Cerebrospinal Fluid and Blood Biomarkers in Patients with Post-Traumatic Disorders of Consciousness: A Scoping Review
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Eligibility Criteria and Data Extraction
3. Results
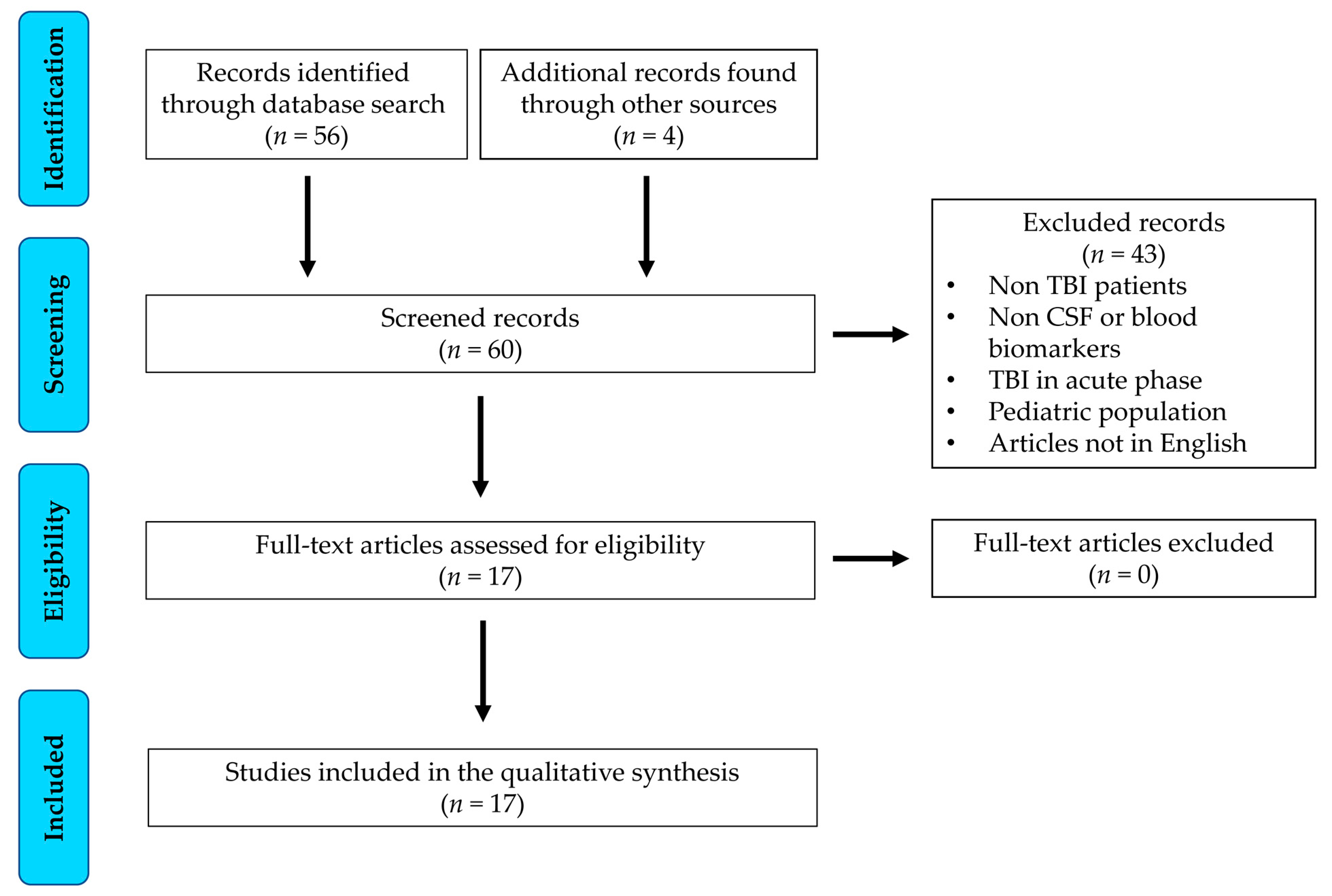
3.1. Study Selection and Characteristics
3.2. Neurofilament Light Chain
3.3. Proteins, Metabolites, and Lipids
3.4. Amyloid-β and Tau Proteins
3.5. Melatonin and Thyroid Hormones
3.6. Microtubule-Associated Protein 2
3.7. Neuron-Specific Enolase
3.8. Brain-Derived Neurotrophic Factor
3.9. Soluble Neural Cell Adhesion Molecule
3.10. MicroRNAs
4. Discussion
4.1. Monitoring of Ongoing Secondary Brain Injury and Recovery Mechanisms
4.2. Support of Diagnoses among DoC
4.3. Refinement of Prognostic Judgments
4.4. Study Limitations and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [PubMed]
- Royal College of Physicians. Prolonged Disorders of Consciousness: National Clinical Guidelines; RCP: London, UK, 2013. [Google Scholar]
- Laureys, S.; Celesia, G.G.; Cohadon, F.; Lavrijsen, J.; León-Carrión, J.; Sannita, W.G.; Sazbon, L.; Schmutzhard, E.; von Wild, K.R.; Zeman, A.; et al. Unresponsive wakefulness syndrome: A new name for the vegetative state or apallic syndrome. BMC Med. 2010, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Ashwal, S.; Childs, N.; Cranford, R.; Jennett, B.; Katz, D.I.; Kelly, J.P.; Rosenberg, J.H.; Whyte, J.; Zafonte, R.D.; et al. The minimally conscious state: Definition and diagnostic criteria. Neurology 2002, 58, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Goyal, A.; Failla, M.D.; Niyonkuru, C.; Amin, K.; Fabio, A.; Berger, R.P.; Wagner, A.K. S100b as a prognostic biomarker in outcome prediction for patients with severe traumatic brain injury. J. Neurotrauma 2013, 30, 946–957. [Google Scholar] [CrossRef]
- Dzierzęcki, S.; Ząbek, M.; Zaczyński, A.; Tomasiuk, R. Prognostic properties of the association between the S-100B protein levels and the mean cerebral blood flow velocity in patients diagnosed with severe traumatic brain injury. Biomed. Rep. 2022, 17, 58. [Google Scholar] [CrossRef]
- Mondello, S.; Linnet, A.; Buki, A.; Robicsek, S.; Gabrielli, A.; Tepas, J.; Papa, L.; Brophy, G.M.; Tortella, F.; Hayes, R.L.; et al. Clinical utility of serum levels of ubiquitin C-terminal hydrolase as a biomarker for severe traumatic brain injury. Neurosurgery 2012, 70, 666–675. [Google Scholar]
- Pei, Y.; Tang, X.; Zhang, E.; Lu, K.; Xia, B.; Zhang, J.; Huang, Y.; Zhang, H.; Dong, L. The diagnostic and prognostic value of glial fibrillary acidic protein in traumatic brain injury: A systematic review and meta-analysis. Eur. J. Trauma Emerg. Surg. 2022. [Google Scholar] [CrossRef]
