A New Smart 2-Min Mobile Alerting Method for Mild Cognitive Impairment Due to Alzheimer’s Disease in the Community
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Design of the Paradigm and Experimental Process
2.3. Data Acquisition
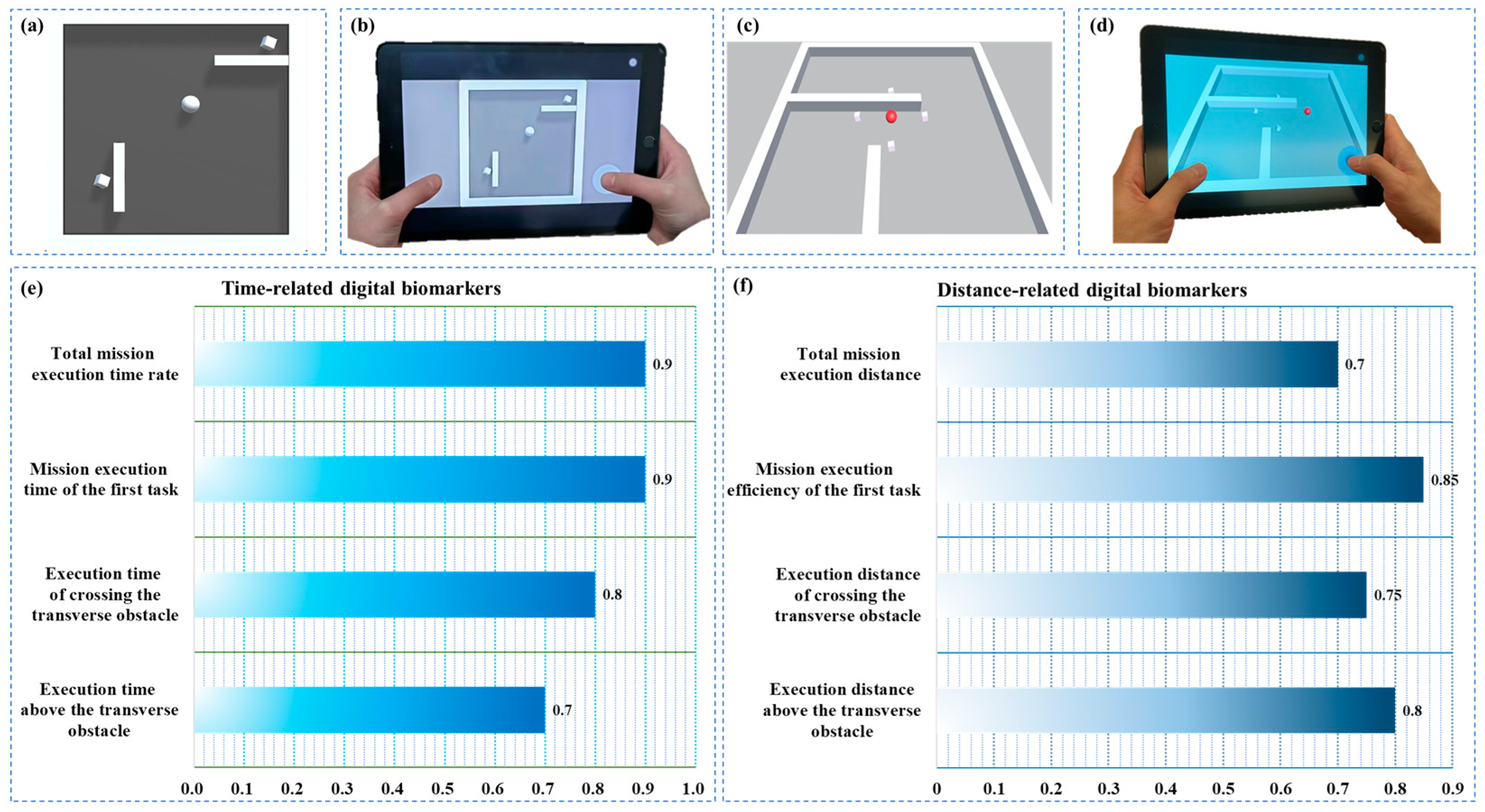
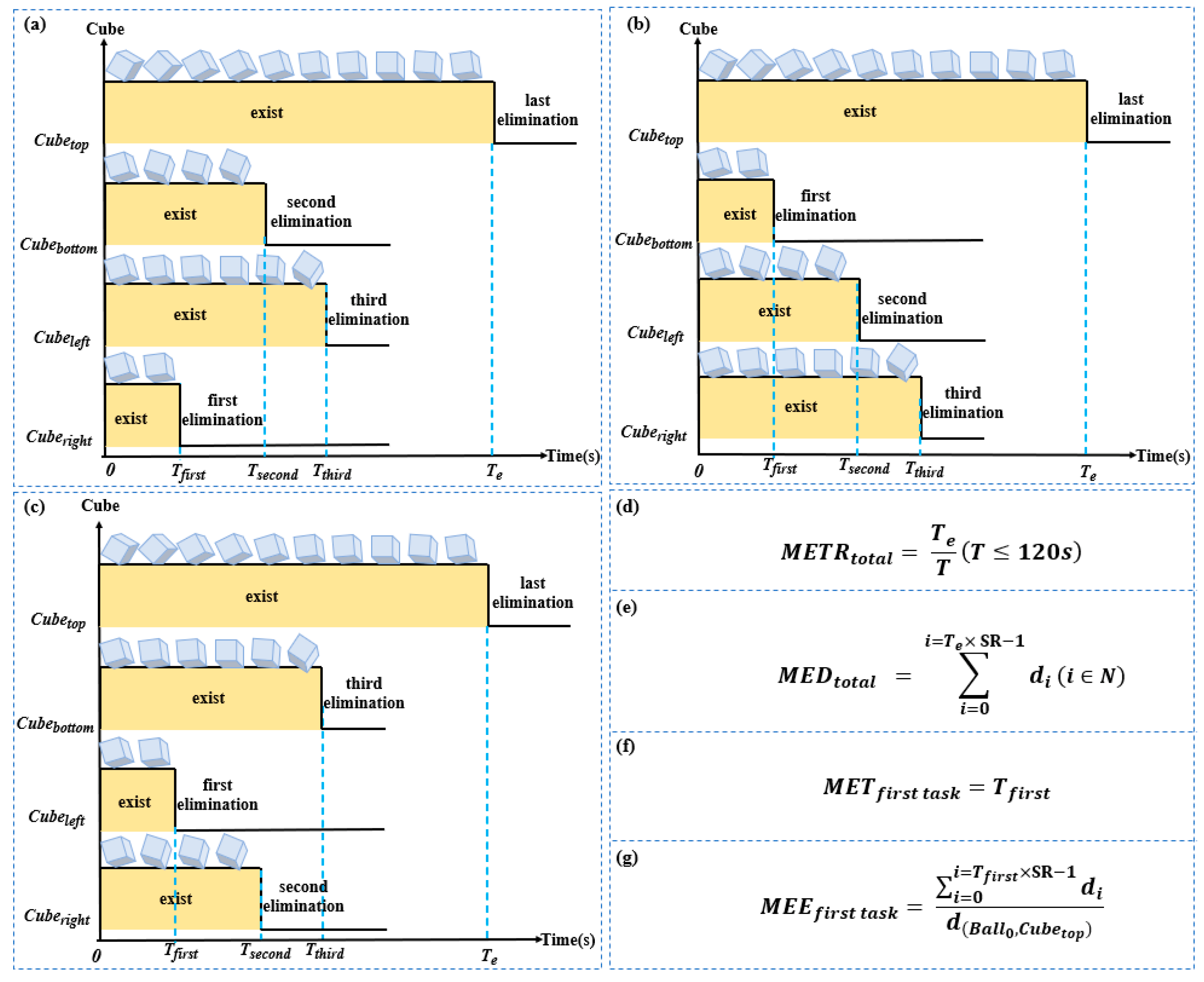
2.4. Definition and Quantitative Analysis of Digital Biomarkers
2.5. Statistical Analysis
3. Results
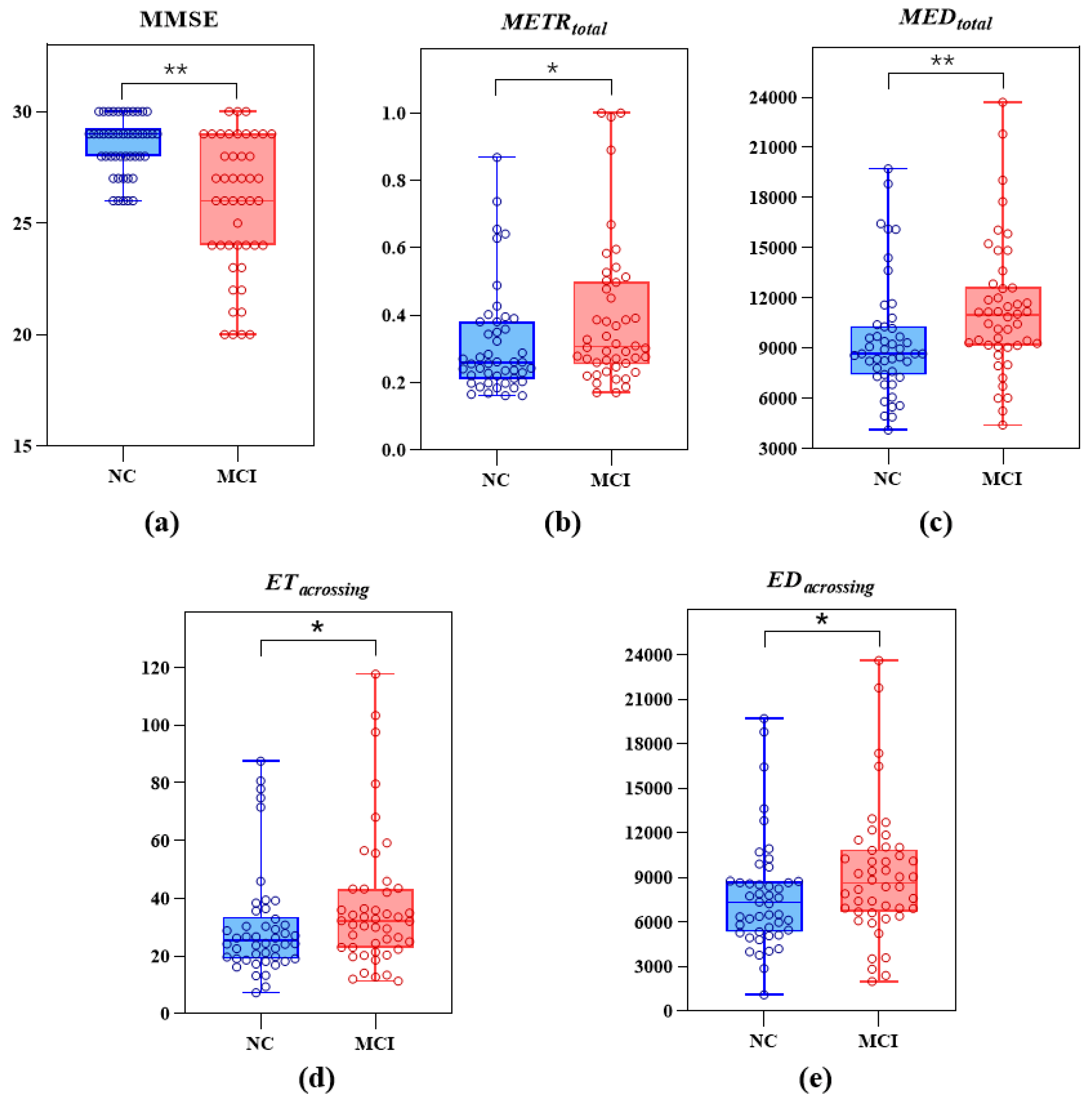
3.1. Demographic and Differential Analysis of All Participants
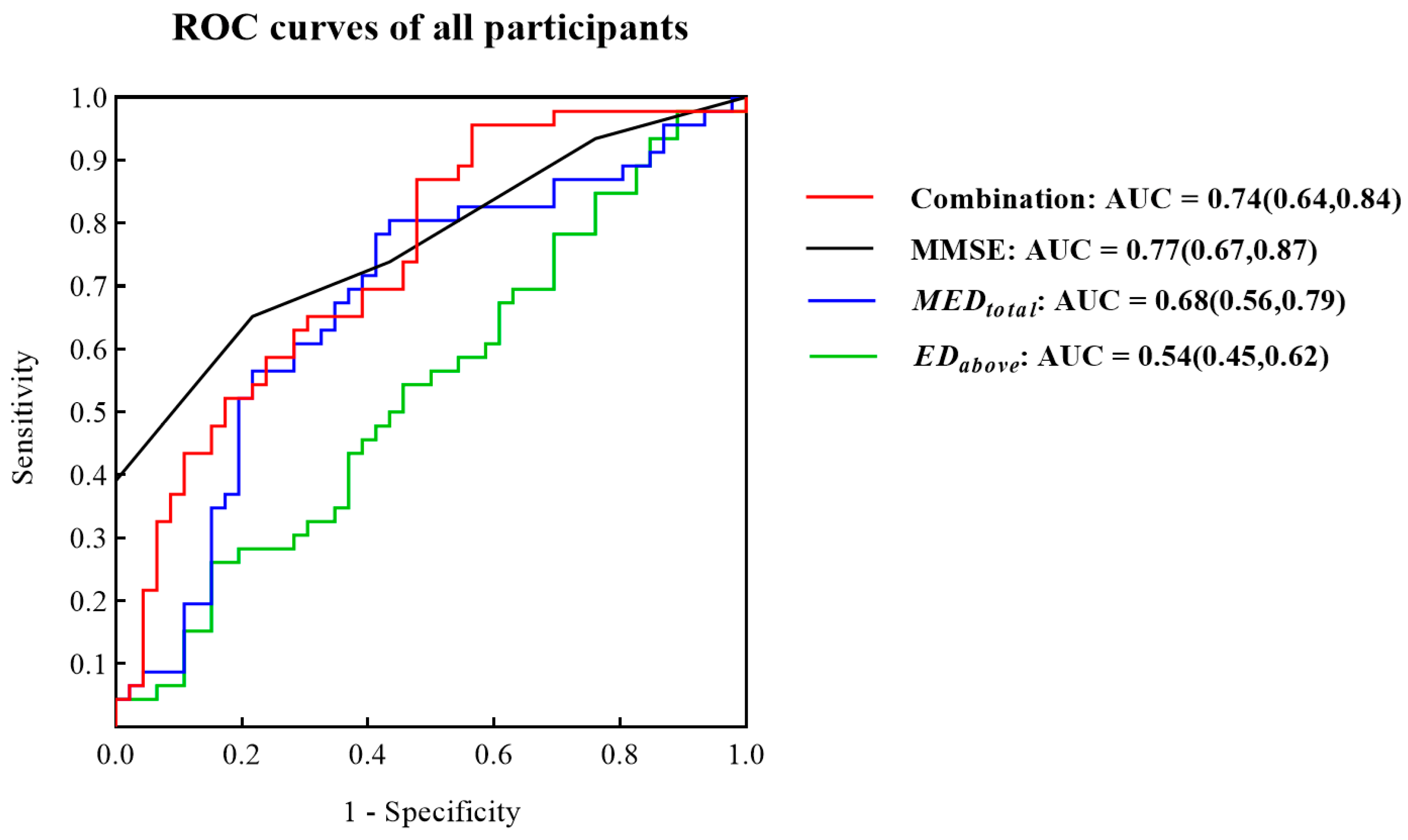
3.2. ROC Curves for Identifying MCI Patients from All Participants
3.3. Demographic and Differential Analysis of the Participants with Basic Education
3.4. ROC Curves for Identifying Participants with Basic Education
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, L.; Quan, M.; Fu, Y.; Zhao, T.; Li, Y.; Wei, C.; Tang, Y.; Qin, Q.; Wang, F.; Qiao, Y.; et al. Dementia in China: Epidemiology, clinical management, and research advances. Lancet Neurol. 2020, 19, 81–92. [Google Scholar] [CrossRef]
- Alzheimer’s Association. Alzheimer’s Association Report 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Phelps, C.H. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Phelps, C.H. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Knopman, D.S.; Amieva, H.; Petersen, R.C.; Chételat, G.; Holtzman, D.M.; Hyman, B.T.; Jones, D.T. Alzheimer disease. Nat. Rev. Dis. Prim. 2021, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Phelps, C.H. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Studenski, S.A.; An, Y.; Kuo, P.L.; Schrack, J.A.; Wanigatunga, A.A.; Ferrucci, L. Association of Combined Slow Gait and Low Activity Fragmentation With Later Onset of Cognitive Impairment. JAMA Netw. Open 2021, 4, e2135168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.L. The nonlinear effects of population aging, industrial structure, and urbanization on carbon emissions: A panel threshold regression analysis of 137 countries. J. Clean. Prod. 2021, 287, 125381. [Google Scholar] [CrossRef]
- Bruno, A.; Galbraith, K.; McKinnon, R. Global Supply of Health Professionals. N. Engl. J. Med. 2014, 370, 2246. [Google Scholar]
- Burgess, D.C.H.; Wasserman, J.; Dahl, C.A. Global health diagnostics. Nature 2006, 444 (Suppl. 1), 1–2. [Google Scholar] [CrossRef] [PubMed]
