiatry 2002, 72, 162–178. [Google Scholar] [CrossRef]
- Moreaud, O. Balint syndrome. Arch. Neurol. 2003, 60, 1329–1331. [Google Scholar] [CrossRef]
- Michel, F.; Henaff, M.A. Seeing without the occipito-parietal cortex: Simultagnosia as a shrinkage of the attentional visual field. Behav. Neurol. 2004, 15, 3–13. [Google Scholar] [CrossRef]
- Farah, M.J. Visual Agnosia, 2nd ed.; MIT Press: Cambridge, MA, USA; London, UK, 2004; pp. 43–58. [Google Scholar]
- Chechlacz, M.; Rotshtein, P.; Hansen, P.C.; Riddoch, J.M.; Deb, S.; Humphreys, G.W. The neural underpinings of simultanagnosia: Disconnecting the visuospatial attention network. J. Cogn. Neurosci. 2012, 247, 18–35. [Google Scholar] [CrossRef]
- Chechlacz, M.; Humphreys, G.W. The enigma of Balint’s syndrome: Neural substrates and cognitive deficits. Front. Hum. Neurosci. 2014, 8, 123. [Google Scholar] [CrossRef] [PubMed]
- Chechlacz, M. Bilateral parietal dysfunctions and disconnections in simultanagnosia and Bálint syndrome. Handb. Clin. Neurol. 2018, 151, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Haslerud, G.M. Influence on extreme peripheral vision of attention to a visual or auditory task. J. Exp. Psychol. 1964, 68, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Engel, F.L. Visual conspicuity, directed attention and retinal locus. Vision Res. 1971, 11, 563–576. [Google Scholar] [CrossRef]
- Ikeda, M.; Takeuchi, T. Influence of foveal load on the functional visual field. Perc. Psychophys. 1975, 18, 225–260. [Google Scholar] [CrossRef]
- Henderson, J.M.; Ferreira, F. Effects of foveal processing difficulty on the perceptual span in reading: Implication for attention and eye movement control. J. Exp. Psychol. Learn. Mem. Cogn. 1990, 16, 417–429. [Google Scholar] [CrossRef]
- Handy, T.C.; Kingstone, A.; Mangun, G.R. Spatial distribution of visual attention: Perceptual sensitivity and response latency. Percept. Psychophys. 1996, 58, 613–627. [Google Scholar] [CrossRef]
- Werth, R. Therapie von Lesestörungen durch Erkennen und Beheben der Ursachen. Ergother. Rehabil. 2006, 9, 6–11. [Google Scholar]
- Klische, A. Leseschwächen gezielt beheben. Individuelle Diagnose und Therapie mit dem Programm Celeco; Tectum: Marburg, Germany, 2007. [Google Scholar]
- Gabay, Y.; Thiessen, E.D.; Holt, L.L. Impaired statistical learning in developmental dyslexia. J. Speech Lang. Hear. Res. 2015, 58, 934–945. [Google Scholar] [CrossRef]
- Adlof, S.M.; Baron, L.S.; Bell, B.A.; Scoggins, J. Spoken Word Learning in Children With Developmental Language Disorder or Dyslexia. J. Speech Lang. Hear. Res. 2021, 64, 2734–2749. [Google Scholar] [CrossRef]
- Alt, M.; Gray, S.; Hogan, T.P.; Schlesinger., N.; Cowan, N. Spoken Word Learning Differences Among Children With Dyslexia, Concomitant Dyslexia and Developmental Language Disorder, and Typical Development. Lang. Speech. Hear. Serv. Sch. 2019, 50, 540–561. [Google Scholar] [CrossRef] [PubMed]
- Melby-Lervåg, M.; Lyster, S.A.; Hulme, C. Phonological skills and their role in learning to read: A meta-analytic review. Psychol. Bull. 2012, 138, 322–352. [Google Scholar] [CrossRef] [PubMed]
