Abstract
Finding a link between a hormone and microRNAs (miRNAs) is of great importance since it enables the adjustment of genetic composition or cellular functions without needing gene-level interventions. The dicer-mediated cleavage of precursor miRNAs is an interface link between miRNA and its regulators; any disruption in this process can affect neurogenesis. Besides, the hormonal regulation of miRNAs can occur at the molecular and cellular levels, both directly, through binding to the promoter elements of miRNAs, and indirectly, via regulation of the signaling effects of the post-transcriptional processing proteins. Estrogenic hormones have many roles in regulating miRNAs in the brain. This review discusses miRNAs, their detailed biogenesis, activities, and both the general and estrogen-dependent regulations. Additionally, we highlight the relationship between miR-29, miR-9, and estrogens in the nervous system. Such a relationship could be a possible etiological route for developing various neurodegenerative disorders.
1. Introduction
Non-coding RNAs (ncRNAs) include miRNAs, short-interfering RNAs (siRNAs), and PIWI-interacting RNA (piRNAs) [1]. MiRNAs are small single-stranded, non-coding RNAs that are 22–23 nucleotides in length. More than 2000 miRNA genes were identified [2]. The primary function of miRNAs is to regulate gene expression, each by binding to their specific messenger RNA (mRNA) through its 3’-untranslated region complementary sequence, which leads to degradation of the mRNA or blockage of translation [3].
miRNAs were first discovered in 1993 by Ambros and Ruvkun, who found the Lin-4 miRNA in Caenorhabditis elegans and revealed that it is the first gene in the pathways directing cell division and regulating the process of differentiation of hypodermal stem cells into skin cells [4]. Since then, thousands of miRNAs have been discovered in most living organisms, even in plants and viruses, and their roles in numerous functional processes such as apoptosis, immunity, and metabolism are now under further research [5].
The association between the different miRNAs’ expression and androgens has been confirmed in prostate and breast cancers [6,7,8]. Similarly, sexual miRNA expression panels in the brain have been identified in both diseased and normal vertebrates and non-vertebrates [9,10]. Studies have shown a potential effect of sex chromosomes on miRNA expression [9,11,12,13,14,15].
Sex steroids are mainly synthesized in the gonads. They are also formed in the central nervous system, hence the name “neurosteroids” [16]. Three primary estrogenic steroidal hormones have been identified: estradiol (E2), estrone (E1), and estriol (E3). These have pivotal roles in the processes of fertility, development, and homeostasis in most tissues, including the brain, breast, cardiovascular system, skin, lung, and reproductive tract, both in women and men [17,18,19,20].
Steroidal hormones regulate the expression of miRNA through nuclear receptor-mediated pathways [21,22]. E2, the primary circulating type of estrogen in premenopausal women, is formed from cholesterol in the granulosa cells in the ovary due to the stimulating effect of luteinizing hormone (LH) [23], while E1 is the primary estrogen in women after menopause. E1 is synthesized primarily in adipose tissue from the adrenal androgens in a reaction catalyzed by aromatase enzymes [24]. These enzymes are found in neurons and astrocytes [25]. The relationship between miRNAs and estrogens in the context of neurodegenerative disease requires further understanding. Here, we comprehensively review miRNAs with a focus on miR-29 and miR-9′s functions and highlight their interactions with estrogens and various neurodegenerative disorders.
2. Estrogens in Brain Development and Function
Sex hormones like estradiol, testosterone, and progesterone regulate miRNA expression by binding to nuclear receptors to alter gene expression directly or indirectly [26]. The brain is both a producer and a target of steroids. Astrocytes form E2 through an aromatase-instigated reaction in many human brain regions, such as the hippocampus, thalamus, and hypothalamus. In stressful conditions (e.g., epilepsy), the estrogen-synthesizing enzyme expression was found to be distorted [27]. Besides, there are widespread estrogen receptors in brain areas, such as the hippocampus, and a high level of responsiveness in these regions to sex hormones, including E2 [28]. Accordingly, E2 is expected to be significantly involved in many physiological processes in the brain, including cognition and neural development [29].
The previous literature documented that E2 synthesized in the brain has a core function in synaptogenesis, adjusts the spine’s density, and endorses long-term potentiation [30]. Such observations might have paved the way for some therapeutic applications of E2. For instance, a study demonstrated that E2 injections after a stroke or a brain injury serve neuroprotection, stimulate neurogenesis to generate replacement neurons, and decrease cell death by enhancing neurotrophin support and suppressing pro-inflammatory pathways [31]. Moreover, ovariectomy of middle-aged female rats altered the transcriptome of the hippocampus, which led to a reduction in neurogenesis [32].
A previous study has reported that E2 and progesterone treatment of female rats that had undergone ovariectomy improved spatial reference memory and exerted an antidepressant effect through a rise in the turnover rate of monoamines like serotonin. With age, neurogenesis rates decline in rodents and humans due to a decline in the dividing cell number and an associated decline in gonadal hormone levels [33,34].
One of the most relevant functions of E2 in the brain is neuroprotection. Many studies have suggested the possible role of E2 in protecting the neural tissue against degenerative events observed in pathologies like Parkinson’s and Alzheimer’s [35]. This can be seen in the late onset of symptoms of Parkinson’s in women. In another context, more severe symptoms of Alzheimer’s were found in postmenopausal women compared to men of the same age group [36,37,38]. However, some contradictory results suggest that the cumulative estrogenic effect during a woman’s fertile life could significantly reduce the severity of the neurodegeneration-related symptoms, thus preserving the observed sex-dependent differences in disease prevalence and life expectancy, which persist even after the age of menopause [39].
3. miRNA Processing
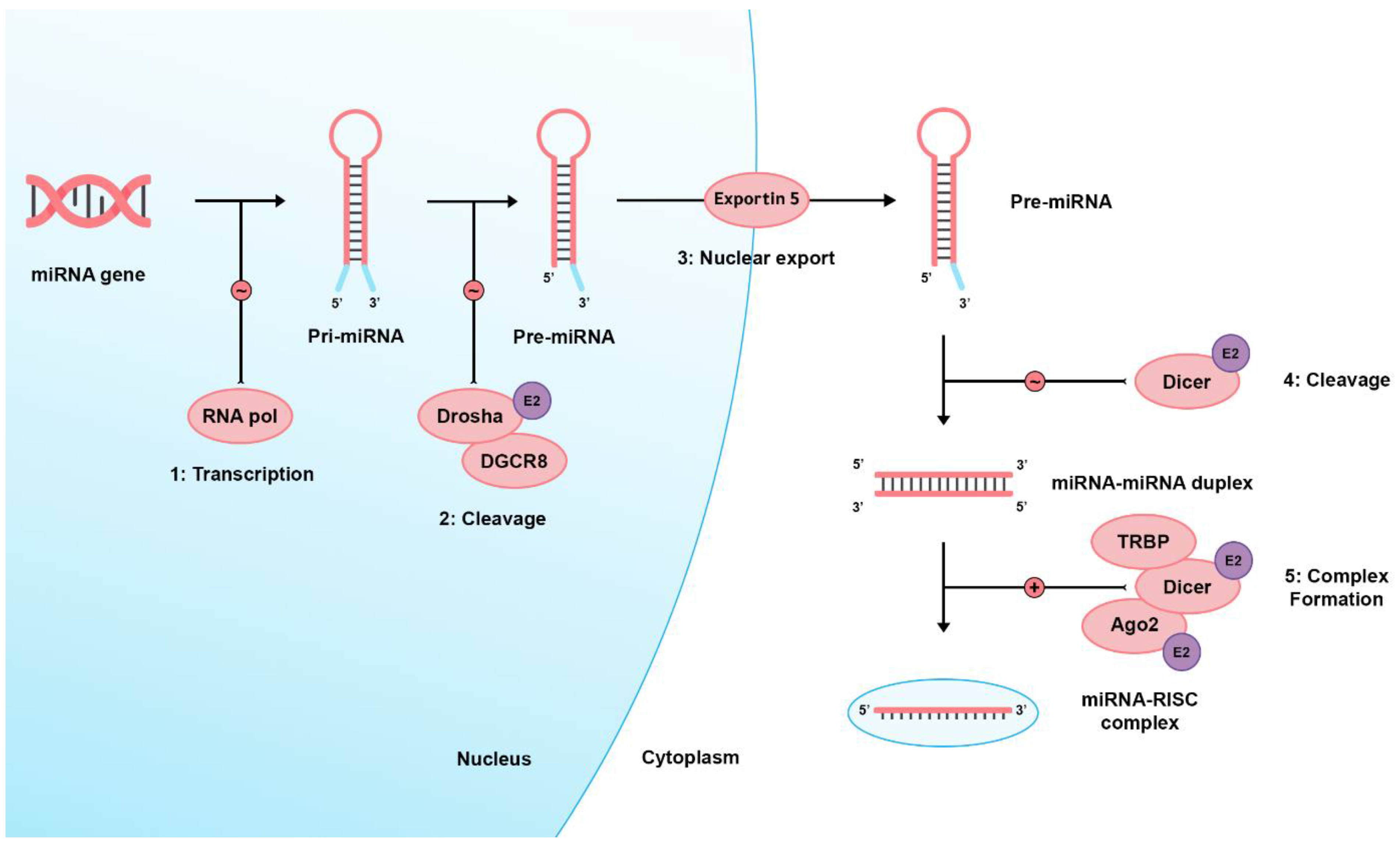
There are two pathways regarding miRNA biogenesis: the canonical and noncanonical pathways [40]. In the dominant canonical pathway, miRNA processing starts with RNA polymerase II, transcribing miRNA from the genome into long precursor molecules called primary miRNAs (pri-miRNA). The pri-miRNAs are cleaved by the ribonuclease (RNAase) III family enzyme, Drosha, into precursor miRNAs (pre-miRNAs). Afterward, they form a microprocessor complex by binding with the DiGeorge Syndrome Critical Region 8 (DGCR8), an RNA-binding protein. Then, it is transported to the cytoplasm through active transport via the Ran-GTP-dependent dsRNA-binding protein, Exportin-5, to be processed by the Dicer enzyme (an RNAase III endonuclease), the transactivating response RNA-binding protein (TRBP), and the Kinase R-activating protein. Mature miRNAs are then formed and loaded on an RNA-induced silencing complex (RISC) consisting of Dicer, TRBP, the Kinase R-activating protein, and Argonaute 2 (Ago2). Eventually, they bind to the 3′UTR of target genes, inducing mRNA degradation or translational inhibition [1,41,42,43,44,45]. E2 signaling can alter Ago2, Drosha, and Dicer’s expression [46]. Figure 1 shows a brief and simplified illustration of miRNA biogenesis.