- Shemilt, M.; Boutin, A.; Lauzier, F.; Zarychanski, R.; Moore, L.; McIntyre, L.A.; Nadeau, L.; Fergusson, D.A.; Mercier, E.; Archambault, P.; et al. Prognostic Value of Glial Fibrillary Acidic Protein in Patients With Moderate and Severe Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Crit. Care Med. 2019, 47, e522–e529. [Google Scholar] [CrossRef]
- Vos, P.E.; Lamers, K.J.; Hendriks, J.C.; van Haaren, M.; Beems, T.; Zimmerman, C.; van Geel, W.; de Reus, H.; Biert, J.; Verbeek, M.M. Glial and neuronal proteins in serum predict outcome after severe traumatic brain injury. Neurology 2004, 62, 1303–1310. [Google Scholar] [CrossRef]
- Böhmer, A.E.; Oses, J.P.; Schmidt, A.P.; Perón, C.S.; Krebs, C.L.; Oppitz, P.P.; D’Avila, T.T.; Souza, D.O.; Portela, L.V.; Stefani, M.A. Neuron-specific enolase, S100B, and glial fibrillary acidic protein levels as outcome predictors in patients with severe traumatic brain injury. Neurosurgery 2011, 68, 1624–1630, discussion 1630–1631. [Google Scholar] [CrossRef]
- Liliang, P.C.; Liang, C.L.; Weng, H.C.; Lu, K.; Wang, K.W.; Chen, H.J.; Chuang, J.H. Tau proteins in serum predict outcome after severe traumatic brain injury. J. Surg. Res. 2010, 160, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Singh, K.; Sharma, V.; Pandey, D.; Jha, R.P.; Rai, S.K.; Chauhan, R.S.; Singh, R. A prospective pilot study on serum cleaved tau protein as a neurological marker in severe traumatic brain injury. Br. J. Neurosurg. 2017, 31, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Castaño-Leon, A.M.; Sánchez Carabias, C.; Hilario, A.; Ramos, A.; Navarro-Main, B.; Paredes, I.; Munarriz, P.M.; Panero, I.; Eiriz Fernández, C.; García-Pérez, D.; et al. Serum assessment of traumatic axonal injury: The correlation of GFAP, t-Tau, UCH-L1, and NfL levels with diffusion tensor imaging metrics and its prognosis utility. J. Neurosurg. 2022, 138, 454–464. [Google Scholar] [CrossRef] [PubMed]
- Giacino, J.T.; Katz, D.I.; Schiff, N.D.; Whyte, J.; Ashman, E.J.; Ashwal, S.; Barbano, R.; Hammond, F.M.; Laureys, S.; Ling, G.S.F.; et al. Practice guideline update recommendations summary: Disorders of consciousness: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology; the American Congress of Rehabilitation Medicine; and the National Institute on Disability, Independent Living, and Rehabilitation Research. Neurology 2018, 91, 450–460. [Google Scholar]
- Bagnato, S.; Boccagni, C. Moderate/severe traumatic brain injury as a trigger of chronic neurodegeneration in humans. Neural. Regen. Res. 2020, 15, 1247–1248. [Google Scholar] [CrossRef]
- Brett, B.L.; Gardner, R.C.; Godbout, J.; Dams-O’Connor, K.; Keene, C.D. Traumatic Brain Injury and Risk of Neurodegenerative Disorder. Biol. Psychiatry 2022, 91, 498–507. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Bagnato, S.; D’Ippolito, M.E.; Boccagni, C.; De Tanti, A.; Lucca, L.F.; Pingue, V.; Colombo, V.; Rubino, F.; Andriolo, M. Six-month outcomes in patients with hemorrhagic and non-hemorrhagic traumatic disorders of consciousness. Neurol. Sci. 2022, 43, 6511–6516. [Google Scholar] [CrossRef]
