Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease?
Abstract
1. Introduction
2. Oxidative Stress and Neuroinflammation at the Core of PD Pathogenesis
3. Role of Vitamins in the Pathogenesis of PD
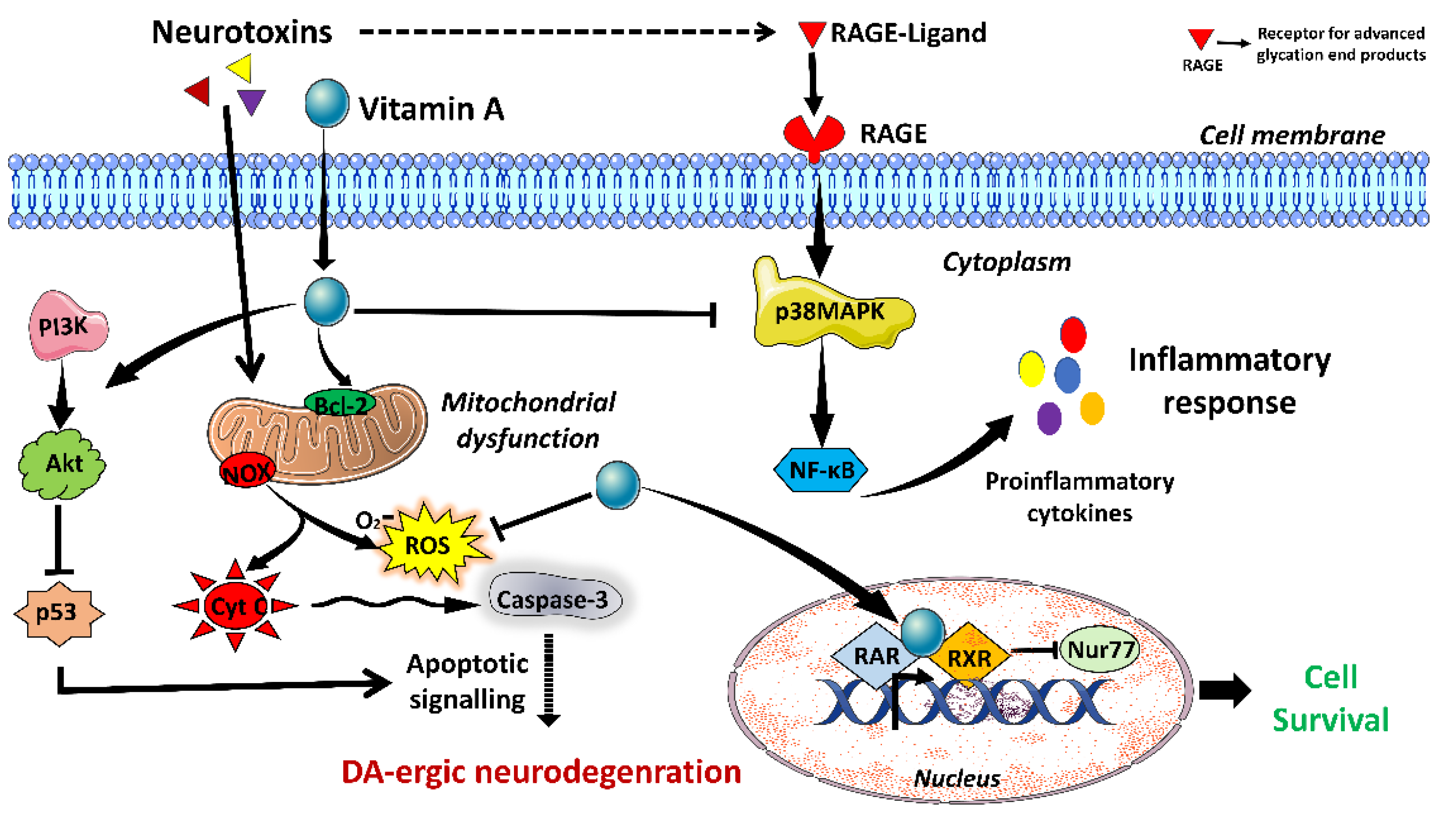
3.1. Vitamin A
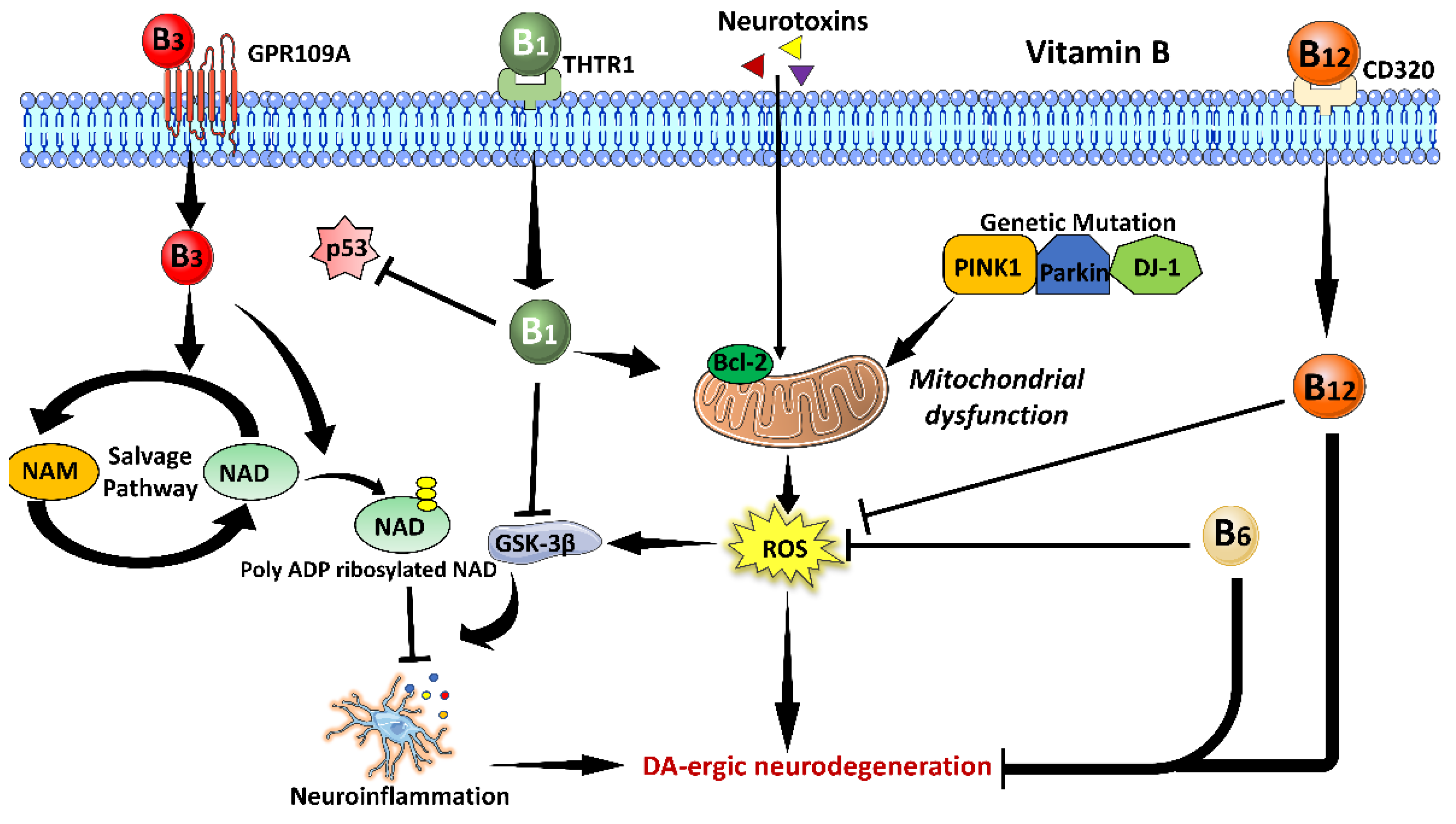
3.2. Vitamin B Family
3.2.1. Vitamin B1
3.2.2. Vitamin B3
3.2.3. Vitamin B6
3.2.4. Vitamin B12
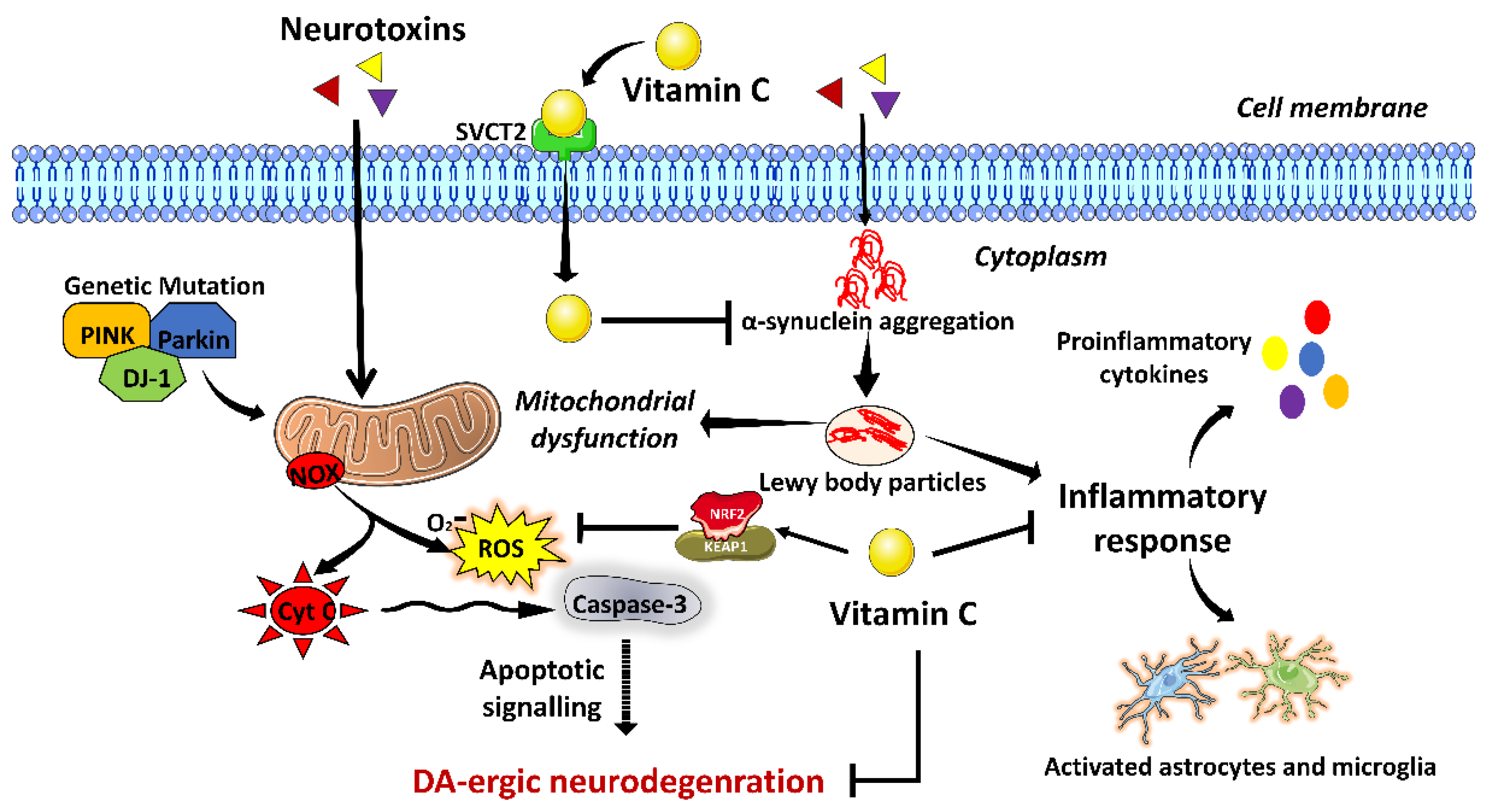
3.3. Vitamin C
3.4. Vitamin D
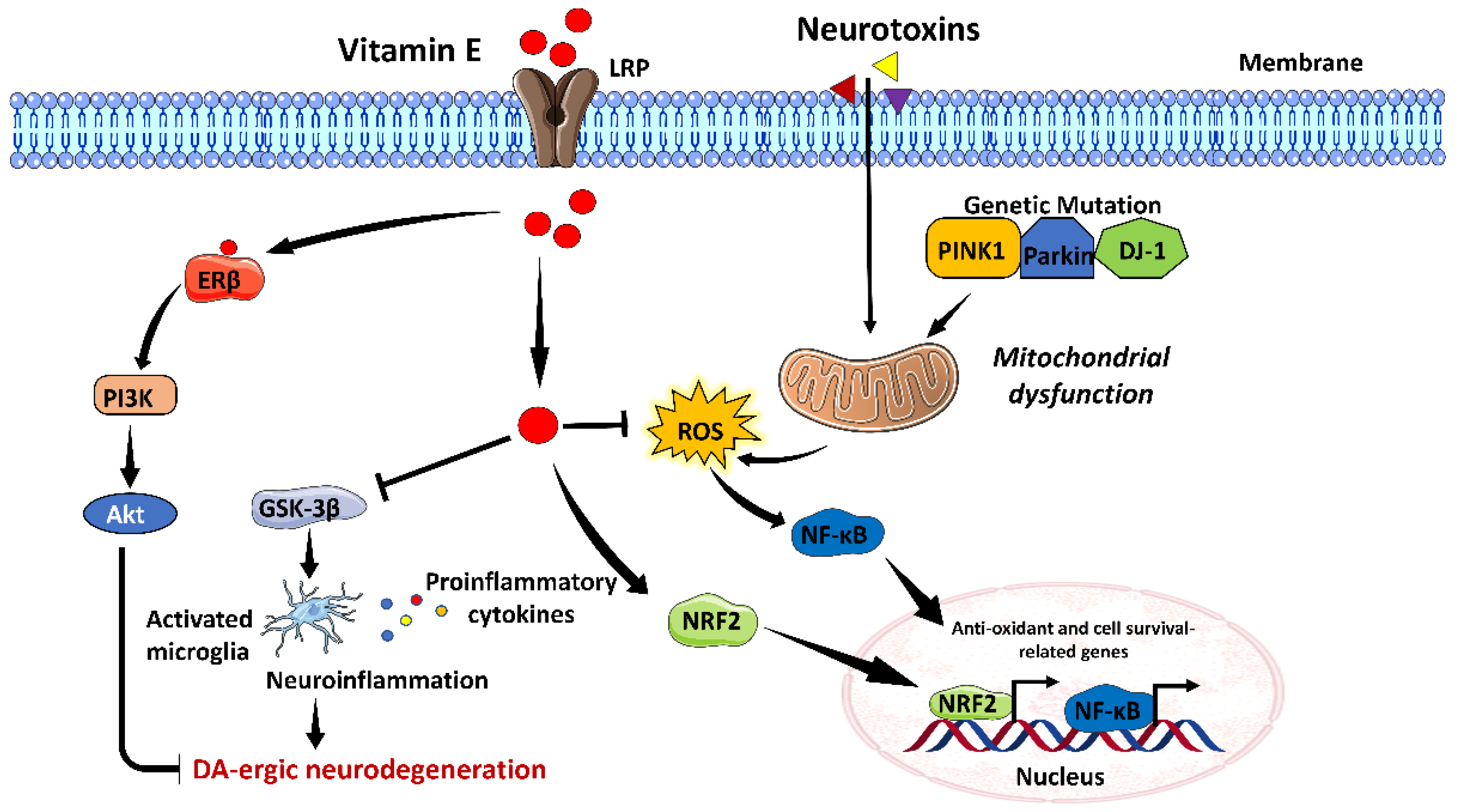
3.5. Vitamin E
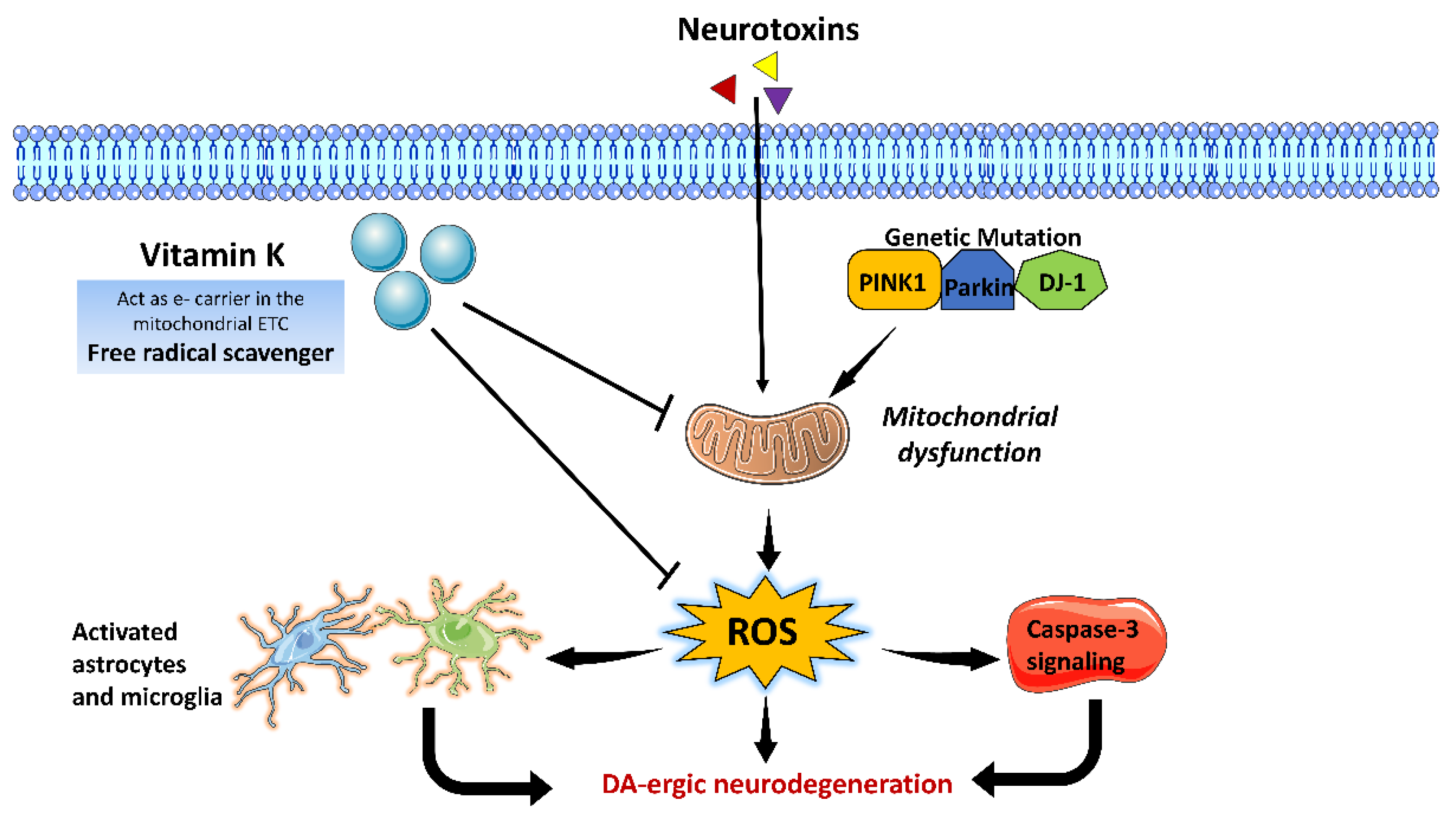
3.6. Vitamin K
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunzler, A.; Kolling, E.A.; da Silva, J.D., Jr.; Gasparotto, J.; de Bittencourt Pasquali, M.A.; Moreira, J.C.F.; Gelain, D.P. Retinol (vitamin A) increases α-synuclein, β-amyloid peptide, tau phosphorylation and RAGE content in human SH-SY5Y neuronal cell line. Neurochem. Res. 2017, 42, 2788–2797. [Google Scholar] [CrossRef]
- Man Anh, H.; Linh, D.M.; My Dung, V.; Thi Phuong Thao, D. Evaluating dose-and time-dependent effects of vitamin c treatment on a parkinson’s disease fly model. Park. Dis. 2019, 2019, 9720546. [Google Scholar] [CrossRef]
- Kunzler, A.; Ribeiro, C.T.; Gasparotto, J.; Petiz, L.L.; da Rosa Silva, H.T.; da Silva, J.D., Jr.; Bortolin, R.; de Souza, P.O.; Barreto, F.; Espitia-Perez, P. The effects of retinol oral supplementation in 6-hydroxydopamine dopaminergic denervation model in Wistar rats. Neurochem. Int. 2019, 125, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Giri, B.; Belanger, K.; Seamon, M.; Bradley, E.; Purohit, S.; Chong, R.; Morgan, J.C.; Baban, B.; Wakade, C. Niacin ameliorates neuro-inflammation in Parkinson’s disease via GPR109A. Int. J. Mol. Sci. 2019, 20, 4559. [Google Scholar] [CrossRef]
- Alster, P.; Madetko, N.; Friedman, A. Neutrophil-to-lymphocyte ratio (NLR) at boundaries of Progressive Supranuclear Palsy Syndrome (PSPS) and Corticobasal Syndrome (CBS). Neurol. I Neurochir. Pol. 2021, 55, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Madetko, N.; Migda, B.; Alster, P.; Turski, P.; Koziorowski, D.; Friedman, A. Platelet-to-lymphocyte ratio and neutrophil-tolymphocyte ratio may reflect differences in PD and MSA-P neuroinflammation patterns. Neurol. I Neurochir. Pol. 2022, 56, 148–155. [Google Scholar] [CrossRef]
- Ganguly, J.; Bernaola, M.T.; Jog, M. Role of Vitamins in Advanced therapy for Parkinson’s disease: Decoding the paradox. Can. J. Neurol. Sci. 2022, 49, 3–4. [Google Scholar] [CrossRef]
- Barmaki, H.; Morovati, A.; Eydivandi, Z.; Naleshkenani, F.J.; Saedi, S.; Musavi, H.; Abbasi, M.; Hemmati-Dinarvand, M. The association between serum oxidative stress indexes and pathogenesis of Parkinson’s disease in the northwest of Iran. Iran. J. Public Health 2021, 50, 606. [Google Scholar] [CrossRef] [PubMed]
- Karahalil, B.; Miser Salihoğlu, E.; Elkama, A.; Orhan, G.; Saygın, E.; Yardim Akaydin, S. Individual susceptibility has a major impact on strong association between oxidative stress, defence systems and Parkinson’s disease. Basic Clin. Pharmacol. Toxicol. 2022, 130, 158–170. [Google Scholar] [CrossRef]
- Chia, S.J.; Tan, E.-K.; Chao, Y.-X. Historical perspective: Models of Parkinson’s disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef]
- Chang, K.-H.; Chen, C.-M. The role of oxidative stress in Parkinson’s disease. Antioxidants 2020, 9, 597. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef]