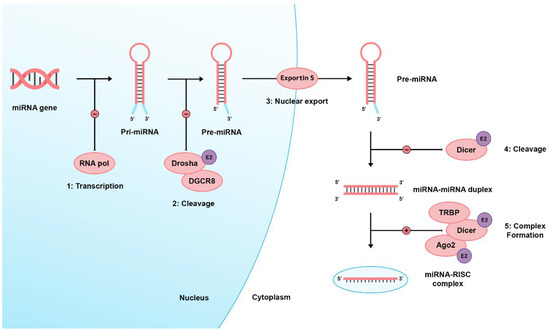
Figure 1.
Shows the miRNA biogenesis. Firstly, a large primary (pri-) miRNA is transcribed by RNA polymerase II then pri-miRNA is cleaved by the RNase III enzyme Drosha and coupled with the microprocessor complex subunit DGCR8 to produce pre-miRNA. Pre-miRNAs range from 70 to 90 nucleotides in length and contain a stem-loop structure for their transport to the cell cytoplasm by Exportin-5. In the cytoplasm, this hairpin structure is cleaved by the RNase III enzyme (Dicer), producing the double-stranded miRNA: miRNA duplex. Eventually, a mature miRNA strand is incorporated into a miRNA-associated RNA-induced silencing complex (miRISC), which targets complementary mRNA sequences, producing its cellular effects via transcriptional cleavage or repression. RNA pol: RNA polymerase II, DGCR8: DiGeorge Syndrome Critical Region 8, TRBP: transactivating response RNA-binding protein, Ago2: Argonaute 2, E2: Estrogen 2 signaling, ∼ icon: catalytic process, + icon: complex formation.
4. miRNA Action
miRISC and target mRNA have been detected in several organelles, including lysosomes, rough endoplasmic reticulum, stress granules, processing (P)-bodies, early/late endosomes, trans-Golgi network multivesicular bodies, mitochondria, and the nucleus. Their gene regulatory activity keeps gene expression in a steady state. The availability of miRNAs and their target mRNAs contributes to electing specific genes to be regulated [47]. About 60% of all protein-coding genes in mammals are under miRNA regulation [48]. The dysregulation of miRNAs leads to the disarray of their target genes. Such disruption is clinically correlated to pathological conditions, including neurological disorders, cardiovascular diseases, and tumors [49]. Cytoplasmic miRISC can diffuse through the cytosol or undergo shuttling via microtubules. After combining with polysomes in the cytosol, miRISC can cause mRNA decline, inhibit translation initiation, or improve translational activation. The rough endoplasmic reticulum is usually where translation inhibition occurs through the interaction with target mRNA [50]. Similar processes take place in other organelles [e.g., P-bodies, mitochondria] with the aid of organelle-specific proteins (TNRC6A in the case of P-bodies) [51,52]. The next steps involve the decoupling with Ago2, and the deadenylation and decapping of target mRNA, which have been posited to take place in multivesicular bodies and endosomes [53]. miRISC then has two options: go back to the cytosol or degrade after being shuttled to lysosomes.
miRNA’s actions are not exclusively autocrine; they can have paracrine and endocrine effects with the help of exosomes and other extracellular vesicles [54]. Exosomes are 40 to 100-nanometer membrane-bound vesicles containing different growth factors, cytoplasmic proteins, cytokines, lipids, and nucleic acids (including miRNAs). They circulate in the lymph and blood to dispense those molecules to tissues, thereby mediating the paracrine and endocrine effects. Exosomes can affect tumor progression via factors that promote proliferation, invasion, drug resistance, and metastasis. In this context, circulating miRNAs in exosomes control cellular mechanisms in recipient cells [55]. Extracellular vesicles were generally shown to facilitate miRNA’s endocrine effects related to immunity [56,57,58], endothelial physiology [59], cell differentiation [60,61], synaptic plasticity [62], neural trauma response [63], mitophagy [64], and follicular maturation [65].
5. miRNA General Regulation
Hormonal regulation of miRNAs can occur at the molecular and cellular levels, both directly and indirectly. It occurs directly when hormones bind to the promoter elements of miRNAs and indirectly when they regulate post-transcriptional processing proteins’ signaling [66]. A tightly balanced and regulated ratio between Drosha and DGCR8 is always achieved in the microprocessor complex: DGCR8 stabilizes Drosha cleaves and inactivates it, generating a stringent feedback loop [67].
Estrogen receptor α (ERα) interacts with p68 to inhibit Drosha complex formation, leading to suppressing pri-miRNA processing [68]. Dicer processes pre-miRNA to mature miRNA. Dicer activity is enhanced by MAPK-phosphorylation (mitogen-activated protein kinase) of TRBP (TAR RNA-binding protein), which promotes miRNA processing and suppresses the maturation of specific tumor suppressor miRNAs under hypoxic conditions [69].
Ago2 is the catalytic protein part of the miRISC complex, serving as an additional regulator of mRNA stability [48]. Ago2 is regulated at the transcriptional and post-transcriptional levels by multiple mechanisms, including arginine methylation, Met1-linked linear ubiquitination, DDX21, and E-cadherin [70,71,72,73]. Nucleolin is a multifunctional protein found mainly in the nucleolus. It has roles in transcription, ribosome biogenesis, DNA replication, chromatin remodeling, apoptosis, and micropinocytosis [74]. Nucleolin works as a transcription factor and as a regulator through its interactions with other proteins. It promotes the maturation of specific miRNAs implicated in carcinogenesis cells [75].
6. Regulation of miRNA by Estrogens
Estrogens act on ERα and ERβ both on genomic and membrane-initiated, non-genomic levels. This review focuses on the genomic activity of ERs; the effects of estrogens on non-genomic levels have been reviewed sufficiently elsewhere [76,77]. ERs belong to the superfamily of nuclear receptors (NRs) [78]. Any NR has a generally outlined structural organization made up of six regions named A-F, each of which has a specific function that varies significantly amongst different receptors’ subtypes. They also exhibit a common structure, with four similar functional domains: the NH2-terminal regulatory domain (the A/B domain), which is quite variable compared to the highly conserved DNA binding domain (C domain), the hinge region (D domain), and the E domain, which contains a C terminal ligand-binding domain (LBD) [79,80].