- Kimel, E.; Lieder, I.; Ahissar, M. Repeated series learning revisited with a novel prediction on the reduced effect of item frequency in dyslexia. Sci. Rep. 2022, 12, 13521. [Google Scholar] [CrossRef] [PubMed]
- Dębska, A.; Łuniewska, M.; Zubek, J.; Chyl, K.; Dynak, A.; Dzięgiel-Fivet, G.; Plewko, J.; Jednoróg, K.; Grabowska, A. The cognitive basis of dyslexia in school-aged children: A multiple case study in a transparent orthography. Dev. Sci. 2022, 25, e13173. [Google Scholar] [CrossRef] [PubMed]
- van Rijthoven, R.; Kleemans, T.; Segers, E.; Verhoeven, L. Compensatory role of verbal learning and consolidation in reading and spelling of children with dyslexia. Ann. Dyslexia. 2022, 72, 461–486. [Google Scholar] [CrossRef] [PubMed]
- Lazzaro, G.; Varuzza, C.; Costanzo, F.; Fucà, E.; Di Vara, S.; De Matteis, M.E.; Vicari, S.; Menghini, D. Memory Deficits in Children with Developmental Dyslexia: A Reading-Level and Chronological-Age Matched Design. Brain Sci. 2021, 11, 40. [Google Scholar] [CrossRef]
- Torgesen, J.K.; Wagner, R.K.; Rashotte, C.A.; Herron, J.; Lindamood, P. Computer-assisted instruction to prevent early reading difficulties in students at risk for dyslexia: Outcomes from two instructional approaches. Ann. Dyslexia 2010, 60, 40–56. [Google Scholar] [CrossRef]
- Galuschka, K.; Ise, E.; Krick, K.; Schulte-Körne, G. Effectiveness of treatment approaches for children and adolescents with reading disabilities: A meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e89900. [Google Scholar] [CrossRef]
- Tilanus, E.A.T.; Segers, E.; Verhoeven, L. Predicting responsiveness to a sustained reading and spelling intervention in children with dyslexia. Dyslexia 2019, 25, 190–206. [Google Scholar] [CrossRef]
- Forné, S.; López-Sala, A.; Mateu-Estivill, R.; Adan, A.; Caldú, X.; Rifà-Ros, X.; Serra-Grabulosa, J.M. Improving Reading Skills Using a Computerized Phonological Training Program in Early Readers with Reading Difficulties. Int. J. Environ. Res. Public Health 2022, 19, 11526. [Google Scholar] [CrossRef]
- Hughes, J.A.; Phillips, G.; Reed, P. Brief exposure to a self-paced computer-based reading programme and how it impacts reading ability and behaviour problems. PLoS ONE 2013, 8, e77867. [Google Scholar] [CrossRef] [PubMed]
- Pulvermüller, F. The case of CAUSE: Neurobiological mechanisms for grounding an abstract concept. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373, 20170129. [Google Scholar] [CrossRef] [PubMed]
- Weichwald, S.; Peters, J. Causality in Cognitive Neuroscience: Concepts, Challenges, and Distributional Robustness. J. Cogn. Neurosci. 2021, 33, 226–247. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Dehaene, S.; Naccache, L.; Lehéricy, S.; Dehaene-Lambertz, G.; Hénaff, M.A.; Michel, F. The visual word form area: Spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain 2000, 123, 291–307. [Google Scholar] [CrossRef]
- Xu, B.; Grafman, J.; Gaillard, W.D.; Ishii, K.; Vega-Bermudez, F.; Pietrini, P.; Reeves-Tyer, P.; DiCamillo, P.; Theodore, W. Conjoint and extended neural networks for the computation of speechcodes: The neural basis of selective impairment in reading words and pseudowords. Cereb. Cortex 2001, 11, 267–277. [Google Scholar] [CrossRef]