- Jenner, P.; Dexter, D.; Sian, J.; Schapira, A.; Marsden, C. Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1992, 32, S82–S87. [Google Scholar] [CrossRef]
- Ciulla, M.; Marinelli, L.; Cacciatore, I.; Stefano, A.D. Role of dietary supplements in the management of Parkinson’s disease. Biomolecules 2019, 9, 271. [Google Scholar] [CrossRef] [PubMed]
- Kalampokini, S.; Becker, A.; Fassbender, K.; Lyros, E.; Unger, M.M. Nonpharmacological modulation of chronic inflammation in Parkinson’s disease: Role of diet interventions. Park. Dis. 2019, 2019, 7535472. [Google Scholar] [CrossRef] [PubMed]
- Yemula, N.; Dietrich, C.; Dostal, V.; Hornberger, M. Parkinson’s disease and the gut: Symptoms, nutrition, and microbiota. J. Park. Dis. 2021, 11, 1491–1505. [Google Scholar] [CrossRef]
- Al-Amin, M.; Bradford, D.; Sullivan, R.K.; Kurniawan, N.D.; Moon, Y.; Han, S.H.; Zalesky, A.; Burne, T.H. Vitamin D deficiency is associated with reduced hippocampal volume and disrupted structural connectivity in patients with mild cognitive impairment. Hum. Brain Mapp. 2019, 40, 394–406. [Google Scholar] [CrossRef]
- Allan Butterfield, D.; Castegna, A.; Drake, J.; Scapagnini, G.; Calabrese, V. Vitamin E and neurodegenerative disorders associated with oxidative stress. Nutr. Neurosci. 2002, 5, 229–239. [Google Scholar] [CrossRef]
- De Lau, L.; Koudstaal, P.; Witteman, J.; Hofman, A.; Breteler, M. Dietary folate, vitamin B12, and vitamin B6 and the risk of Parkinson disease. Neurology 2006, 67, 315–318. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin K, an emerging nutrient in brain function. Biofactors 2012, 38, 151–157. [Google Scholar] [CrossRef]
- Takeda, A.; Nyssen, O.P.; Syed, A.; Jansen, E.; Bueno-de-Mesquita, B.; Gallo, V. Vitamin A and carotenoids and the risk of Parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 2014, 42, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.-H.; Shen, H.; Diaz-Ruiz, O.; Bäckman, C.M.; Bae, E.; Yu, S.-J.; Wang, Y. Early post-treatment with 9-cis retinoic acid reduces neurodegeneration of dopaminergic neurons in a rat model of Parkinson’s disease. BMC Neurosci. 2012, 13, 120. [Google Scholar] [CrossRef] [PubMed]
- Gelain, D.P.; de Bittencourt Pasquali, M.A.; Caregnato, F.F.; Moreira, J.C.F. Vitamin A (retinol) up-regulates the receptor for advanced glycation endproducts (RAGE) through p38 and Akt oxidant-dependent activation. Toxicology 2011, 289, 38–44. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Molina, J.; Fernández-Calle, P.; Vázquez, A.; Pondal, M.; del Ser, T.; Gómez-Pastor, A.; Codoceo, R. Serum levels of vitamin A in Parkinson’s disease. J. Neurol. Sci. 1992, 111, 73–76. [Google Scholar] [CrossRef]
- Ying, A.F.; Khan, S.; Wu, Y.; Jin, A.; Wong, A.S.; Tan, E.K.; Yuan, J.M.; Koh, W.P.; Tan, L.C. Dietary antioxidants and risk of Parkinson’s disease in the Singapore Chinese Health Study. Mov. Disord. 2020, 35, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Ke, Z.; Luo, J. Thiamine deficiency and neurodegeneration: The interplay among oxidative stress, endoplasmic reticulum stress, and autophagy. Mol. Neurobiol. 2017, 54, 5440–5448. [Google Scholar] [CrossRef]
- Lương, K.v.; Nguyễn, L.T. The beneficial role of thiamine in Parkinson disease. CNS Neurosci. Ther. 2013, 19, 461–468. [Google Scholar] [CrossRef]
- Costantini, A.; Pala, M.I.; Compagnoni, L.; Colangeli, M. High-dose thiamine as initial treatment for Parkinson’s disease. Case Rep. 2013, 2013, bcr2013009289. [Google Scholar] [CrossRef]