- Qiang, Q.; Skudder-Hill, L.; Adachi, H. CSF GAP-43 as a biomarker of synaptic dysfunction is associated with tau pathology in Alzheimer’s disease. Sci. Rep. 2022, 12, 17392. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling data from four cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Villemagne, V.L.; Burnham, S.; Bourgeat, P.; Brown, B.; Ellis, K.A.; Salvado, O.; Masters, C.L. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013, 12, 357–367. [Google Scholar] [CrossRef]
- Sharma, S.; Guleria, K.; Tiwari, S.; Kumar, S. A deep learning based convolutional neural network model with VGG16 feature extractor for the detection of Alzheimer Disease using MRI scans. Meas. Sens. 2022, 24, 100506. [Google Scholar] [CrossRef]
- Patnode, C.D.; Perdue, L.A.; Rossom, R.C.; Rushkin, M.C.; Redmond, N.; Thomas, R.G.; Lin, J.S. Screening for Cognitive Impairment in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2020, 323, 764–785. [Google Scholar] [PubMed]
- Saxton, J.; Lopez, O.L.; Ratcliff, G.; Dulberg, C.; Fried, L.P.; Carlson, M.C.; Kuller, L. Preclinical Alzheimer disease: Neuropsychological test performance 1.5 to 8 years prior to onset. Neurology 2004, 63, 2341–2347. [Google Scholar] [CrossRef]
- Pinto, T.C.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.L.; Ximenes, R.C.; Sougey, E.B. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 2019, 31, 491–504. [Google Scholar] [CrossRef]
- Trzepacz, P.T.; Hochstetler, H.; Wang, S.; Walker, B.; Saykin, A.J.; Alzheimer’s Disease Neuroimaging Initiative. Relationship between the Montreal Cognitive Assessment and Mini-mental State Examination for assessment of mild cognitive impairment in older adults. Bmc Geriatr. 2015, 15, 107. [Google Scholar] [CrossRef] [PubMed]
- Kourtis, L.C.; Regele, O.B.; Wright, J.M.; Jones, G.B. Digital biomarkers for Alzheimer’s disease: The mobile/ wearable devices opportunity. NPJ Digit. Med. 2019, 2, 9. [Google Scholar] [CrossRef]
- Piau, A.; Wild, K.; Mattek, N.; Kaye, J. Current State of Digital Biomarker Technologies for Real-Life, Home-Based Monitoring of Cognitive Function for Mild Cognitive Impairment to Mild Alzheimer Disease and Implications for Clinical Care: Systematic Review. J. Med. Internet Res. 2019, 21, e12785. [Google Scholar] [CrossRef] [PubMed]