- Bagnato, S.; D’Ippolito, M.E.; Boccagni, C.; De Tanti, A.; Lucca, L.F.; Nardone, A.; Salucci, P.; Fiorilla, T.; Pingue, V.; Gennaro, S.; et al. Sustained Axonal Degeneration in Prolonged Disorders of Consciousness. Brain Sci. 2021, 11, 1068. [Google Scholar] [CrossRef]
- Bagnato, S.; Grimaldi, L.M.E.; Di Raimondo, G.; Sant’Angelo, A.; Boccagni, C.; Virgilio, V.; Andriolo, M. Prolonged Cerebrospinal Fluid Neurofilament Light Chain Increase in Patients with Post-Traumatic Disorders of Consciousness. J. Neurotrauma 2017, 34, 2475–2479. [Google Scholar] [CrossRef]
- Coppola, L.; Mirabelli, P.; Baldi, D.; Smaldone, G.; Estraneo, A.; Soddu, A.; Grimaldi, A.M.; Mele, G.; Salvatore, M.; Cavaliere, C. An innovative approach for the evaluation of prolonged disorders of consciousness using NF-L and GFAP biomarkers: A pivotal study. Sci. Rep. 2022, 12, 18446. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; He, F.; Yu, L.; Gao, J.; Meng, F.; Ding, Y.; Zou, H.; Luo, B. Complement cascade on severe traumatic brain injury patients at the chronic unconscious stage: Implication for pathogenesis. Expert Rev. Mol. Diagn. 2018, 18, 761–766. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, T.; Xiong, Y.; Xia, X.; Dang, Y.; Chen, X.; Geng, X.; He, J.; Yang, Y.; Zhao, J. Elevated cerebrospinal fluid protein levels associated with poor short-term outcomes after spinal cord stimulation in patients with disorders of consciousness. Front. Aging Neurosci. 2022, 14, 1032740. [Google Scholar] [CrossRef] [PubMed]
- Romaniello, C.; Bertoletti, E.; Matera, N.; Farinelli, M.; Pedone, V. Morfeo Study II: Clinical Course and Complications in Patients with Long-Term Disorders of Consciousness. Am. J. Med. Sci. 2016, 351, 563–569. [Google Scholar] [CrossRef]
- Yu, J.; Meng, F.; He, F.; Chen, F.; Bao, W.; Yu, Y.; Zhou, J.; Gao, J.; Li, J.; Yao, Y.; et al. Metabolic Abnormalities in Patients with Chronic Disorders of Consciousness. Aging Dis. 2021, 12, 386–403. [Google Scholar] [CrossRef]
- Bagnato, S.; Andriolo, M.; Boccagni, C.; Sant’Angelo, A.; D’Ippolito, M.E.; Galardi, G. Dissociation of cerebrospinal fluid amyloid-β and tau levels in patients with prolonged posttraumatic disorders of consciousness. Brain Inj. 2018, 32, 1056–1060. [Google Scholar] [CrossRef]
- Bagnato, S.; Andriolo, M.; Boccagni, C.; Galardi, G. Prolonged changes in amyloid-β metabolism after a severe traumatic brain injury. Neuroreport 2017, 28, 250–252. [Google Scholar] [CrossRef]
- Guaraldi, P.; Sancisi, E.; La Morgia, C.; Calandra-Buonaura, G.; Carelli, V.; Cameli, O.; Battistini, A.; Cortelli, P.; Piperno, R. Nocturnal melatonin regulation in post-traumatic vegetative state: A possible role for melatonin supplementation? Chronobiol. Int. 2014, 31, 741–745. [Google Scholar] [CrossRef]