- Håglin, L.; Johansson, I.; Forsgren, L.; Bäckman, L. Intake of vitamin B before onset of Parkinson’s disease and atypical parkinsonism and olfactory function at the time of diagnosis. Eur. J. Clin. Nutr. 2017, 71, 97–102. [Google Scholar] [CrossRef]
- Håglin, L.; Domellöf, M.; Bäckman, L.; Forsgren, L. Low plasma thiamine and phosphate in male patients with Parkinson’s disease is associated with mild cognitive impairment. Clin. Nutr. ESPEN 2020, 37, 93–99. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, M.; Li, C.; Jiang, X.; Su, Y.; Zhang, Y. Benefits of Vitamins in the Treatment of Parkinson’s Disease. Oxidative Med. Cell. Longev. 2019, 2019, 9426867. [Google Scholar] [CrossRef]
- Griffin, S.M.; Pickard, M.R.; Hawkins, C.P.; Williams, A.C.; Fricker, R.A. Nicotinamide restricts neural precursor proliferation to enhance catecholaminergic neuronal subtype differentiation from mouse embryonic stem cells. PLoS ONE 2020, 15, e0233477. [Google Scholar] [CrossRef] [PubMed]
- Jia, H.; Li, X.; Gao, H.; Feng, Z.; Li, X.; Zhao, L.; Jia, X.; Zhang, H.; Liu, J. High doses of nicotinamide prevent oxidative mitochondrial dysfunction in a cellular model and improve motor deficit in a Drosophila model of Parkinson’s disease. J. Neurosci. Res. 2008, 86, 2083–2090. [Google Scholar] [CrossRef]
- Lehmann, S.; Loh, S.H.; Martins, L.M. Enhancing NAD+ salvage metabolism is neuroprotective in a PINK1 model of Parkinson’s disease. Biol. Open 2017, 6, 141–147. [Google Scholar] [CrossRef]
- Wakade, C.; Chong, R. A novel treatment target for Parkinson’s disease. J. Neurol. Sci. 2014, 347, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Sadik, N.A.; Hamed, M.A.; Ali, S.A.; Khalil, W.K.; Ahmed, Y.R. Potential therapeutic effects of antagonizing adenosine A2A receptor, curcumin and niacin in rotenone-induced Parkinson’s disease mice model. Mol. Cell. Biochem. 2020, 465, 89–102. [Google Scholar] [CrossRef]
- Wakade, C.; Giri, B.; Malik, A.; Khodadadi, H.; Morgan, J.C.; Chong, R.K.; Baban, B. Niacin modulates macrophage polarization in Parkinson’s disease. J. Neuroimmunol. 2018, 320, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Rojo-Sebastián, A.; González-Robles, C.; de Yébenes, J.G. Vitamin B6 deficiency in patients with Parkinson disease treated with levodopa/carbidopa. Clin. Neuropharmacol. 2020, 43, 151–157. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Safo, M.K.; Contestabile, R. Biomedical aspects of pyridoxal 5’-phosphate availability. Front. Biosci. -Elite 2012, 4, 897–913. [Google Scholar]
- Elstner, M.; Morris, C.M.; Heim, K.; Lichtner, P.; Bender, A.; Mehta, D.; Schulte, C.; Sharma, M.; Hudson, G.; Goldwurm, S. Single-cell expression profiling of dopaminergic neurons combined with association analysis identifies pyridoxal kinase as Parkinson’s disease gene. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2009, 66, 792–798. [Google Scholar] [CrossRef]
- Modica, J.S.; Bonno, D.; Lizarraga, K.J. Pearls and Oy-sters: Vitamin B6 deficiency presenting with new-onset epilepsy and status epilepticus in a patient with Parkinson disease. Neurology 2020, 94, e2605–e2607. [Google Scholar] [CrossRef]
- Murakami, K.; Miyake, Y.; Sasaki, S.; Tanaka, K.; Fukushima, W.; Kiyohara, C.; Tsuboi, Y.; Yamada, T.; Oeda, T.; Miki, T. Dietary intake of folate, vitamin B6, vitamin B12 and riboflavin and risk of Parkinson’s disease: A case–control study in Japan. Br. J. Nutr. 2010, 104, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wang, Y.; Wei, W.; Zhao, W.; Lu, F.; Liu, F. Vitamin B12 inhibits α-synuclein fibrillogenesis and protects against amyloid-induced cytotoxicity. Food Funct. 2019, 10, 2861–2870. [Google Scholar] [CrossRef] [PubMed]