- Coravos, A.; Khozin, S.; Mandl, K.D. Developing and adopting safe and effective digital biomarkers to improve patient outcomes. Npj Digit. Med. 2019, 2, 14. [Google Scholar] [CrossRef] [PubMed]
- Poos, J.M.; van der Ham, I.J.M.; Leeuwis, A.E.; Pijnenburg, Y.A.L.; van der Flier, W.M.; Postma, A. Short Digital Spatial Memory Test Detects Impairment in Alzheimer’s Disease and Mild Cognitive Impairment. Brain Sci. 2021, 11, 1350. [Google Scholar] [CrossRef]
- Seixas, A.A.; Rajabli, F.; Pericak-Vance, M.A.; Jean-Louis, G.; Harms, R.L.; Tarnanas, I. Associations of digital neuro-signatures with molecular and neuroimaging measures of brain resilience: The altoida large cohort study. Front. Psychiatry 2022, 13, 899080. [Google Scholar] [CrossRef]
- Tadokoro, K.; Yamashita, T.; Fukui, Y.; Nomura, E.; Ohta, Y.; Ueno, S.; Abe, K. Early detection of cognitive decline in mild cognitive impairment and Alzheimer’s disease with a novel eye tracking test. J. Neurol. Sci. 2021, 427, 117529. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Yang, X.; Du, W.; Ogihara, A.; Zhou, S.; Ma, X.; Wang, Y.; Li, S.; Li, K. Exploratory Research on Key Technology of Human-Computer Interactive 2.5-Minute Fast Digital Early Warning for Mild Cognitive Impairment. Comput. Intell. Neurosci. 2022, 2022, 2495330. [Google Scholar] [CrossRef]
- Kalafatis, C.; Modarres, M.H.; Apostolou, P.; Marefat, H.; Khanbagi, M.; Karimi, H.; Khaligh-Razavi, S.M. Validity and Cultural Generalisability of a 5-Minute AI-Based, Computerised Cognitive Assessment in Mild Cognitive Impairment and Alzheimer’s Dementia. Front. Psychiatry 2021, 12, 706695. [Google Scholar] [CrossRef]
- Cheah, W.-T.; Hwang, J.-J.; Hong, S.-Y.; Fu, L.-C.; Chang, Y.-L.; Chen, T.-F.; Chen, I.-A.; Chou, C.-C. A Digital Screening System for Alzheimer Disease Based on a Neuropsychological Test and a Convolutional Neural Network: System Development and Validation. Jmir Med. Inform. 2022, 10, e31106. [Google Scholar] [CrossRef] [PubMed]
- Backman, L.; Small, B.J.; Fratiglioni, L. Stability of the preclinical episodic memory deficit in Alzheimer’s disease. Brain A J. Neurol. 2001, 124, 96–102. [Google Scholar] [CrossRef]