- Cohen, L.; Lehericy, S.; Chochon, F.; Lemer, C.; Rivaud, S.; Dehaene, S. Language-specific tuning of visual cortex? Functional properties of the visual word form area. Brain 2002, 125, 1054–1069. [Google Scholar]
- Polk, T.A.; Stallcup, M.; Aguirre, G.K.; Alsop, D.C.; D’Esposito, M.; Detre, J.A.; Farah, M.J. Neural specialization for letter recognition. J. Cogn. Neurosci. 2002, 14, 145–159. [Google Scholar] [CrossRef]
- Dehaene, S.; Le Clec´H., G.; Poline, J.B.; Le Bihan, D.; Cohen, L. The visual word-form area: A prelexical representation of visualwords in the fusiform gyrus. Neuroreport 2002, 13, 321–325. [Google Scholar] [CrossRef]
- Wydell, T.N.; Vuorinen, T.; Helenius, P.; Salmelin, R. Neural correlates of letter-string length and lexicality during reading in a regular orthography. J. Cogn. Neurosci. 2003, 15, 1052–1062. [Google Scholar] [CrossRef]
- McCandliss, B.D.; Cohen, L.; Dehaene, S. The visual word form area: Expertise for reading in the fusiform gyrus. Trends Cogn. Sci. 2003, 7, 293–299. [Google Scholar] [CrossRef]
- Cohen, L.; Martinaud, O.; Lemer, C.; Lehéricy, S.; Samson, Y.; Obadia, M.; Slachevsky, A.; Dehaene, S. Visual word recognition in the left and right hemispheres: Anatomical and functional correlates of peripheral alexias. Cereb. Cortex 2003, 13, 1313–1333. [Google Scholar] [CrossRef]
- Binder, J.R.; Medler, D.A.; Desai, R.; Conant, L.L.; Liebenthal, E. Some neurophysiological constraints on models of word naming. Neuroimage 2005, 27, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Binder, J.R.; Medler, D.A.; Westbury, C.F.; Liebenthal, E.; Buchanan, L. Tuning of the human left fusiform gyrus to sublexical orthographic structure. Neuroimage 2006, 33, 739–748. [Google Scholar] [CrossRef]
- Martens, V.E.; de Jong, P.F. The effect of word length on lexical decision in dyslexic and normal reading children. Brain Lang. 2006, 98, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Kronbichler, M.; Bergmann, J.; Hutzler, F.; Staffen, W.; Mair, A.; Ladurner, G.; Wimmer, H. Taxi vs. Taksi: On orthographic word recognition in the left ventral occitiotemporal cortex. J. Cogn. Neurosci. 2007, 19, 1584–1594. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.I.; Liu, J.; Wald, L.L.; Kwong, K.K.; Benner, T.; Kanwisher, N. Visual word processing and experiential origins of functional selectivity in human extrastriate cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 9087–9092. [Google Scholar] [CrossRef] [PubMed]
- Epelbaum, S.; Pinel, P.; Gaillard, R.; Delmaire, C.; Perrin, M.; Dupont, S.; Dehaene, S.; Cohen, L. Pure alexia as a disconnection syndrome: New diffusion imaging evidence for an old concept. Cortex 2008, 44, 962–974. [Google Scholar] [CrossRef]
- Graves, W.W.; Desai, R.; Humphries, C.; Seidenberg, M.S.; Binder, J.R. Neural systems for reading aloud: A multiparametric approach. Cereb. Cortex 2010, 20, 1799–1815. [Google Scholar] [CrossRef]
- Church, J.A.; Balota, D.A.; Petersen, S.E.; Schlaggar, B.L. Manipulation of length and lexicality localizes the functional neuroanatomy of phonological processing in adult readers. J. Cogn. Neurosci. 2011, 23, 1475–1493. [Google Scholar] [CrossRef]
- Ben-Shachar, M.; Dougherty, R.F.; Deutsch, G.K.; Wandell, B.A. The development of cortical sensitivity to visual word forms. J. Cogn. Neurosci. 2011, 23, 2387–2399. [Google Scholar] [CrossRef]