- Ozer, F.; Meral, H.; Hanoglu, L.; Aydemir, T.; Yilsen, M.; Cetin, S.; Ozturk, O.; Seval, H.; Koldas, M. Plasma homocysteine levels in patients treated with levodopa: Motor and cognitive associations. Neurol. Res. 2006, 28, 853–858. [Google Scholar] [CrossRef]
- McCarter, S.J.; Teigen, L.M.; McCarter, A.R.; Benarroch, E.E.; Louis, E.K.S.; Savica, R. Low vitamin B12 and Parkinson disease: Potential link to reduced cholinergic transmission and severity of disease. Mayo Clin. Proc. 2019, 94, 757–762. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.J.; Stang, C.; Turcano, P.; Mielke, M.M.; Ali, F.; Bower, J.H.; Savica, R. Higher vitamin B12 level at Parkinson’s disease diagnosis is associated with lower risk of future dementia. Park. Relat. Disord. 2020, 73, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Dietiker, C.; Kim, S.; Zhang, Y.; Christine, C.W.; Investigators, N.N.-P. Characterization of vitamin b12 supplementation and correlation with clinical outcomes in a large longitudinal study of early Parkinson’s disease. J. Mov. Disord. 2019, 12, 91. [Google Scholar] [CrossRef]
- Orozco-Barrios, C.E.; Battaglia-Hsu, S.-F.; Arango-Rodriguez, M.L.; Ayala-Davila, J.; Chery, C.; Alberto, J.-M.; Schroeder, H.; Daval, J.-L.; Martinez-Fong, D.; Gueant, J.-L. Vitamin B12-impaired metabolism produces apoptosis and Parkinson phenotype in rats expressing the transcobalamin-oleosin chimera in substantia nigra. PLoS ONE 2009, 4, e8268. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhao, Z.; Yang, N.; Xin, C.; Li, Z.; Xu, J.; Ma, B.; Lim, K.-L.; Li, L.; Wu, Q. Vitamin B12 Ameliorates the Pathological Phenotypes of Multiple Parkinson’s Disease Models by Alleviating Oxidative Stress. Antioxidants 2023, 12, 153. [Google Scholar] [CrossRef]
- Uddin, M.S.; Millat, M.; Baral, P.K.; Ferdous, M.; Uddin, M.; Sarwar, M.; Islam, M.S. The protective role of vitamin C in the management of COVID-19: A Review. J. Egypt. Public Health Assoc. 2021, 96, 33. [Google Scholar] [CrossRef]
- He, X.-B.; Kim, M.; Kim, S.-Y.; Yi, S.-H.; Rhee, Y.-H.; Kim, T.; Lee, E.-H.; Park, C.-H.; Dixit, S.; Harrison, F.E. Vitamin C facilitates dopamine neuron differentiation in fetal midbrain through TET1-and JMJD3-dependent epigenetic control manner. Stem Cells 2015, 33, 1320–1332. [Google Scholar] [CrossRef]
- Paraskevas, G.P.; Kapaki, E.; Petropoulou, O.; Anagnostouli, M.; Vagenas, V.; Papageorgiou, C. Plasma levels of antioxidant vitamins C and E are decreased in vascular parkinsonism. J. Neurol. Sci. 2003, 215, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Casani, S.; Gómez-Pastor, R.; Matallana, E.; Paricio, N. Antioxidant compound supplementation prevents oxidative damage in a Drosophila model of Parkinson’s disease. Free Radic. Biol. Med. 2013, 61, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, W.; Hu, Z.-J.; Ge, S.-M.; Huo, Y.; Liu, L.-X.; Gao, B.-L. Protective effects and mechanisms of high-dose vitamin C on sepsis-associated cognitive impairment in rats. Sci. Rep. 2021, 11, 14511. [Google Scholar] [CrossRef]
- Lee, J.-M.; Lee, J.-H.; Song, M.-K.; Kim, Y.-J. Nxp031 improves cognitive impairment in a chronic cerebral hypoperfusion-induced vascular dementia rat model through nrf2 signaling. Int. J. Mol. Sci. 2021, 22, 6285. [Google Scholar] [CrossRef]