- Parizkova, M.; Lerch, O.; Moffat, S.D.; Andel, R.; Mazancova, A.F.; Nedelska, Z.; Vyhnalek, M.; Hort, J.; Laczó, J. The effect of Alzheimer’s disease on spatial navigation strategies. Neurobiol. Aging 2018, 64, 107–115. [Google Scholar] [CrossRef]
- Benke, T.; Karner, E.; Petermichl, S.; Prantner, V.; Kemmler, G. Neuropsychological Deficits Associated With Route Learning in Alzheimer Disease, MCI, and Normal Aging. Alzheimer Dis. Assoc. Disord. 2014, 28, 162–167. [Google Scholar] [CrossRef]
- Vlcek, K.; Laczo, J. Neural correlates of spatial navigation changes in mild cognitive impairment and Alzheimer’s disease. Front. Behav. Neurosci. 2014, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Lithfous, S.; Dufour, A.; Després, O. Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: Insights from imaging and behavioral studies. Ageing Res. Rev. 2013, 12, 201–213. [Google Scholar] [CrossRef]
- Gazova, I.; Laczó, J.; Rubínová, E.; Mokrisova, I.; Hyncicova, E.; Andel, R.; Vyhnalek, M.; Sheardova, K.; Coulson, E.J.; Hort, J. Spatial navigation in young versus older adults. Front. Aging Neurosci. 2013, 5, 94. [Google Scholar] [CrossRef] [PubMed]
- Storandt, M.; Mintun, M.M.A.; Head, D.; Morris, J.C. Cognitive Decline and Brain Volume Loss as Signatures of Cerebral Amyloid-beta Peptide Deposition Identified With Pittsburgh Compound B Cognitive Decline Associated With A beta Deposition. Arch. Neurol. 2009, 66, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 2015, 138, 2814–2833. [Google Scholar] [CrossRef] [PubMed]
- Coughlan, G.; Laczo, J.; Hort, J.; Minihane, A.; Hornberger, M. Spatial navigation deficits—Overlooked cognitive marker for preclinical Alzheimer disease? Nat. Rev. Neurol. 2018, 14, 496–506. [Google Scholar] [PubMed]
- Verghese, J.; Lipton, R.; Ayers, E. Spatial navigation and risk of cognitive impairment: A prospective cohort study. Alzheimers Dement. 2017, 13, 985–992. [Google Scholar] [CrossRef]
- Tangen, G.G.; Engedal, K.; Bergland, A.; Moger, T.A.; Hansson, O.; Mengshoel, A.M. Spatial navigation measured by the Floor Maze Test in patients with subjective cognitive impairment, mild cognitive impairment, and mild Alzheimer’s disease. Int. Psychogeriatr. 2015, 27, 1401–1409. [Google Scholar] [CrossRef]