E2 has a high affinity for binding to the LBD of ERs. Such binding forces ER to become more specific, as a transcription factor, to DNA regions that contain the following sequence: 5’-GGTCAnnnTGACC-3′, the so-called “estrogen-responsive element” (ERE) [81]. A group of factors called “pioneer factors” enhances the binding ERs to the ERE. These include FoxA1, PBX, TLE1, AP2g, and GATA3 [82,83,84]. They remodel compacted and condensed chromatin into open chromatin. The compaction of chromatin has been proven to stand as a barrier against transcription. Remodeling of the condensed chromatin increases the accessibility to the DNA and increases target gene transcription by allowing RNA polymerase II to act on DNA and form miRNAs [85].
7. miRNA and the Nervous System
The last step in the processing of miRNAs is the Dicer-mediated cleavage. This final step is considered the interface link between miRNA and its regulators (e.g., E2 and androgens) on one hand and neurons on the other. Any disruption in the dicer-mediated cleavage of pre-miRNAs will affect mature miRNA production, which could propagate a negative effect on both cortical neurogenesis and the embryonic development of the nervous system [86]. Several studies concluded that the disruption of mature miRNAs would probably affect the function of the nervous system by causing a reduction in neural progenitor cells’ proliferation, a delay in the cell cycle, a disturbance in neural migration, an induction of apoptosis by activation of caspase 3, the stimulation of astrocyte differentiation, and the inhibition of neuronal differentiation [86,87,88,89].
Four highly conserved and distinguishable processes constitute the general term “neurogenesis” or “neural maturation”. The first is axonal and neurite outgrowth, followed by dendritic growth and synaptogenesis, neuronal maturation, and finally, cell apoptosis. Each process is regulated by a specific miRNA or a subset of miRNAs [90].
7.1. miR-9 Activity and Regulation by Estrogens (Table 1)
The activity of E2 and its action on miRNA are mainly mediated by the two ER subtypes (ERα, ERβ), both of which are expressed in the ventral hippocampus [91,92]. E2 participates in miRNA processing through ERα’s interaction with the Dorsha complex. Many studies discussed the regulation of different miRNAs by steroid hormones. They showed that miR-9 expression could be regulated by E2. The availability of binding sites for ER within miR-9 promoters increases the possibility that E2 directly impacts miR-9 transcription [93].
In a study performed to assess the correlation between miR-9 and breast cancer progression, the authors observed high variability in miR-9 expression in human samples; this variability was strongly related to the expression of ERs, where ERα binds to Drosha during the maturation of miRNAs [94]. The ingenuity pathway analysis (IPA) further delineated a regulatory loop of miR-9-5p expression influenced by ERs [95,96,97].
The Nuclear Factor kappa-light-chain-enhancer of activated B-cells activates miR-9. Then, a negative feedback loop occurs where miR-9-5p directly targets the mRNA of ERα [93]. Another study assessed E2 regulation for miRNAs, especially in the brain. E2 played a critical role in expressing mature functional miRNA in the brain, which was age- and brain region-specific. The regulation of miR-9 expression by E2 exhibited similar age changes; the three-month-old rats showed that E2 reduces miR-9 expression levels in the dorsal hippocampus, with miR-9-3p expression increased in the same region. However, in rats aged 18 months or more, E2 continued reducing miR-9 expression, while the controlling effect of E2 on miR-9-3p could not be detected anymore [98].
Sirtuin 1 (SIRT1) is a protein deacetylase that has a role in aging, and is a target gene of E2 [99,100]. It partakes in many vital functions of the brain, such as energy balance, memory processing, and neuroprotection [101,102]. miR-9 expression is inversely correlated with that of SIRT1, in which the 3′ UTR region complements with the miR-9 sequence [103].

Table 1.
Molecules interacting with miR-9 and estrogen.
Table 1.
Molecules interacting with miR-9 and estrogen.
| Molecule | Interaction with miR-9 | Reference |
|---|---|---|
| NF-Kβ | 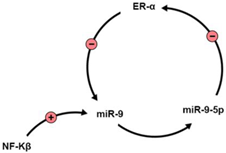 | [93] |
| NEFH | miR-9 binds to the 3′UTR region of NEFH. miR-9-5p is significantly downregulated in ALS. | [104] |
| Serine Palmitoyltransferase [SPT] | SPT regulates Aβ in Alzheimer’s disease, and is correlated with miR-9 serum and cortical levels. | [105] |
| SIRT1 | Negative correlation with miR-9 levels. SIRT1 gene is a target of E2. | [99,100,101,102,103] |
| E2 |
| [95,96,97,98] |
| REST/CoREST | They regulate, and are regulated by miR-9. In HD, mutant huntingtin fails to regulate REST/CoREST, disrupting miR-9 activity. | [97,106,107] |
7.2. miR-29 Family and Neurodegenerative Disorders (Table 2)
Principally, miRNAs have significant roles in gene expression regulation in different brain diseases and were suggested as therapeutic approaches in a wide range of human diseases because of their ability to deliver factors that would enhance repair mechanisms [108,109,110].
The miR-29 family is a group of miRNAs that is highly involved in developing mature and fully functional neurons. The miR-29 family consists of four transcripts; miR-29a, miR-29b-1, miR-29b-2 and miR-29c. Human miR-29a and miR-29b-1 are transcribed from chromosome 7, while miR-29b-2 and miR-29c are transcribed from chromosome 1. Both miR-29b-1 and 2 have the same mature sequence [111].
The first of the three sequences in this group is miR-29a. It has been suggested that miR-29a can be of great importance in the process of neural maturation, based on the observed upregulation of miR-29a gene expression during the differentiation of neural stem cells (NSCs). Infecting the NSCs with lentiviral-mediated miR-29a was associated with a significant increase in microtubule-associated protein 2 positive neurons, as well as a significant reduction in astrocytes. This is mediated through targeting the phosphatase and tensin homolog, commonly known as PTEN, which plays a vital role in the differentiation and growth of NSCs, making its deletion an essential step in optimizing neural maturation [112,113,114,115].
Other direct targets for miR-29a have been proposed, such as the expression of the protein Doublecortin, which restricts axonal branching [116]. By inhibiting this protein when it is overly expressed in mice’s cortical neurons, miR-29a increased the rates of axon branching in these cells [117]. Moreover, the widespread death of neurons was observed in mice following the knockdown of miR-29a using a chemically engineered oligonucleotide, i.e., locked nucleic acid antagomir (blockmir) called LNA29a/c. It is believed that the effect of miR-29a knockdown might be propagated through multiple mediators, like the voltage-dependent anion channel 1, a pro-apoptotic mediator that is inhibited by miR-29a [118,119]. miR-29b has been suggested as a rescue factor for neuronal cells, as it silences the pro-apoptotic BH3-only gene family [120]. In principle, miRNAs have significant roles in gene expression regulation in different brain diseases and were suggested as therapeutic approaches in a wide range of human diseases because of their ability to deliver factors that would enhance repair mechanisms.
The expression of miRNAs in embryonic and adult brains is controversial. It has been suggested that the miR-29 family is not expressed in the embryonic brain. However, further investigation showed that miR-29b is over-expressed in NSCs, and regulates embryonic proliferation and neurogenesis by targeting T-cell factor-mediated inhibitors, and the Wnt/β-catenin signaling pathway [121,122]. It was suggested that sex chromosomes might control miRNAs in neurodegenerative disorders [12,123]. In the brains of patients with neurological diseases such as Alzheimer’s disease (AD) or schizophrenia, only a few human studies have assessed sex differences in miRNA expression [124,125].

Table 2.
Molecules interacting with miR-29.
Table 2.