- De Nuccio, F.; Cianciulli, A.; Porro, C.; Kashyrina, M.; Ruggiero, M.; Calvello, R.; Miraglia, A.; Nicolardi, G.; Lofrumento, D.D.; Panaro, M.A. Inflammatory Response Modulation by Vitamin C in an MPTP Mouse Model of Parkinson’s Disease. Biology 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Adams, L.; Lee, J.H.; Kim, Y.-S. NXP031 prevents dopaminergic neuronal loss and oxidative damage in the AAV-WT-α-synuclein mouse model of Parkinson’s disease. PLoS ONE 2022, 17, e0272085. [Google Scholar] [CrossRef]
- Wąsik, A.; Antkiewicz-Michaluk, L. The mechanism of neuroprotective action of natural compounds. Pharmacol. Rep. 2017, 69, 851–860. [Google Scholar] [CrossRef]
- Moretti, M.; Fraga, D.B.; Rodrigues, A.L.S. Preventive and therapeutic potential of ascorbic acid in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 921–929. [Google Scholar] [CrossRef]
- Bayo-Olugbami, A.; Nafiu, A.B.; Amin, A.; Ogundele, O.M.; Lee, C.C.; Owoyele, B.V. Vitamin D attenuated 6-OHDA-induced behavioural deficits, dopamine dysmetabolism, oxidative stress, and neuro-inflammation in mice. Nutr. Neurosci. 2022, 25, 823–834. [Google Scholar] [CrossRef]
- Kimlin, M.G. Geographic location and vitamin D synthesis. Mol. Asp. Med. 2008, 29, 453–461. [Google Scholar] [CrossRef]
- Yeum, K.-J.; Song, B.C.; Joo, N.-S. Impact of geographic location on vitamin D status and bone mineral density. Int. J. Environ. Res. Public Health 2016, 13, 184. [Google Scholar] [CrossRef]
- Lv, L.; Tan, X.; Peng, X.; Bai, R.; Xiao, Q.; Zou, T.; Tan, J.; Zhang, H.; Wang, C. The relationships of vitamin D, vitamin D receptor gene polymorphisms, and vitamin D supplementation with Parkinson’s disease. Transl. Neurodegener. 2020, 9, 34. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, J.R.; Mao, C.J.; Li, K.; Wang, F.; Chen, J.; Liu, C.F. Relationship between 25-Hydroxyvitamin D, bone density, and Parkinson’s disease symptoms. Acta Neurol. Scand. 2019, 140, 274–280. [Google Scholar] [CrossRef]
- Sleeman, I.; Aspray, T.; Lawson, R.; Coleman, S.; Duncan, G.; Khoo, T.K.; Schoenmakers, I.; Rochester, L.; Burn, D.; Yarnall, A. The role of vitamin D in disease progression in early Parkinson’s disease. J. Park. Dis. 2017, 7, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; He, Y.; Beck, J.; da Silva Teixeira, S.; Harrison, K.; Xu, Y.; Sisley, S. Defining vitamin D receptor expression in the brain using a novel VDRCre mouse. J. Comp. Neurol. 2021, 529, 2362–2375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, N.; Lu, Y.; Tan, K. Vitamin D receptor polymorphisms and the susceptibility of Parkinson’s disease. Neurosci. Lett. 2019, 699, 206–211. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, R.; Zhang, Z.; Li, K. The association between vitamin D status, vitamin D supplementation, sunlight exposure, and Parkinson’s disease: A systematic review and meta-analysis. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 666. [Google Scholar] [CrossRef] [PubMed]
- Gatto, N.M.; Paul, K.C.; Sinsheimer, J.S.; Bronstein, J.M.; Bordelon, Y.; Rausch, R.; Ritz, B. Vitamin D receptor gene polymorphisms and cognitive decline in Parkinson’s disease. J. Neurol. Sci. 2016, 370, 100–106. [Google Scholar] [CrossRef]
- Kim, J.E.; Oh, E.; Park, J.; Youn, J.; Kim, J.S.; Jang, W. Serum 25-hydroxyvitamin D3 level may be associated with olfactory dysfunction in de novo Parkinson’s disease. J. Clin. Neurosci. 2018, 57, 131–135. [Google Scholar] [CrossRef]