- Mele, C.; De Tanti, A.; Bagnato, S.; Lucca, L.F.; Saviola, D.; Estraneo, A.; Moretta, P.; Marcuccio, L.; Lanzillo, B.; Aimaretti, G.; et al. Thyrotropic Axis and Disorders of Consciousness in Acquired Brain Injury: A Potential Intriguing Association? Front. Endocrinol. 2022, 13, 887701. [Google Scholar] [CrossRef]
- Mondello, S.; Gabrielli, A.; Catani, S.; D’Ippolito, M.; Jeromin, A.; Ciaramella, A.; Bossù, P.; Schmid, K.; Tortella, F.; Wang, K.K.; et al. Increased levels of serum MAP-2 at 6-months correlate with improved outcome in survivors of severe traumatic brain injury. Brain Inj. 2012, 26, 1629–1635. [Google Scholar] [CrossRef]
- Bagnato, S.; Andriolo, M.; Boccagni, C.; Lucca, L.F.; De Tanti, A.; Pistarini, C.; Barone, T.; Galardi., G. Reduced Neuron-Specific Enolase Levels in Chronic Severe Traumatic Brain Injury. J. Neurotrauma 2020, 37, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Bagnato, S.; Galardi, G.; Ribaudo, F.; Boccagni, C.; Fiorilla, T.V.; Rubino, F.; D’Ippolito, M.E.; Andriolo, M. Serum BDNF Levels Are Reduced in Patients with Disorders of Consciousness and Are Not Modified by Verticalization with Robot-Assisted Lower-Limb Training. Neural Plast. 2020, 2020, 5608145. [Google Scholar] [CrossRef] [PubMed]
- Ziliotto, N.; Marchetti, G.; Straudi, S.; Tisato, V.; Lavezzi, S.; Manfredini, F.; Basaglia, N.; Bernardi, F. Soluble neural cell adhesion molecule and behavioural recovery in minimally conscious patients undergoing transcranial direct current stimulation. Clin. Chim. Acta. 2019, 495, 374–376. [Google Scholar] [CrossRef]
- Zilliox, M.J.; Foecking, E.M.; Kuffel, G.R.; Conneely, M.; Saban, K.L.; Herrold, A.A.; Kletzel, S.L.; Radke, J.R.; Walsh, E.; Guernon, A.; et al. An Initial miRNA Profile of Persons with Persisting Neurobehavioral Impairments and States of Disordered Consciousness After Severe Traumatic Brain Injury. J. Head Trauma Rehabil. 2022. [Google Scholar] [CrossRef] [PubMed]
- Johnson, V.E.; Stewart, J.E.; Begbie, F.D.; Trojanowski, J.Q.; Smith, D.H.; Stewart, W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain 2013, 136, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H.; Jolly, A.; de Simoni, S.; Bourke, N.; Patel, M.C.; Scott, G.; Sharp, D.J. Spatial patterns of progressive brain volume loss after moderate-severe traumatic brain injury. Brain 2018, 141, 822–836. [Google Scholar] [CrossRef]
- Bagnato, S. The role of plasticity in the recovery of consciousness. Handb. Clin. Neurol. 2022, 184, 375–395. [Google Scholar]
- Khalil, M.; Teunissen, C.E.; Otto, M.; Piehl, F.; Sormani, M.P.; Gattringer, T.; Barro, C.; Kappos, L.; Comabella, M.; Fazekas, F.; et al. Neurofilaments as biomarkers in neurological disorders. Nat. Rev. Neurol. 2018, 14, 577–589. [Google Scholar] [CrossRef]