Molecules interacting with miR-29.
| Molecule | Interaction with miR-29 | Reference |
|---|---|---|
| Doublecortin | miR-29a targets doublecortin expression, reducing axonal branching | [116] |
| Voltage-dependent anion channel 1 | miR-29a regulates this molecule, reducing apoptosis | [118,119] |
| BH3-only family | miR-29b silences this proapoptotic gene family | [120] |
| Wnt/βcatenin signaling | miR-29b regulates this pathway, hereby affecting embryonic proliferation and neurogenesis | [121,122] |
| BACE | Negative correlation with miR-29a, miR-29b and miR-29c-3p expression in AD | [126] |
| DNA methyltransferase III beta (DNMT3B) | miR-29c acts on DNMT3B to reduce BDNF levels in AD invitro models. | [127] |
| Parkinsonism-associated Deglycase (PARK7) | miR-29 regulates this molecule. It is also regulated by estrogens and is implicated in PD pathology. | [128,129] |
| BcI2L2 | BcI2L2 gene, which is antiapoptotic, is regulated by miR-29b. | [111,130,131,132] |
7.3. Alzheimer’s Disease
The progression of AD is characterized by the accumulation of plaques made of short β-amyloid peptides. These peptides emerge from proteolytic cleavage of the β-amyloid precursor protein (APP) by a β-secretase known as the β-site APP-cleaving enzyme (BACE) [133,134]. It has been observed that miR-29a, miR-29b and miR-29c-3p have low expression levels in AD with abnormally high levels of BACE [126]. Despite the low expression levels, the miR-29 clad were found to be abnormally high in the CSF of two cohorts of AD patients [135,136], achieving 89% sensitivity and 70% specificity for the miR-29a [135], making it a potential candidate for future AD biomarker research [137]. However, some potentially contradicting evidence found low CSF miR-29c levels in AD patients. These were found to be linked to the decreased levels of BDNF expression, an effect that was posited to be mediated by DNA methyltransferase 3 through some in vitro experiments [127]. Besides, estrogens are essential in AD pathogenesis since they might decrease β-amyloid protein levels as a neuroprotective mechanism against the disease [125,138]. Pan and colleagues showed that estradiol’s neuroprotective effect against amyloid pathology in AD is potentially mediated by the miR-106b-5p/TXNIP axis in a neuroblastoma cell line [139], while another study showed that estradiol treatment on ovarectomized AD model mice slowed the pathological conformational changes of tau. This change was potentiated by the decreased expression of miR-218 [140]. Sedghi et al. showed that when levels of miR-29a are raised it produces a neuroprotective effect in the peripheral blood mononuclear cells of AD patients [141]. While this effect was realized using klotho and linagliptin treatment on isolated cells, it paves the road for testing other interventions with similar effects on the implicated pathways.
7.4. Parkinson Disease
MiR-29a, miR-29b-1, and miR-29b-2 are over-expressed in the brains of Parkinson’s disease (PD) patients [142], while miR-29b-2-5p was under-expressed in another study [143]. miR-29 has significant roles in PD pathology, such as apoptosis and neuronal survival, motor function tuning, the immune response (by regulating T1 helper cells), and genetic modulation [144,145]. Inhibiting miR-29 expression in the mouse brain caused massive rates of neuronal death, especially in the hippocampus and cerebellum [119]. miR-29 also targets Parkinsonism Associated Deglycase, which is thought to be regulated by androgens and estrogens [128,129]. We believe that the role of miR-29 in the pathophysiology of PD needs further investigation in the clinical setting [144,146]. As for other miRNAs, Liu and colleagues used computational methods to study the differentially-expressed genes in PD patients. By constructing a miRNA-mRNA regulatory network, they found that has-miR-142 was the most vital miRNA in the network, carrying out its effects on GNAQ, TMTC2, KYNU, and BEND2 [147].
7.5. Huntington’s Disease
Pre-clinical studies done on HD showed a consistent downregulation of miR-9 and miR-29; this effect was constant across different animal and animal cell models [148]. One of miR-9/9*’s functions is regulating the function of REST/CoREST in the cell. Because REST/CoREST targets miR-9/9* as well, and because mutant huntingtin in HD patients fails to regulate the levels of REST in the cell, levels of REST become unusually high in the neurons of HD patients. Ultimately, this leads to the misregulation of gene expression in those neurons [106,107]. Another study found that miR-9*, but not miR-9 or miR-29b, were significantly downregulated in the peripheral leukocytes of HD patients compared to controls [97]. However, the level of miR-9* was not correlated to the UHDRS score in HD patients.
7.6. Other Brain Diseases
Owing to their potent antifibrotic effect, miR-29s have been strongly linked to the pathophysiology and management of stroke. At the infarction region, miR-29b levels were notably lower than in other healthy brain areas. Studies showed significant improvement in patients’ outcomes when utilizing a special approach to deliver a miR-29b mimic to combat the stroke-induced loss of miR-29b. It was also postulated that miR-29b levels were significantly increased during ischemic brain injuries. A recent study found that the overexpression of miR-29b reduced the neuronal cell death observed during brain ischemia. Conversely, the downregulation of miR-29b was accompanied by increased rates of neuronal cell death. It was proposed that miR-29b carries its action through inhibiting the expression of the antiapoptotic gene BcI2L2. Therefore, when BcI2L2 is overexpressed, it leads to increased neuronal cell survival [111,130,131,132].
The modulation of post-stroke-induced neurogenesis by miR-9, and Histone Deacetylase 4 was documented [149]. In brain neurogenic areas, miR-9 is widely expressed, which has ramifications for the differentiation of embryonic, and adult progenitor cells [150]. The effect of miR-9 in reducing neuronal apoptosis after ischemic stroke was investigated in a few studies [151,152]. Wei et al. found that miR-9 directly targets Bcl2l11, and that altering miR-9 causes changes to Bcl2l11 protein levels [151]. The authors concluded that miR-9 targets Bcl2l11 to facilitate cell apoptosis. Another study showed that miR-9 upregulation promoted neuronal survival, and regeneration following ischemic stroke [153]. The authors put forth a key mechanism by which HDAC4 inactivation positively regulated the expression of miR-9 and ameliorated ischemic insult in vitro. This may contribute to overcoming the hurdles hampering the adoption of miRNA-based therapeutics for ischemic stroke. A recent study by Wang et al. showed that, compared to healthy people, early-stage acute ischemic stroke patients had higher blood levels of miR-9-5p. Serum levels of miR-9-5p were significantly correlated with patient prognosis, with high concentrations being linked to unfavorable patient outcomes [154].
In gliomas, Wu et al. documented that elevated miR-9 expression might be involved in tumour progression, and proposed miR-9 as a valuable marker to predict the clinical prognosis of glioma patients, particularly those with advanced subtypes [155]. Another study by Tan et al. [156] showed that miR-9 is substantially expressed in glioma cells. miR-9 suppressed glioma cell growth, and increased migration by directly targeting cAMP-response element binding protein as well as neurofibromin 1. Further studies also documented that miR-9 plays an important role in glioma pathogenesis, and might be used as a prognostic marker, and possible therapeutic target for gliomas [157,158]. On the other hand, miR-9 overexpression significantly inhibited the growth of U87 glioma cells. The growth limitation was primarily attributable to the stimulation of apoptosis, which coincided with an increase in the Bax/Bcl-2 ratio [159]. miR-9 overexpression caused cell cycle arrest in U87 glioma cells at the G2/M checkpoint, and miR-9 inhibited the migration as well as invasion of U87 glioma cells [159]. In their study, Shi et al. investigated miR-29s as a tumor suppressor in gliomas using 187 human glioma specimens as well as 20 nontumoral brain specimens. The authors documented that when glioma grade and the Ki-67 index rose, the expression of miR-29a/b/c substantially decreased [160]. This highlights the importance of miR-29a/b/c and TRAF4 in predicting prognosis and their possible therapeutic role in malignant gliomas.
Research has gradually moved away from safeguarding neurons and toward investigating the combined effects of the neurovascular unit on traumatic brain injury (TBI) in recent years as a result of the ongoing investigation of the pathological process [161,162,163]. This shift highlights the necessity of angiogenesis for neurological functional recovery after TBI. By triggering the Hedgehog pathway, and enhancing the p-AKT expression, Wu et al. documented that miR-9-5p stimulates angiogenesis in the injured cerebral cortex. This highlights the possibility that miR-9-5p may be a useful therapeutic target for TBI. Mu et al. provided a new function for miR-29a in controlling NSC development and neurite outgrowth. They also provided a potential theoretical base for how NSC migration contributes to brain growth as well as damage repair [164]. By inhibiting NLRP3 expression and activation, miR-29a-5p mimics have been documented to protect against TBI-induced enhanced endothelial cell permeability, and BBB dysfunction [165].
8. Future Directions
Limited in silico research highlighted the possibility of interactions between the miR-29 family and estrogens in a neurodegenerative context [166,167]; such interactions have yet to be verified experimentally. Most of the studies reviewed here have been carried out in cell cultures, rats, or mice. There is a need to use other animal models, including non-human primates, to gain more reliable insights into the mechanisms highlighted in this review. Recent research showcased the prevalence of sex differences among neurodegeneration patients; such differences have been partly attributed to sex hormones, including estrogens [168,169,170], but have yet to be connected to miRNAs in a clinical setting. While the use of estrogen replacement therapy for treating Alzheimer’s [171], Parkinson’s [172,173,174,175], and Huntington’s [176] diseases was thoroughly tested in previous work, more precise therapies targeting miR-9/29 pathways need to be explored.