- Calvello, R.; Cianciulli, A.; Nicolardi, G.; De Nuccio, F.; Giannotti, L.; Salvatore, R.; Porro, C.; Trotta, T.; Panaro, M.A.; Lofrumento, D.D. Vitamin D treatment attenuates neuroinflammation and dopaminergic neurodegeneration in an animal model of Parkinson’s disease, shifting M1 to M2 microglia responses. J. Neuroimmune Pharmacol. 2017, 12, 327–339. [Google Scholar] [CrossRef]
- Lima, L.A.; Lopes, M.J.P.; Costa, R.O.; Lima, F.A.V.; Neves, K.R.T.; Calou, I.B.; Andrade, G.M.; Viana, G.S. Vitamin D protects dopaminergic neurons against neuroinflammation and oxidative stress in hemiparkinsonian rats. J. Neuroinflammation 2018, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elfotuh, K.; Hamdan, A.M.E.; Abbas, A.N.; Alahmre, A.T.S.; Elewa, M.A.; Masoud, R.A.E.; Ali, A.A.; Othman, M.; Kamal, M.M.; Hassan, F.A.M. Evaluating the neuroprotective activities of vinpocetine, punicalagin, niacin and vitamin E against behavioural and motor disabilities of manganese-induced Parkinson’s disease in Sprague Dawley rats. Biomed. Pharmacother. 2022, 153, 113330. [Google Scholar] [CrossRef]
- Iqbal, A.; Anwar, F.; Saleem, U.; Khan, S.S.; Karim, A.; Ahmad, B.; Gul, M.; Iqbal, Z.; Ismail, T. Inhibition of Oxidative Stress and the NF-κB Pathway by a Vitamin E Derivative: Pharmacological Approach against Parkinson’s Disease. ACS Omega 2022, 7, 45088–45095. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Nehru, B. Beneficial effect of vitamin E in rotenone induced model of PD: Behavioural, neurochemical and biochemical study. Exp. Neurobiol. 2013, 22, 214. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-M.; Elliot, J.; Hobson, P.; O’Hare, E. Effects of intrahippocampal NAC61–95 injections on memory in the rat and attenuation with vitamin E. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2009, 33, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.C.; Kwak, S.G.; Kwak, S. Effect of dietary vitamins C and E on the risk of Parkinson’s disease: A meta-analysis. Clin. Nutr. 2021, 40, 3922–3930. [Google Scholar] [CrossRef]
- Ricciarelli, R.; Argellati, F.; Pronzato, M.A.; Domenicotti, C. Vitamin E and neurodegenerative diseases. Mol. Asp. Med. 2007, 28, 591–606. [Google Scholar] [CrossRef] [PubMed]
- Taghizadeh, M.; Tamtaji, O.R.; Dadgostar, E.; Kakhaki, R.D.; Bahmani, F.; Abolhassani, J.; Aarabi, M.H.; Kouchaki, E.; Memarzadeh, M.R.; Asemi, Z. The effects of omega-3 fatty acids and vitamin E co-supplementation on clinical and metabolic status in patients with Parkinson’s disease: A randomized, double-blind, placebo-controlled trial. Neurochem. Int. 2017, 108, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-X.; Yu, X.-D.; Cheng, Q.-Z.; Tang, L.; Shen, M.-Q. The association of serum vitamin K2 levels with Parkinson’s disease: From basic case-control study to big data mining analysis. Aging 2020, 12, 16410. [Google Scholar] [CrossRef]
- Vos, M.; Esposito, G.; Edirisinghe, J.N.; Vilain, S.; Haddad, D.M.; Slabbaert, J.R.; Van Meensel, S.; Schaap, O.; De Strooper, B.; Meganathan, R. Vitamin K2 is a mitochondrial electron carrier That rescues pink1 deficiency. Science 2012, 336, 1306–1310. [Google Scholar] [CrossRef] [PubMed]
- da Silva, F.L.; Cerqueira, E.C.; de Freitas, M.S.; Gonçalves, D.L.; Costa, L.T.; Follmer, C. Vitamins K interact with N-terminus α-synuclein and modulate the protein fibrillization in vitro. Exploring the interaction between quinones and α-synuclein. Neurochem. Int. 2013, 62, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-X.; Li, Y.-P.; Gao, F.; Hu, Q.-S.; Zhang, Y.; Chen, D.; Wang, G.-H. Vitamin K2 suppresses rotenone-induced microglial activation in vitro. Acta Pharmacol. Sin. 2016, 37, 1178–1189. [Google Scholar] [CrossRef] [PubMed]