- Goodson, H.V.; Jonasson, E.M. Microtubules and Microtubule-Associated Proteins. Cold Spring Harb. Perspect. Biol. 2018, 10, a022608. [Google Scholar] [CrossRef]
- Isgrò, M.A.; Bottoni, P.; Scatena, R. Neuron-Specific Enolase as a Biomarker: Biochemical and Clinical Aspects. Adv. Exp. Med. Biol. 2015, 867, 125–143. [Google Scholar]
- Mielke, M.M.; Ransom, J.E.; Mandrekar, J.; Turcano, P.; Savica, R.; Brown, A.W. Traumatic Brain Injury and Risk of Alzheimer’s Disease and Related Dementias in the Population. J. Alzheimers Dis. 2022, 88, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P. Neurotrophins Time Point Intervention after Traumatic Brain Injury: From Zebrafish to Human. Int. J. Mol. Sci. 2021, 22, 1585. [Google Scholar] [CrossRef] [PubMed]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Kondziella, D.; Bender, A.; Diserens, K.; van Erp, W.; Estraneo, A.; Formisano, R.; Laureys, S.; Naccache, L.; Ozturk, S.; Rohaut, B.; et al. European Academy of Neurology guideline on the diagnosis of coma and other disorders of consciousness. Eur. J. Neurol. 2020, 27, 741–756. [Google Scholar] [CrossRef] [PubMed]
| Reference | Biomarker | Fluid | Population (n) | Etiology (n) | Time Post-Injury at Study Entry | Main Findings |
|---|---|---|---|---|---|---|
| Bagnato et al., 2022 [19] | NFL | Serum | UWS (25) MCS (27) HC (52) | Traumatic (52) | 28–90 days | NFL level higher in patients vs. controls at baseline and 6 months post-injury, no difference according to intracranial hematoma |
| Bagnato et al., 2021 [20] | NFL | Serum | UWS (45) MCS (25) HC (70) | Traumatic (48) Hypoxic (22) | 28–90 days | NFL levels higher in patients vs. controls at baseline and 6 months post-injury, patients with UWS vs. those in MCS at baseline, patients with hypoxic brain injury vs. those with TBI 6 months post-injury |
| Bagnato et al., 2017 [21] | NFL | CSF | UWS (3) MCS (7) AD (9) | Traumatic (10) | 95–581 days | NFL level higher in patients vs. normal limit and patients with AD |
| Coppola et al., 2022 [22] | NFL and GFAP | Serum | UWS (7) MCS (9) HC (6) | Traumatic (3) Hypoxic (6) Vascular (7) | 1–14 months | NFL and GFAP levels higher in patients vs. controls |
| Bao et al., 2018 [23] | 300 different proteins | Plasma | UWS (13) MCS (5) HC (6) | Traumatic (18) | 30–254 days | 32 proteins, especially those involved in complement cascade, differentially expressed in patients vs. controls |
| He et al., 2022 [24] | Proteins | CSF | UWS (24) MCS (42) | Traumatic (27) Hypoxic (11) Vascular (28) | 3–>12 months | High protein levels associated with poor outcomes after spinal cord stimulation |
| Romaniello et al., 2016 [25] | Albumin, hemoglobin, white blood cells count | Serum, whole blood | UWS (N/A) MCS (N/A) | Traumatic (25) Hypoxic (42) Vascular (40) Other (5) | 11 months (average) | Albumin and hemoglobin levels, white blood cell count correlate with mortality |