9. Conclusions
The miRNA–estrogen interaction has been studied thoroughly over the past few decades. The hypothesis that this relation is somehow related to developing some major neurological disorders constitutes a starting point for further clinical research within the same subject area. A proper understanding of the nature of this tri-axial system will contribute to the development of more advanced diagnostic techniques and more convenient treatment options in a variety of neurological disorders. We summarized the different regulation routes that estrogens impose on miRNAs, specifically miR-9 and miR-29. Furthermore, we overviewed the multiple effects for altering miRNAs’ transcription, maturation, and gene expression in different neural tissues. We then examined the possible benefits for this hormone-induced transcriptional modification in some neurological conditions using the currently available evidence.
Author Contributions
Conceptualization, M.A.E., G.M.A. and M.S.; methodology, M.A.E. and A.M.; visualization and figures, A.M., A.-H.A.G. and A.W.-A.; resources, A.-H.A.G., S.A., A.W.-A. and A.M.; writing—original draft preparation, all authors; writing—review and editing, M.A.E., A.M. and M.S.; supervision, G.M.A. and M.S.; funding acquisition, A.M.A. and G.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was funded by the Institutional Fund Projects under grant no. (IFPDP-66-22).
Institutional Review Board Statement
This article does not contain any studies with human or animal subjects performed by any of the authors.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research work was funded by the Institutional Fund Projects under grant no. (IFPDP-66-22). Therefore, the authors gratefully acknowledge technical and financial support from the Ministry of Education and Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, Saudi Arabia.
Conflicts of Interest
We all confirm that this article’s content has no financial or personal conflict of interest.
References
- Carthew, R.W.; Sibtgeuner, E.J. Origins and Mechanisms of miRNAs and siRNAs Richard. Cell 2010, 136, 642–655. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Wadaa Allah, A.; Yahya, M.; Elsaeidy, K.S.; Alkanj, S.; Hamam, K.; El-Saady, M.; Ebada, M.A. Clinical assessment of miRNA-23b as a prognostic factor for various carcinomas: A systematic review and meta-analysis. Meta Gene 2020, 24, 100651. [Google Scholar] [CrossRef]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A. The lin-4 microRNA. Cell Cycle 2014, 13, 1060–1061. [Google Scholar] [CrossRef]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- O’Kelly, F.; Marignol, L.; Meunier, A.; Lynch, T.H.; Perry, A.S.; Hollywood, D. MicroRNAs as putative mediators of treatment response in prostate cancer. Nat. Rev. Urol. 2012, 9, 397–407. [Google Scholar] [CrossRef]
- Lin, S.-C.; Kao, C.-Y.; Lee, H.-J.; Creighton, C.J.; Ittmann, M.M.; Tsai, S.-J.; Tsai, S.Y.; Tsai, M.-J. Dysregulation of miRNAs-COUP-TFII-FOXM1-CENPF axis contributes to the metastasis of prostate cancer. Nat. Commun. 2016, 7, 11418. [Google Scholar] [CrossRef]
- Rao, Y.S.; Pak, T.R. microRNAs and the adolescent brain: Filling the knowledge gap. Neurosci. Biobehav. Rev. 2016, 70, 313–322. [Google Scholar] [CrossRef]
- Khan, D.; Dai, R.; Ansar Ahmed, S. Sex differences and estrogen regulation of miRNAs in lupus, a prototypical autoimmune disease. Cell. Immunol. 2015, 294, 70–79. [Google Scholar] [CrossRef]
- Bizuayehu, T.T.; Babiak, J.; Norberg, B.; Fernandes, J.M.O.; Johansen, S.D.; Babiak, I. Sex-Biased miRNA Expression in Atlantic Halibut (Hippoglossus hippoglossus) Brain and Gonads. Sex. Dev. 2012, 6, 257–266. [Google Scholar] [CrossRef]
- Morgan, C.P.; Bale, T.L. Early prenatal stress epigenetically programs dysmasculinization in second-generation offspring via the paternal lineage. J. Neurosci. 2011, 31, 11748–11755. [Google Scholar] [CrossRef]
- Rao, Y.S.; Shults, C.L.; Pinceti, E.; Pak, T.R. Prolonged ovarian hormone deprivation alters the effects of 17β-estradiol on microRNA expression in the aged female rat hypothalamus. Oncotarget 2015, 6, 36965–36983. [Google Scholar] [CrossRef]
- Siegel, C.; Li, J.; Liu, F.; Benashski, S.E.; McCullough, L.D. miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proc. Natl. Acad. Sci. USA 2011, 108, 11662–11667. [Google Scholar] [CrossRef]
- Pak, T.R.; Rao, Y.S.; Prins, S.A.; Mott, N.N. An emerging role for microRNAs in sexually dimorphic neurobiological systems. Pflugers Arch. Eur. J. Physiol. 2013, 465, 655–667. [Google Scholar] [CrossRef]
- Mellon, S.H.; Griffin, L.D. Neurosteroids: Biochemistry and clinical significance. Trends Endocrinol. Metab. 2002, 13, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.K.; Jones, C.K.; Newhouse, P.A. The Role of Estrogen in Brain and Cognitive Aging. Neurother. J. Am. Soc. Exp. Neurother. 2019, 16, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef] [PubMed]
- Iorga, A.; Cunningham, C.M.; Moazeni, S.; Ruffenach, G.; Umar, S.; Eghbali, M. The protective role of estrogen and estrogen receptors in cardiovascular disease and the controversial use of estrogen therapy. Biol. Sex Differ. 2017, 8, 33. [Google Scholar] [CrossRef]
- Rothenberger, N.J.; Somasundaram, A.; Stabile, L.P. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef]
- Klinge, C.M. miRNAs and estrogen action. Trends Endocrinol. Metab. 2012, 23, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen action: Receptors, transcripts, cell signaling, and non-coding RNAs in normal physiology and disease. Mol. Cell. Endocrinol. 2015, 418 Pt 3, 191–192. [Google Scholar] [CrossRef] [PubMed]
- Jaubert, A.-M.; Mehebik-Mojaat, N.; Lacasa, D.; Sabourault, D.; Giudicelli, Y.; Ribière, C. Nongenomic estrogen effects on nitric oxide synthase activity in rat adipocytes. Endocrinology 2007, 148, 2444–2452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gasperino, J. Gender is a risk factor for lung cancer. Med. Hypotheses 2011, 76, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, M.F.; Cambiasso, M.J.; Holschbach, M.A.; Cabrera, R. Oestrogens and Progestagens: Synthesis and Action in the Brain. J. Neuroendocrinol. 2016, 28, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.W.-F.; Shah, K.; Brosens, J.J. The diversity of sex steroid action: The role of micro-RNAs and FOXO transcription factors in cycling endometrium and cancer. J. Endocrinol. 2012, 212, 13–25. [Google Scholar] [CrossRef]
- Azcoitia, I.; Yague, J.G.; Garcia-Segura, L.M. Estradiol synthesis within the human brain. Neuroscience 2011, 191, 139–147. [Google Scholar] [CrossRef]
- Hara, Y.; Waters, E.M.; McEwen, B.S.; Morrison, J.H. Estrogen effects on cognitive and synaptic health over the lifecourse. Physiol. Rev. 2015, 95, 785–807. [Google Scholar] [CrossRef]
- Hojo, Y.; Murakami, G.; Mukai, H.; Higo, S.; Hatanaka, Y.; Ogiue-Ikeda, M.; Ishii, H.; Kimoto, T.; Kawato, S. Estrogen synthesis in the brain--role in synaptic plasticity and memory. Mol. Cell. Endocrinol. 2008, 290, 31–43. [Google Scholar] [CrossRef]