| Vitamin | Study Design | Sample Size | Results | Vitamin and PD Association | Ref. |
|---|---|---|---|---|---|
| Vitamin A | Cohort-study | 42 PD patients and 42 healthy controls | No significant difference in the serum levels of vitamin A and retinol-binding protein between the control and PD group | No association between vitamin A and the risk of PD | [24] |
| Population-based cohort study | 63,257 men and women, including 544 patients with incident PD | No precise dose-dependent association between dietary intake of vitamin A, E, and C and the risk of PD | No association between vitamin A and the risk of PD | [25] | |
| Vitamin B1 | Case-study | 5 PD patients | Intake of daily 100–200 mg doses of parenteral thiamine improved movement, arm swings, and tremors in thiamine deficient PD patients | TD is associated with an increased risk of PD, and its supplementation may be beneficial | [27] |
| Case-study | 3 PD patients | A high dose of thiamine intake significantly improved motor coordination-related UPDRS, ranging from 31.3% to 77.3% | A high dose of thiamine intake is associated with improvement in PD symptoms | [28] | |
| Case-controlled study | 96 PD patients and 375 control subjects | Deficiency of thiamine and folate caused olfactory dysfunction in PD patients | TD is associated with an increased risk of PD | [29] | |
| Case-controlled study | 75 PD patients and 24 control subjects | In male PD patients, higher levels of phosphate and thiamine concentration, as well as higher MNA-total score, were correlated with a lower risk of MCI | Thiamine insufficiency and low phosphate levels increase the risk for PD-associated cognitive deficits | [30] | |
| Vitamin B3 | Case-controlled study | 46 PD patients | PD patients with vitamin B3 deficiency were associated with GPR109A-mediated inflammation. Supplementation with 100 mg and 200 mg doses showed ameliorative effect | Vitamin B3 deficiency is associated with an increased risk of PD. | [38] |
| Vitamin B6 | Case-controlled study | 249 PD patients and 368 control subjects | Low consumption of vitamin B6 was associated with an elevated risk of PD | A deficiency of vitamin B6 increases the risk of PD | [51] |
| Case-study | 83-year-old woman with hypertension, coronary artery disease, and PD | Early detection and treatment of pyridoxine deficiency may reduce new-onset epileptic seizures and status epilepticus in PD patients | Vitamin B6 deficiency is associated with an increased risk of PD | [42] | |
| Population based cohort-study | 5289 total participants, including 72 patients with incident PD | No association between dietary folate and vitamin B12 and the risk of PD. Vitamin B6 decreased the risk of PD. | Dietary vitamin B6 correlated with reduced risk of PD | [19] | |
| Vitamin B12 | Population-based cohort study | NA | Higher serum vitamin B12 at baseline level of PD diagnosis was correlated with a reduced risk of dementia | Vitamin B12 is associated with decreased risk of PD | [46] |
| Longitudinal cohort- study | 1741 participants | A low hazard ratio in subjects taking vitamin B12 + MVI and MVI groups for developing sensory symptoms of PD | Vitamin B12 is associated with a reduced risk of PD | [47] | |