- Stigliani, A.; Weiner, K.S.; Grill-Spector, K. Temporal Processing Capacity in High-Level Visual Cortex Is Domain Specific. J. Neurosci. 2015, 35, 12412–12424. [Google Scholar] [CrossRef] [PubMed]
- Stevens, W.D.; Kravitz, D.J.; Peng, C.S.; Tessler, M.H.; Martin, A. Privileged Functional Connectivity between the Visual Word Form Area and the Language System. J. Neurosci. 2017, 37, 5288–5297. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Baum, S.; Kook, J.H.; Lee, Y.; Ramos-Nuñez, A.; Vannucci, M. Individual Differences in the Neural and Cognitive Mechanisms of Single Word Reading. Front. Hum. Neurosci. 2018, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Dębska, A.; Chyl, K.; Dzięgiel, G.; Kacprzak, A.; Łuniewska, M.; Plewko, J.; Marchewka, A.; Grabowska, A.; Jednoróg, K. Reading and spelling skills are differentially related to phonological processing: Behavioral and fMRI study. Dev. Cogn. Neurosci. 2019, 39, 100683. [Google Scholar] [CrossRef] [PubMed]
- Ostertag, C.; Reynolds, J.E.; Dewey, D.; Landman, B.; Huo, Y.; Lebel, C. Altered gray matter development in pre-reading children with a family history of reading disorder. Dev Sci. 2022, 25, e13160. [Google Scholar] [CrossRef]
- Eckert, M.A.; Vaden, K.I., Jr.; Iuricich, F. Dyslexia Data Dyslexia Data Consortium. Cortical asymmetries at different spatial hierarchies relate to phonological processing ability. PLoS Biol. 2022, 20, e3001591. [Google Scholar] [CrossRef]
- Hedenius, M.; Persson, J. Neural correlates of sequence learning in children with developmental dyslexia. Hum. Brain Mapp. 2022, 43, 3559–3576. [Google Scholar] [CrossRef]
- Yu, X.; Ferradal, S.; Dunstan, J.; Carruthers, C.; Sanfilippo, J.; Zuk, J.; Zöllei, L.; Gagoski, B.; Ou, Y.; Grant, P.E.; et al. Patterns of Neural Functional Connectivity in Infants at Familial Risk of Developmental Dyslexia. JAMA Netw. Open 2022, 5, e2236102. [Google Scholar] [CrossRef]
- Zhao, J.; Song, Z.; Zhao, Y.; Thiebaut de Schotten, M.; Altarelli, I.; Ramus, F. White matter connectivity in uncinate fasciculus accounts for visual attention span in developmental dyslexia. Neuropsychologia 2022, 177, 108414. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Peng, P. Reading real words versus pseudowords: A meta-analysis of research in developmental dyslexia. Dev. Psychol. 2022, 58, 1035–1050. [Google Scholar] [CrossRef] [PubMed]
- Braid, J.; Richlan, F. The Functional Neuroanatomy of Reading Intervention. Front. Neurosci. 2022, 16, 921931. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K. The neural basis of the blood-oxygen-level-dependent functional magnetic resonance imaging signal. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2002, 357, 1003–1037. [Google Scholar] [CrossRef] [PubMed]
- Goense, J.B.; Logothetis, N.K. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr. Biol. 2008, 18, 631–640. [Google Scholar] [CrossRef] [PubMed]
- Werth, R. Visual functions without the occipital lobe or after cerebral hemispherectomy in infancy. Eur. J. Neurosci. 2006, 24, 2932–2944. [Google Scholar] [CrossRef] [PubMed]