| Yu et al., 2021 [26] | Metabolomic and lipidomic profiles | Plasma | Metabolomic profile
| Traumatic (92; some patients studied both in UWS and MCS) | N/A | Purine metabolism pathway suppressed in patients with UWS and MCS; some lipids distinguish these patients |
| Bagnato et al., 2018 [27] | Amyloid-β, total tau, phosphorylated tau | CSF | UWS (3) MCS (12) | Traumatic (15) | 92–578 days | Amyloid-β level reduced |
| Bagnato et al., 2017 [28] | Amyloid-β | CSF | UWS (1) MCS (7) | Traumatic (8) | 95–578 days | Amyloid-β level reduced |
| Guaraldi et al., 2014 [29] | Melatonin | Plasma | UWS (6) HC (9) | TBI (6) | 6–18 months | Melatonin synthesis reduced at night, not suppressed by light |
| Mele et al., 2022 [30] | TSH, fT3, fT4 | Serum | UWS (94) MCS (57) | Traumatic (45) Hypoxic (33) Vascular (73) | 28–90 days | Lower baseline TSH level, greater TSH increment after rehabilitation predict good outcomes |
| Mondello et al., 2012 [31] | MAP-2 | Serum | UWS (5) MCS (4) EMCS (7) HC (16) | Traumatic (16) | 6 months | MAP-2 level lower in patients with UWS vs. those with higher levels of consciousness |
| Bagnato et al., 2020 [32] | NSE | Serum | UWS (14) MCS (21) EMCS (16) HC (30) | Traumatic (51) | 23 months (average) | NSE level lower at longer intervals after TBI |
| Bagnato et al., 2020 [33] | BDNF | Serum | UWS (10) MCS (8) HC 16) | Traumatic (8) Hypoxic (6) Vascular (4) | 1–7 months | BDNF level reduced in patients, not modified by verticalization with robot-assisted lower-limb training |
| Ziliotto et al., 2019 [34] | sNCAM | Plasma | MCS (8) HC (39) | Traumatic (8) | 1–19 years | Low sNCAM level associated with better outcomes after transcranial direct current stimulation |
| Zilliox et al., 2022 [35] | miRNAs | Whole blood | UWS (2) MCS (4) | Traumatic (6) | 399–730 days | 41 miRNAs differentially expressed in patients vs. controls |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bagnato, S.; Boccagni, C. Cerebrospinal Fluid and Blood Biomarkers in Patients with Post-Traumatic Disorders of Consciousness: A Scoping Review. Brain Sci. 2023, 13, 364. https://doi.org/10.3390/brainsci13020364
Bagnato S, Boccagni C. Cerebrospinal Fluid and Blood Biomarkers in Patients with Post-Traumatic Disorders of Consciousness: A Scoping Review. Brain Sciences. 2023; 13(2):364. https://doi.org/10.3390/brainsci13020364
Chicago/Turabian StyleBagnato, Sergio, and Cristina Boccagni. 2023. "Cerebrospinal Fluid and Blood Biomarkers in Patients with Post-Traumatic Disorders of Consciousness: A Scoping Review" Brain Sciences 13, no. 2: 364. https://doi.org/10.3390/brainsci13020364
APA StyleBagnato, S., & Boccagni, C. (2023). Cerebrospinal Fluid and Blood Biomarkers in Patients with Post-Traumatic Disorders of Consciousness: A Scoping Review. Brain Sciences, 13(2), 364. https://doi.org/10.3390/brainsci13020364