| Vitamin C | Cohort-study | 75 PD patients and 75 healthy subjects | Patients with PD had considerably increased nitrite oxide and peroxynitrite but low vitamin C levels in the serum | Vitamin C deficiency is associated with an increased risk of PD | [8] |
| Vitamin D | Cohort-study | 182 PD patients and 185 control subjects | PD patients had lower serum levels of 25 (OH)D than healthy controls | Vitamin D deficiency is associated with an increased risk of PD | [64] |
| Patients with MCI were categorized as serum 25 (OH)D deficient (n = 27) or not deficient (n = 29) based on serum 25 (OH)D levels. | In older persons with MCI, low vitamin D levels were related with lower volumes of hippocampus subfields and connection impairments, which aggravated neurocognitive results. | Low vitamin D is associated with progression from MCI to major cognitive disorders. | [17] | ||
| Observational study | 145 PD patients and 94 control subjects | PD patients had lower serum levels of 25 (OH)D than healthy controls at baseline and at 18th-month follow-up session | A deficiency of 25 (OH)D is associated with increased motor severity and risk of bone fracture in PD patients | [65] | |
| Meta-analysis | NA | Significant associations between rs2228570 and PD risk were found in allelic, dominant, and additive models but not in the recessive model. | VDR polymorphism is associated with an increased risk of PD | [67] | |
| Meta-analysis | NA | Both 25 (OH)D insufficiency and deficiency were correlated with an increased risk of PD. However, vitamin D supplementation did not improve motor symptoms in PD patients | Deficiency of 25 (OH)D and reduced exposure to sunlight is associated with an increased risk of PD | [68] | |
| Observational study | 39 drug-naive, de novo PD patients | Vitamin D was involved in the etiology of olfactory impairment in PD | Vitamin D deficiency increases the risk of olfactory dysfunction | [70] | |
| Vitamin E | Meta-analysis | NA | High vitamin E consumption considerably decreased the chance of developing PD | High vitamin E intake is associated with a reduced risk of PD | [77] |
| Randomized double-blind placebo-controlled study | 60 PD patients | Co-supplementation with omega-3 fatty acids and vitamin E improved UPDRS in persons with PD | Vitamin E supplementation is associated with decreased risk of PD in older adults | [79] | |
| Vitamin K | Case-controlled study | 93 PD patients and 95 healthy controls | PD patients were deficient in serum vitamin K2 level | Vitamin K2 deficiency is associated with an increased risk of PD | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandeep; Sahu, M.R.; Rani, L.; Kharat, A.S.; Mondal, A.C. Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease? Brain Sci. 2023, 13, 272. https://doi.org/10.3390/brainsci13020272
Sandeep, Sahu MR, Rani L, Kharat AS, Mondal AC. Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease? Brain Sciences. 2023; 13(2):272. https://doi.org/10.3390/brainsci13020272
Chicago/Turabian StyleSandeep, Manas Ranjan Sahu, Linchi Rani, Arun S. Kharat, and Amal Chandra Mondal. 2023. "Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease?" Brain Sciences 13, no. 2: 272. https://doi.org/10.3390/brainsci13020272
APA StyleSandeep, Sahu, M. R., Rani, L., Kharat, A. S., & Mondal, A. C. (2023). Could Vitamins Have a Positive Impact on the Treatment of Parkinson’s Disease? Brain Sciences, 13(2), 272. https://doi.org/10.3390/brainsci13020272