- Zanco, M.; Plácido, J.; Marinho, V.; Ferreira, J.V.; de Oliveira, F.; Monteiro-Junior, R.S.; Barca, M.L.; Engedal, K.; Laks, J.; Deslandes, A. Spatial Navigation in the Elderly with Alzheimer’s Disease: A Cross-Sectional Study. J. Alzheimers Dis. 2018, 66, 1683–1694. [Google Scholar] [CrossRef] [PubMed]
- Grahn, J.A.; Parkinson, J.A.; Owen, A.M. The role of the basal ganglia in learning and memory: Neuropsychological studies. Behav. Brain Res. 2009, 199, 53–60. [Google Scholar] [PubMed]
- McCabe, D.P.; Roediger, H.L.; McDaniel, M.A.; Balota, D.A.; Hambrick, D.Z. The Relationship Between Working Memory Capacity and Executive Functioning: Evidence for a Common Executive Attention Construct. Neuropsychology 2010, 24, 222–243. [Google Scholar] [CrossRef]
- Allain, P.; Chaudet, H.; Nicoleau, S.; Etcharry-Bouyx, F.; Barré, J.; Dubas, F.; Le Gall, D. A study of action planning in patients with Alzheimer’s disease using the zoo map test. Rev. Neurol. 2007, 163, 222–230. [Google Scholar] [CrossRef] [PubMed]
- Burkart, M.; Heun, R.; Maier, W.; Benkert, O. Dementia screening in routine clinical practice. A comparative analysis of MMSE, SIDAM and ADAS. Der Nervenarzt 1998, 69, 983–990. [Google Scholar] [CrossRef]
- Ghosh, A.; Puthusseryppady, V.; Chan, D.; Mascolo, C.; Hornberger, M. Machine learning detects altered spatial navigation features in outdoor behaviour of Alzheimer’s disease patients. Sci. Rep. 2022, 12, 3160. [Google Scholar] [CrossRef] [PubMed]
- Hartley, T.; Lever, C.; Burgess, N.; O’Keefe, J. Space in the brain: How the hippocampal formation supports spatial cognition. Philos. Trans. R. Soc. B-Biol. Sci. 2014, 369, 20120510. [Google Scholar] [CrossRef]
- Deipolyi, A.; Rankin, K.P.; Mucke, L.; Miller, B.L.; Gorno-Tempini, M.L. Gorno-Tempini. Spatial cognition and the human navigation network in AD and MCI. Neurology 2007, 69, 986–997. [Google Scholar] [CrossRef] [PubMed]



| Researcher | Method | Limitation |
|---|---|---|
| Li Nan et al. [26] | The 2.5-min interactive rapid digital human–computer early warning technology was developed to accurately distinguish elderly NC patients and MCI patients within 2.5 min, with an AUC of 0.824 by means of an eye tracking device. | (1) The total sample size was limited, (2) only 32 participants were included, and the mean age was 84.5 years, (3) eye tracking equipment and gamepads were required. |