- Werth, R. Cerebral blindness and plasticity oft he visual system in children. A review of visual capacities in patients with occipital lesions, hemispherectomy or hydranencephaly. Rest. Neurol. Neurosci. 2008, 26, 377–389. [Google Scholar]
- Seghier, M.L.; Neufeld, N.H.; Zeidman, P.; Leff, A.P.; Mechelli, A.; Nagendran, A.; Riddoch, J.M.; Humphreys, G.W.; Price, C.J. Reading without the left ventral occipito-temporal cortex. Neuropsychologia 2012, 50, 3621–3635. [Google Scholar] [CrossRef]
- Krafnick, A.J.; Napoliello, E.M.; Flowers, D.L.; Eden, G.F. The role of brain activity in characterizing successful reading intervention in children with dyslexia. Front. Neurosci. 2022, 16, 898661. [Google Scholar] [CrossRef]
- Warrington, E.K.; Shallice, T. Word-form dyslexia. Brain 1980, 103, 99–112. [Google Scholar] [CrossRef]
- Salmelin, R.; Service, E.; Kiesilä, P.; Uutela, K.; Salonen, O. Impaired visual word processing in dyslexia revealed with magnetoencephalography. Ann. Neurol. 1996, 40, 157–162. [Google Scholar] [CrossRef]
- Rumsey, J.M.; Horwitz, B.; Donohue, B.C.; Nace, K.; Maisog, J.M.; Andreason, P. Phonological and orthographic components of word recognition. A PET-rCBF study. Brain 1997, 120, 739–759. [Google Scholar] [CrossRef]
- Tarkiainen, A.; Helenius, P.; Hansen, P.C.; Cornelissen, P.L.; Salmelin, R. Dynamics of letter string perception in the human occipitotemporal cortex. Brain 1999, 122, 2119–2131. [Google Scholar] [CrossRef] [PubMed]
- Paulesu, E.; Démonet, J.F.; Fazio, F.; McCrory, E.; Chanoine, V.; Brunswick, N.; Cappa, S.F.; Cossu, G.; Habib, M.; Frith, C.D.; et al. Dyslexia: Cultural diversity and biological unity. Science 2001, 291, 2165–2167. [Google Scholar] [CrossRef] [PubMed]
- Shaywitz, B.A.; Shaywitz, S.E.; Pugh, K.R.; Mencl, W.E.; Fulbright, R.K.; Skudlarski, P.; Constable, R.T.; Marchione, K.E.; Fletcher, J.M.; Lyon, G.R.; et al. Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry 2002, 52, 101–110. [Google Scholar] [CrossRef]
- Polk, T.A.; Farah, M.J. Functional MRI evidence for an abstract, not perceptual, word-form area. J. Exp. Psychol. Gen. 2002, 131, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Cohen, L.; Dehaene, S. Specialization within the ventral stream: The case for the visual word form area. Neuroimage 2004, 22, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Mechelli, A.; Crinion, J.T.; Long, S.; Friston, K.J.; Ralph, M.A.L.; Patterson, K.; McClelland, J.L.; Price, C.J. Dissociating reading processes on the basis of neuronal interactions. J. Cogn. Neurosci. 2005, 17, 1753–1765. [Google Scholar] [CrossRef] [PubMed]
- Cao, F.; Bitan, T.; Chou, T.; Burman, D.D.; Booth, J.R. Deficient orthographic and phonological representations in children with dyslexia revealed by brain activation patterns. J. Child Psychol. Psychiatry 2006, 47, 1041–1050. [Google Scholar] [CrossRef]
- Maurer, U.; Brem, S.; Bucher, K.; Kranz, F.; Benz, R.; Steinhausen, H.C.; Brandeis, D. Impaired tuning of a fast occipito-temporal response for print in dyslexic children learning to read. Brain 2007, 130, 3200–3210. [Google Scholar] [CrossRef]
- Ben-Shachar, M.; Dougherty, R.F.; Deutsch, G.K.; Wandell, B.A. Differential sensitivity to words and shapes in ventral occipito-temporal cortex. Cereb. Cortex 2007, 17, 1604–1611. [Google Scholar] [CrossRef] [PubMed]