- Vierk, R.; Glassmeier, G.; Zhou, L.; Brandt, N.; Fester, L.; Dudzinski, D.; Wilkars, W.; Bender, R.A.; Lewerenz, M.; Gloger, S.; et al. Aromatase inhibition abolishes LTP generation in female but not in male mice. J. Neurosci. 2012, 32, 8116–8126. [Google Scholar] [CrossRef]
- Brown, C.M.; Suzuki, S.; Jelks, K.A.B.; Wise, P.M. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin. Reprod. Med. 2009, 27, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Sárvári, M.; Kalló, I.; Hrabovszky, E.; Solymosi, N.; Liposits, Z. Ovariectomy Alters Gene Expression of the Hippocampal Formation in Middle-Aged Rats. Endocrinology 2017, 158, 69–83. [Google Scholar] [CrossRef]
- Kiss, A.; Delattre, A.M.; Pereira, S.I.R.; Carolino, R.G.; Szawka, R.E.; Anselmo-Franci, J.A.; Zanata, S.M.; Ferraz, A.C. 17β-estradiol replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Behav. Brain Res. 2012, 227, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Moraga, A.; Pradillo, J.M.; García-Culebras, A.; Palma-Tortosa, S.; Ballesteros, I.; Hernández-Jiménez, M.; Moro, M.A.; Lizasoain, I. Aging increases microglial proliferation, delays cell migration, and decreases cortical neurogenesis after focal cerebral ischemia. J. Neuroinflamm. 2015, 12, 1. [Google Scholar] [CrossRef]
- Ebada, M.A.; Alkanj, S.; Ebada, M.; Abdelkarim, A.H.; Diab, A.; Aziz, M.A.E.; Soliman, A.M.; Fayed, N.; Bahbah, E.I.; Negida, A. Safety and Efficacy of Levetiracetam for the Management of Levodopa- Induced Dyskinesia in Patients with Parkinson’s Disease: A Systematic Review. CNS Neurol. Disord. Drug Targets 2019, 18, 317–325. [Google Scholar] [CrossRef]
- Li, R.; Singh, M. Sex differences in cognitive impairment and Alzheimer’s disease. Front. Neuroendocrinol. 2014, 35, 385–403. [Google Scholar] [CrossRef] [PubMed]
- Kowal, S.L.; Dall, T.M.; Chakrabarti, R.; Storm, M.V.; Jain, A. The current and projected economic burden of Parkinson’s disease in the United States. Mov. Disord. 2013, 28, 311–318. [Google Scholar] [CrossRef]
- Rocca, W.A.; Bower, J.H.; Maraganore, D.M.; Ahlskog, J.E.; Grossardt, B.R.; De Andrade, M.; Melton, L.J. Increased risk of parkinsonism in women who underwent oophorectomy before menopause. Neurology 2008, 70, 200–209. [Google Scholar] [CrossRef]
- Shadyab, A.H.; MacEra, C.A.; Shaffer, R.A.; Jain, S.; Gallo, L.C.; Gass, M.L.S.; Waring, M.E.; Stefanick, M.L.; LaCroix, A.Z. Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: The Women’s Health Initiative. Menopause 2017, 24, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Kim, V.N. Processing of intronic microRNAs. EMBO J. 2007, 26, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. miRNAs regulated by estrogens, tamoxifen, and endocrine disruptors and their downstream gene targets. Mol. Cell. Endocrinol. 2015, 418, 273–297. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef]
- Denli, A.M.; Tops, B.B.J.; Plasterk, R.H.A.; Ketting, R.F.; Hannon, G.J. Processing of primary microRNAs by the Microprocessor complex. Nature 2004, 432, 231–235. [Google Scholar] [CrossRef]
- Okada, C.; Yamashita, E.; Lee, S.J.; Shibata, S.; Katahira, J.; Nakagawa, A.; Yoneda, Y.; Tsukihara, T. A High-Resolution Structure of the Pre-microRNA Nuclear Export Machinery. Science 2009, 326, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Ling, H.; Doki, Y.; Mori, M.; Calin, G. MicroRNA Processing and Human Cancer. J. Clin. Med. 2015, 4, 1651–1667. [Google Scholar] [CrossRef]
- Gregory, R.I.; Yan, K.-P.; Amuthan, G.; Chendrimada, T.; Doratotaj, B.; Cooch, N.; Shiekhattar, R. The Microprocessor complex mediates the genesis of microRNAs. Nature 2004, 432, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Bottini, S.; Hamouda-Tekaya, N.; Mategot, R.; Zaragosi, L.-E.; Audebert, S.; Pisano, S.; Grandjean, V.; Mauduit, C.; Benahmed, M.; Barbry, P.; et al. Post-transcriptional gene silencing mediated by microRNAs is controlled by nucleoplasmic Sfpq. Nat. Commun. 2017, 8, 1189. [Google Scholar] [CrossRef]
- Finnegan, E.F.; Pasquinelli, A.E. MicroRNA biogenesis: Regulating the regulators. Crit. Rev. Biochem. Mol. Biol. 2013, 48, 51–68. [Google Scholar] [CrossRef]
- Abe, M.; Bonini, N.M. MicroRNAs and neurodegeneration: Role and impact. Trends Cell Biol. 2013, 23, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Barman, B.; Bhattacharyya, S.N. mRNA Targeting to Endoplasmic Reticulum Precedes Ago Protein Interaction and MicroRNA (miRNA)-mediated Translation Repression in Mammalian Cells. J. Biol. Chem. 2015, 290, 24650–24656. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Takahashi, T.; Suzawa, M.; Miyakawa, T.; Nagasawa, T.; Ming, Y.; Tanokura, M.; Ui-Tei, K. Control of the localization and function of a miRNA silencing component TNRC6A by Argonaute protein. Nucleic Acids Res. 2015, 43, 9856–9873. [Google Scholar] [CrossRef] [PubMed]
- Barrey, E.; Saint-Auret, G.; Bonnamy, B.; Damas, D.; Boyer, O.; Gidrol, X. Pre-microRNA and Mature microRNA in Human Mitochondria. PLoS ONE 2011, 6, e20220. [Google Scholar] [CrossRef]
- Bose, M.; Barman, B.; Goswami, A.; Bhattacharyya, S.N. Spatiotemporal Uncoupling of MicroRNA-Mediated Translational Repression and Target RNA Degradation Controls MicroRNP Recycling in Mammalian Cells. Mol. Cell. Biol. 2017, 37, e00464-16. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015, 22, 34–45. [Google Scholar] [CrossRef]
- Ouyang, Y.; Mouillet, J.-F.; Coyne, C.B.; Sadovsky, Y. Review: Placenta-specific microRNAs in exosomes–good things come in nano-packages. Placenta 2014, 35, S69–S73. [Google Scholar] [CrossRef] [PubMed]
- Ekström, K.; Valadi, H.; Sjöstrand, M.; Malmhäll, C.; Bossios, A.; Eldh, M.; Lötvall, J. Characterization of mRNA and microRNA in human mast cell-derived exosomes and their transfer to other mast cells and blood CD34 progenitor cells. J. Extracell. Vesicles 2012, 1, 18389. [Google Scholar] [CrossRef]
- Fernández-Messina, L.; Gutiérrez-Vázquez, C.; Rivas-García, E.; Sánchez-Madrid, F.; de la Fuente, H. Immunomodulatory role of microRNAs transferred by extracellular vesicles. Biol. Cell 2015, 107, 61–77. [Google Scholar] [CrossRef]
- Yáñez-Mó, M.; Siljander, P.R.M.; Andreu, Z.; Zavec, A.B.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 1–60. [Google Scholar] [CrossRef]
- Xu, J.-F.; Yang, G.-H.; Pan, X.-H.; Zhang, S.-J.; Zhao, C.; Qiu, B.-S.; Gu, H.-F.; Hong, J.-F.; Cao, L.; Chen, Y.; et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS ONE 2014, 9, e114627. [Google Scholar] [CrossRef]
- Forterre, A.; Jalabert, A.; Chikh, K.; Pesenti, S.; Euthine, V.; Granjon, A.; Errazuriz, E.; Lefai, E.; Vidal, H.; Rome, S. Myotube-derived exosomal miRNAs downregulate Sirtuin1 in myoblasts during muscle cell differentiation. Cell Cycle 2014, 13, 78–89. [Google Scholar] [CrossRef]