| Koh Tadokoro et al. [25] | A high-performance eye tracking device was used to analyze the subjects’ eye tracking using the novel eye tracking test and to establish machine learning classification of NC, MCI and AD subjects. | (1) It took 3 min, (2) an eye movement meter was needed, which is expensive, (3) the subjects were old, with an average age of 75 years, (4) ROC curves showed that AUC was only 0.75 for MCI patients. |
| Kalafatis Chris, et al. [27] | Integrated Cognitive Assessment, a 5-min computerized cognitive assessment tool based on a rapid visual categorization task. It is a rapid visual classification test, which can test the speed of information processing of the subjects and use artificial intelligence to automatically calculate the results of a cognitive impairment screening. | (1) The test time was 5 min, (2) relatively lower recruitment of young subjects with mild AD and mild-AD subjects with high education. |
| Seixas Azizi, et al. [24] | Sensors such as accelerometers, gyroscopes, magnetic mirrors, cameras, microphones and touch screens capture digital neural biomarkers that can predict the overall cognitive function and changes in older adults with cognitive impairment and cognitive health in a range of motor function tasks and two augmented reality tasks. | (1) The test took 20 min, (2) required multiple sensing devices, (3) there were sample size differences between cognitively impaired and control groups, (4) focused on old adults and lacked an early age group. |
| Poos Jackie M, et al. [23] | Objective Location Memory Test and Virtual Tubingen Test, such as the Short Digital Spatial Memory Test, were used to examine mild AD dementia and MCI patients. | (1) The test took 20 min, (2) it had a small sample size and age mismatch between groups. |
| Cheah Wen-Ting, et al. [28] | A digital screening system was designed based on the Rey-Osterrieth Complex Figure neuropsychological test. A tablet and smart pen were also used for data collection to differentiate between MCI and AD patients and healthy controls. | (1) Includes a delayed recall module with a total completion time of 30 min or more, (2) large age differences between sample groups within the dataset, (3) requires an electronic pen. |
| Digital Biomarker | Abbreviation | Unit | Interpretation |
|---|---|---|---|