- Maisog, J.M.; Einbinder, E.R.; Flowers, D.L.; Turkeltaub, P.E.; Eden, G.F. A meta-analysis of functional neuroimaging studies of dyslexia. Ann. N. Y. Acad. Sci. 2008, 1145, 237–259. [Google Scholar] [CrossRef]
- Glezer, L.S.; Jiang, X.; Riesenhuber, M. Evidence for highly selective neuronal tuning to whole words in the "visual word form area". Neuron 2009, 62, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Paulesu, E.; Danelli, L.; Berlingeri, M. Reading the dyslexic brain: Multiple dysfunctional routes revealed by a new meta-analysis of PET and fMRI activation studies. Front. Hum. Neurosci. 2014, 8, 830. [Google Scholar] [CrossRef] [PubMed]
- Olulade, O.A.; Flowers, D.L.; Napoliello, E.M.; Eden, G.F. Dyslexic children lack word selectivity gradients in occipito-temporal and inferior frontal cortex. Neuroimage Clin. 2015, 7, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Boros, M.; Anton, J.-L.; Pech, C.; Grainger, J.; Szwed, M.; Ziegler, J.C. Orthographic processing deficits in developmental dyslexia: Beyond the ventral visual stream. Neuroimage 2016, 128, 316–327. [Google Scholar] [CrossRef]
- Saygin, Z.M.; Osher, D.E.; Norton, E.S.; Youssoufian, D.A.; Beach, S.D.; Feather, J.; Gaab, N.; Gabrieli, J.D.; Kanwisher, N. Connectivity precedes function in the development of the visual word form area. Nat. Neurosci 2016, 19, 1250–1255. [Google Scholar] [CrossRef]
- Kronbichler, L.; Kronbichler, M. The importance of the left occipitotemporal cortex in developmental dyslexia. Curr. Dev. Disord. Rep. 2018, 5, 1–8. [Google Scholar] [CrossRef][Green Version]
- Rodrigues, A.P.; Rebola, J.; Pereira, M.; van Asselen, M.; Castelo-Branco, M. Neural Responses of the AnteriorVentral Occipitotemporal Cortex in Developmental Dyslexia: Beyond the Visual Word Form Area. IOVS Vis. Neurosci. 2019, 60, 1063–1068. [Google Scholar]
- Dickens, J.V.; Fama, M.E.; DeMarco, A.T.; Lacey, E.H.; Friedman, R.B.; Turkeltaub, P.E. Localization of Phonological and Semantic Contributions to Reading. J. Neurosci. 2019, 39, 5361–5368. [Google Scholar] [CrossRef]
- Brem, S.; Maurer, U.; Kronbichler, M.; Schurz, M.; Richlan, F.; Blau, V.; Reithler, J.; van der Mark, S.; Schulz, E.; Bucher, K.; et al. Visual word form processing deficits driven by severity of reading impairments in children with developmental dyslexia. Sci. Rep. 2020, 10, 18728. [Google Scholar] [CrossRef]
- Liu, T.; Thiebaut de Schotten, M.; Altarelli, I.; Ramus, F.; Zhao, J. Neural dissociation of visual attention span and phonological deficits in developmental dyslexia: A hub-based white matter network analysis. Hum. Brain. Mapp. 2022, 43, 5210–5219. [Google Scholar] [CrossRef]
- Taran, N.; Farah, R.; DiFrancesco, M.; Altaye, M.; Vannest, J.; Holland, S.; Rosch, K.; Schlaggar, B.L.; Horowitz-Kraus, T. The role of visual attention in dyslexia: Behavioral and neurobiological evidence. Hum. Brain Mapp. 2022, 43, 1720–1737. [Google Scholar] [CrossRef] [PubMed]
- Gaillard, R.; Naccache, L.; Pinel, P.; Clémenceau, S.; Volle, E.; Hasboun, D.; Dupont, S.; Baulac, M.; Dehaene, S.; Adam, C.; et al. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron 2006, 50, 191–204. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).