- Gao, Y.-N.; Zhang, Y.-Q.; Wang, H.; Deng, Y.-L.; Li, N.-M. A New Player in Depression: MiRNAs as Modulators of Altered Synaptic Plasticity. Int. J. Mol. Sci. 2022, 23, 4555. [Google Scholar] [CrossRef]
- Simeoli, R.; Montague, K.; Jones, H.R.; Castaldi, L.; Chambers, D.; Kelleher, J.H.; Vacca, V.; Pitcher, T.; Grist, J.; Al-Ahdal, H.; et al. Exosomal cargo including microRNA regulates sensory neuron to macrophage communication after nerve trauma. Nat. Commun. 2017, 8, 1778. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [PubMed]
- Santonocito, M.; Vento, M.; Guglielmino, M.R.; Battaglia, R.; Wahlgren, J.; Ragusa, M.; Barbagallo, D.; Borzì, P.; Rizzari, S.; Maugeri, M.; et al. Molecular characterization of exosomes and their microRNA cargo in human follicular fluid: Bioinformatic analysis reveals that exosomal microRNAs control pathways involved in follicular maturation. Fertil. Steril. 2014, 102, 1751–1761.e1. [Google Scholar] [CrossRef]
- Macias, S.; Michlewski, G.; Cáceres, J.F. Hormonal regulation of microRNA biogenesis. Mol. Cell 2009, 36, 172–173. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Pedersen, J.S.; Kwon, S.C.; Belair, C.D.; Kim, Y.-K.; Yeom, K.-H.; Yang, W.-Y.; Haussler, D.; Blelloch, R.; Kim, V.N. Posttranscriptional crossregulation between Drosha and DGCR8. Cell 2009, 136, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, K.; Fujiyama, S.; Ito, S.; Ueda, T.; Murata, T.; Naitou, M.; Takeyama, K.-I.; Minami, Y.; O’Malley, B.W.; Kato, S. Maturation of microRNA is hormonally regulated by a nuclear receptor. Mol. Cell 2009, 36, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Paroo, Z.; Ye, X.; Chen, S.; Liu, Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell 2009, 139, 112–122. [Google Scholar] [CrossRef]
- Hu, P.; Zhao, H.; Zhu, P.; Xiao, Y.; Miao, W.; Wang, Y.; Jin, H. Dual regulation of Arabidopsis AGO2 by arginine methylation. Nat. Commun. 2019, 10, 844. [Google Scholar] [CrossRef]
- Li, J.-N.; Sun, H.-L.; Wang, M.-Y.; Chen, P.-S. E-cadherin Interacts with Posttranslationally-Modified AGO2 to Enhance miRISC Activity. Front. Cell Dev. Biol. 2021, 9, 671244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, X.; Guo, Y.; Chen, R.; He, J.; Li, L.; Qiang, Z.; Yang, Q.; Liu, X.; Huang, C.; et al. Hypoxia regulates overall mRNA homeostasis by inducing Met(1)-linked linear ubiquitination of AGO2 in cancer cells. Nat. Commun. 2021, 12, 5416. [Google Scholar] [CrossRef]
- Gong, M.; Zhang, X.; Wang, Y.; Mao, G.; Ou, Y.; Wei, C.; Hu, X.; Xiang, S. DDX21 interacts with nuclear AGO2 and regulates the alternative splicing of SMN2. Biosci. Biotechnol. Biochem. 2021, 85, 272–279. [Google Scholar] [CrossRef]
- Bates, P.J.; Laber, D.A.; Miller, D.M.; Thomas, S.D.; Trent, J.O. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009, 86, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zou, C.; Zou, C.; Han, Z.; Xiao, H.; Wei, H.; Wang, W.; Zhang, L.; Zhang, X.; Tang, Q.; et al. MicroRNA-25 functions as a potential tumor suppressor in colon cancer by targeting Smad7. Cancer Lett. 2013, 335, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Eyster, K.M. The Estrogen Receptors: An Overview from Different Perspectives. In Estrogen Receptors; Methods in Molecular Biology; Humana Press: New York, NY, USA, 2016; Volume 1366, pp. 1–10. [Google Scholar] [CrossRef]
- Maglich, J.M.; Sluder, A.; Guan, X.; Shi, Y.; Mckee, D.D.; Carrick, K.; Kamdar, K.; Willson, T.M.; Moore, J.T. Comparison of complete nuclear receptor sets from the human, Caenorhabditis elegans and Drosophila genomes. Genome Biol. 2001, 2, research0029.1. [Google Scholar] [CrossRef]
- Klinge, C. Estrogen Regulation of MicroRNA Expression. Curr. Genom. 2009, 10, 169–183. [Google Scholar] [CrossRef]
- Ruff, M.; Gangloff, M.; Wurtz, J.M.; Moras, D. Estrogen receptor transcription and transactivation: Structure-function relationship in DNA- and ligand-binding domains of estrogen receptors. Breast Cancer Res. 2000, 2, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogen Receptor Interaction with Estrogen Response Elements. Nucleic Acids Res. 2001, 29, 2905–2919. [Google Scholar] [CrossRef]
- Perkins, S.M.; Bales, C.; Vladislav, T.; Althouse, S.; Miller, K.D.; Sandusky, G.; Badve, S.; Nakshatri, H. TFAP2C expression in breast cancer: Correlation with overall survival beyond 10 years of initial diagnosis. Breast Cancer Res. Treat. 2015, 152, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Jeselsohn, R.; Pereira, R.; Hollingsworth, E.F.; Creighton, C.J.; Li, F.; Shea, M.; Nardone, A.; De Angelis, C.; Heiser, L.M.; et al. FOXA1 overexpression mediates endocrine resistance by altering the ER transcriptome and IL-8 expression in ER-positive breast cancer. Proc. Natl. Acad. Sci. USA 2016, 113, E6600–E6609. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Wang, X.; Sheng, D.; Zhou, L.; Liu, Y.; Xu, C.; Liu, S.; Zhang, J. Cooperativity of co-factor NR2F2 with Pioneer Factors GATA3, FOXA1 in promoting ERα function. Theranostics 2019, 9, 6501–6516. [Google Scholar] [CrossRef] [PubMed]
- Magnani, L.; Lupien, M. Chromatin and epigenetic determinants of estrogen receptor alpha (ESR1) signaling. Mol. Cell. Endocrinol. 2014, 382, 633–641. [Google Scholar] [CrossRef]
- McLoughlin, H.S.; Fineberg, S.K.; Ghosh, L.L.; Tecedor, L.; Davidson, B.L. Dicer is required for proliferation, viability, migration and differentiation in corticoneurogenesis. Neuroscience 2012, 223, 285–295. [Google Scholar] [CrossRef]
- Andersson, T.; Rahman, S.; Sansom, S.N.; Alsiö, J.M.; Kaneda, M.; Smith, J.; O’Carroll, D.; Tarakhovsky, A.; Livesey, F.J. Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs. PLoS ONE 2010, 5, e13453. [Google Scholar] [CrossRef]
- Kanellopoulou, C. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes Dev. 2005, 19, 489–501. [Google Scholar] [CrossRef]
- Martino, S.; Di Girolamo, I.; Orlacchio, A.; Datti, A.; Orlacchio, A. MicroRNA implications across neurodevelopment and neuropathology. J. Biomed. Biotechnol. 2009, 2009, 654346. [Google Scholar] [CrossRef]
- Nampoothiri, S.S.; Rajanikant, G.K. Decoding the ubiquitous role of microRNAs in neurogenesis. Mol. Neurobiol. 2017, 54, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Power, B.D.; Mitrofanis, J. Distribution of estrogen receptor β immunoreactivity in the rat central nervous system. J. Comp. Neurol. 2001, 436, 64–81. [Google Scholar] [CrossRef]
- Shima, N.; Yamaguchi, Y.; Yuri, K. Distribution of estrogen receptor β mRNA-containing cells in ovariectomized and estrogen-treated female rat brain. Anat. Sci. Int. 2003, 78, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Barbano, R.; Pasculli, B.; Rendina, M.; Fontana, A.; Fusilli, C.; Copetti, M.; Castellana, S.; Valori, V.M.; Morritti, M.; Graziano, P.; et al. Stepwise analysis of MIR9 loci identifies MIR-9-5p to be involved in Oestrogen regulated pathways in breast cancer patients. Sci. Rep. 2017, 7, 45283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Raz, L.; Wang, R.; Han, D.; De Sevilla, L.; Yang, F.; Vadlamudi, R.K.; Brann, D.W. Estrogen Attenuates Ischemic Oxidative Damage via an Estrogen Receptor -Mediated Inhibition of NADPH Oxidase Activation. J. Neurosci. 2009, 29, 13823–13836. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.Y.; Deatherage, D.E.; Rodriguez, B.A.T.; Liyanarachchi, S.; Weng, Y.I.; Zuo, T.; Liu, J.; Cheng, A.S.L.; Huang, T.H.M. Xenoestrogen-induced epigenetic repression of microRNA-9-3 in breast epithelial cells. Cancer Res. 2009, 69, 5936–5945. [Google Scholar] [CrossRef] [PubMed]