| Total mission execution time rate | / | Means the ratio of the total execution time of the paradigm to the maximum evaluation time of the paradigm. | |
| Mission execution time of the first task | s | Means how long it takes the participants to eliminate the first target cube. | |
| Execution time of crossing the transverse obstacle | s | Means the total amount of time the subject controls the sphere to complete the remaining tasks after crossing the horizontal obstacle in the paradigm scene. | |
| Execution time while above the transverse obstacle | s | Means the total execution time during which the sphere is above the horizontal obstacle in the paradigm scene when the subject controls the sphere to perform the task. |
| Digital Biomarker | Abbreviation | Unit | Interpretation |
|---|---|---|---|
| Total mission execution distance | px | Means the total distance at which the participants manipulate the movement of the sphere during this paradigm | |
| Mission execution efficiency of the first task | / | Means the ratio of the distance the subject controls the movement of the sphere to the shortest distance between the sphere and the cube during the time of | |
| Execution distance of crossing the transverse obstacle | px | Means that the subject controls the sum of the distance the sphere covers after it crosses the horizontal obstacle in the paradigm scene, i.e., the distance the sphere covers during the time of | |
| Execution distance above the transverse obstacle | px | Means the sum of the distances covered by the sphere while moving over the horizontal obstacle in the paradigm scene when the subject controls the sphere to perform the task, i.e., the distance of movement of the sphere during the time of |
| NC n = 46 | MCI n = 46 | p Value | |
|---|---|---|---|
| Age | 68.00 (60.75, 79.00) | 70.00 (64.75, 80.00) | 0.33 |
| Sex (female/male) | 29/17 | 31/15 | 0.66 |
| Years of education | 12.00 (9.00, 15.25) | 12.00 (9.00, 14.25) | 0.46 |
| MMSE ** | 29 (28.00, 29.25) | 26.00 (24.00, 29.00) | <0.01 |
| * | 0.26 (0.21, 0.38) | 0.31 (0.25, 0.50) | 0.02 |
| (px) ** | 8676.08 (7406.04, 10,324.11) | 10,963.08 (9156.78, 12,672.55) | <0.01 |
| (s) | 1.12 (0.78, 1.75) | 1.08 (0.82, 1.96) | 0.75 |
| 1.04 (1.01, 1.06) | 1.03 (1.01, 1.10) | 0.72 | |
| (s) * | 25.32 (19.00, 33.52) | 32.18 (22.83, 43.30) | 0.04 |
| (px) * | 7292.46 (5331.37, 8750.32) | 8606.23 (6675.84, 10,861.47) | 0.04 |
| (s) | 16.51 (12.99, 25.22) | 18.27 (13.40, 29.00) | 0.54 |
| (px) | 4497.86 (3567.56, 6036.56) | 4279.95 (3508.07, 5780.54) | 0.82 |
| Basic-Educated NC n = 29 | Basic-Educated MCI n = 31 | p Value | |
|---|---|---|---|
| Age | 68.00(61.00, 83.00) | 72.00 (64.00, 80.00) | 0.62 |
| Sex (female/male) | 17/12 | 17/14 | 0.77 |
| Years of education | 12.00 (9.00, 16.00) | 12.00 (6.00, 15.00) | 0.08 |
| MMSE ** | 29 (28.00, 30.00) | 27.00 (24.00, 29.00) | <0.01 |
| * | 0.24 (0.20, 0.28) | 0.30 (0.26, 0.50) | <0.01 |
| (px) ** | 8469.29 (7071.54, 9285.65) | 10,473.75 (9189.11, 12,848.72) | <0.01 |
| (s) | 0.95 (0.77, 1.56) | 1.25 (0.85, 2.30) | 0.11 |
| 1.04 (1.01, 1.08) | 1.03 (1.01, 1.09) | 0.60 | |
| (s) | 22.65 (18.37, 28.17) | 30.40 (22.98, 43.30) | <0.01 |
| (px) | 6515.23 (5128.11, 8748.07) | 8391.10 (6912.21, 11,023.95) | <0.01 |
| (s) | 14.03 (12.45, 17.53) | 17.72 (12.75, 29.02) | 0.26 |
| (px) | 4040.34 (2928.82, 5167.38) | 4260.67 (3579.10, 5640.59) | 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, T.; Wang, C.; Ogihara, A.; Ma, X.; Huang, S.; Zhou, S.; Li, S.; Liu, J.; Li, K. A New Smart 2-Min Mobile Alerting Method for Mild Cognitive Impairment Due to Alzheimer’s Disease in the Community. Brain Sci. 2023, 13, 244. https://doi.org/10.3390/brainsci13020244
Wang Y, Chen T, Wang C, Ogihara A, Ma X, Huang S, Zhou S, Li S, Liu J, Li K. A New Smart 2-Min Mobile Alerting Method for Mild Cognitive Impairment Due to Alzheimer’s Disease in the Community. Brain Sciences. 2023; 13(2):244. https://doi.org/10.3390/brainsci13020244
Chicago/Turabian StyleWang, Yujia, Tong Chen, Chen Wang, Atsushi Ogihara, Xiaowen Ma, Shouqiang Huang, Siyu Zhou, Shuwu Li, Jiakang Liu, and Kai Li. 2023. "A New Smart 2-Min Mobile Alerting Method for Mild Cognitive Impairment Due to Alzheimer’s Disease in the Community" Brain Sciences 13, no. 2: 244. https://doi.org/10.3390/brainsci13020244
APA StyleWang, Y., Chen, T., Wang, C., Ogihara, A., Ma, X., Huang, S., Zhou, S., Li, S., Liu, J., & Li, K. (2023). A New Smart 2-Min Mobile Alerting Method for Mild Cognitive Impairment Due to Alzheimer’s Disease in the Community. Brain Sciences, 13(2), 244. https://doi.org/10.3390/brainsci13020244