- Pillai, M.M.; Gillen, A.E.; Yamamoto, T.M.; Kline, E.; Brown, J.; Flory, K.; Hesselberth, J.R.; Kabos, P. HITS-CLIP reveals key regulators of nuclear receptor signaling in breast cancer. Breast Cancer Res. Treat. 2014, 146, 85–97. [Google Scholar] [CrossRef]
- Chang, K.H.; Wu, Y.R.; Chen, C.M. Down-regulation of miR-9∗ in the peripheral leukocytes of Huntington’s disease patients. Orphanet J. Rare Dis. 2017, 12, 1–5. [Google Scholar] [CrossRef]
- Rao, Y.S.; Mott, N.N.; Wang, Y.; Chung, W.C.J.; Pak, T.R. MicroRNAs in the aging female brain: A putative mechanism for age-specific estrogen effects. Endocrinology 2013, 154, 2795–2806. [Google Scholar] [CrossRef]
- Lin, S.J.; Defossez, P.A.; Guarente, L. Requirement of NAD and SIR2 for life-span extension by calorie restriction in saccharomyces cerevisiae. Science 2000, 289, 2126–2128. [Google Scholar] [CrossRef]
- Longo, V.D.; Kennedy, B.K. Sirtuins in Aging and Age-Related Disease. Cell 2006, 126, 257–268. [Google Scholar] [CrossRef]
- Gao, J.; Wang, W.Y.; Mao, Y.W.; Gräff, J.; Guan, J.S.; Pan, L.; Mak, G.; Kim, D.; Su, S.C.; Tsai, L.H. A novel pathway regulates memory and plasticity via SIRT1 and miR-134. Nature 2010, 466, 1105–1109. [Google Scholar] [CrossRef]
- Michan, S.; Li, Y.; Chou, M.M.-H.; Parrella, E.; Ge, H.; Long, J.M.; Allard, J.S.; Lewis, K.; Miller, M.; Xu, W.; et al. SIRT1 Is Essential for Normal Cognitive Function and Synaptic Plasticity. J. Neurosci. 2010, 30, 9695–9707. [Google Scholar] [CrossRef] [PubMed]
- Schonrock, N.; Humphreys, D.T.; Preiss, T.; Götz, J. Target gene repression mediated by miRNAs miR-181c and miR-9 both of which are down-regulated by amyloid-β. J. Mol. Neurosci. 2012, 46, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Campos-Melo, D.; Hawley, Z.C.E.; Strong, M.J. Dysregulation of human NEFM and NEFH mRNA stability by ALS-linked miRNAs. Mol. Brain 2018, 11, 43. [Google Scholar] [CrossRef] [PubMed]
- Geekiyanage, H.; Upadhye, A.; Chan, C. Inhibition of serine palmitoyltransferase reduces Aβ and tau hyperphosphorylation in a murine model: A safe therapeutic strategy for Alzheimer’s disease. Neurobiol. Aging 2013, 34, 2037–2051. [Google Scholar] [CrossRef]
- Packer, A.N.; Xing, Y.; Harper, S.Q.; Jones, L.; Davidson, B.L. The Bifunctional microRNA miR-9/miR-9* Regulates REST and CoREST and Is Downregulated in Huntington’s Disease. J. Neurosci. 2008, 28, 14341–14346. [Google Scholar] [CrossRef]
- Giusti, S.A.; Vogl, A.M.; Brockmann, M.M.; Vercelli, C.A.; Rein, M.L.; Trümbach, D.; Wurst, W.; Cazalla, D.; Stein, V.; Deussing, J.M.; et al. MicroRNA-9 controls dendritic development by targeting REST. Elife 2014, 3, e02755. [Google Scholar] [CrossRef]
- Guo, X.; Liu, T.; Zhao, D.; Wang, X.; Liu, D.; He, Y.; Shan, C.; Kong, Y.; Hu, W.; Tao, B.; et al. FGF18 protects against 6-hydroxydopamine-induced nigrostriatal damage in a rat model of Parkinson’s disease. Neuroscience 2017, 356, 229–241. [Google Scholar] [CrossRef]
- Urbich, C.; Kuehbacher, A.; Dimmeler, S. Role of microRNAs in vascular diseases, inflammation, and angiogenesis. Cardiovasc. Res. 2008, 79, 581–588. [Google Scholar] [CrossRef]
- Juźwik, C.A.; Drake, S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Khanna, S.; Rink, C.; Ghoorkhanian, R.; Gnyawali, S.; Heigel, M.; Wijesinghe, D.S.; Chalfant, C.E.; Chan, Y.C.; Banerjee, J.; Huang, Y.; et al. Loss of miR-29b following Acute Ischemic Stroke Contributes to Neural Cell Death and Infarct Size. J. Cereb. Blood Flow Metab. 2013, 33, 1197–1206. [Google Scholar] [CrossRef]
- Shi, Z.; Zhou, H.; Lu, L.; Pan, B.; Wei, Z.; Liu, J.; Li, J.; Yuan, S.; Kang, Y.; Liu, L.; et al. MicroRNA-29a regulates neural stem cell neuronal differentiation by targeting PTEN. J. Cell. Biochem. 2018, 119, 5813–5820. [Google Scholar] [CrossRef]
- Chen, L.; Guo, D. The functions of tumor suppressor PTEN in innate and adaptive immunity. Cell. Mol. Immunol. 2017, 14, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Waite, K.A.; Eng, C. Protean PTEN: Form and function. Am. J. Hum. Genet. 2002, 70, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Gregorian, C.; Nakashima, J.; Belle, J.L.; Ohab, J.; Kim, R.; Liu, A.; Smith, K.B.; Groszer, M.; Garcia, A.D.; Sofroniew, M.V.; et al. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J. Neurosci. 2009, 29, 1874–1886. [Google Scholar] [CrossRef] [PubMed]
- Bilimoria, P.M.; De La Torre-Ubieta, L.; Ikeuchi, Y.; Becker, E.B.E.; Reiner, O.; Bonni, A. A JIP3-regulated GSK3β/DCX signaling pathway restricts axon branching. J. Neurosci. 2010, 30, 16766–16776. [Google Scholar] [CrossRef]
- Li, H.; Mao, S.; Wang, H.; Zen, K.; Zhang, C.; Li, L. MicroRNA-29a modulates axon branching by targeting doublecortin in primary neurons. Protein Cell 2014, 5, 160–169. [Google Scholar] [CrossRef]
- Bargaje, R.; Gupta, S.; Sarkeshik, A.; Park, R.; Xu, T.; Sarkar, M.; Halimani, M.; Roy, S.S.; Yates, J.; Pillai, B. Identification of novel targets for miR-29a using miRNA proteomics. PLoS ONE 2012, 7, e43243. [Google Scholar] [CrossRef]
- Roshan, R.; Shridhar, S.; Sarangdhar, M.A.; Banik, A.; Chawla, M.; Garg, M.; Singh, V.P.; Pillai, B. Brain-specific knockdown of miR-29 results in neuronal cell death and ataxia in mice. RNA 2014, 20, 1287–1297. [Google Scholar] [CrossRef]
- Kole, A.J.; Swahari, V.; Hammond, S.M.; Deshmukh, M. miR-29b is activated during neuronal maturation and targets BH3-only genes to restrict apoptosis. Genes Dev. 2011, 25, 125–130. [Google Scholar] [CrossRef]
- Shin, J.; Shin, Y.; Oh, S.M.; Yang, H.; Yu, W.J.; Lee, J.P.; Huh, S.O.; Lee, S.H.; Suh, Y.H.; Chung, S.; et al. MiR-29b controls fetal mouse neurogenesis by regulating ICAT-mediated Wnt/β-catenin signaling. Cell Death Dis. 2014, 5, e1473. [Google Scholar] [CrossRef]
- Landgraf, P.; Rusu, M.; Sheridan, R.; Sewer, A.; Iovino, N.; Aravin, A.; Pfeffer, S.; Rice, A.; Kamphorst, A.O.; Landthaler, M.; et al. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007, 129, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.P.; Bale, T.L. Sex differences in microRNA regulation of gene expression: No smoke, just miRs. Biol. Sex Differ. 2012, 3, 22. [Google Scholar] [CrossRef] [PubMed]
- Piscopo, P.; Bellenghi, M.; Manzini, V.; Crestini, A.; Pontecorvi, G.; Corbo, M.; Ortona, E.; Carè, A.; Confaloni, A. A sex perspective in neurodegenerative diseases: Micrornas as possible peripheral biomarkers. Int. J. Mol. Sci. 2021, 22, 4423. [Google Scholar] [CrossRef]
- Ashraf, G.M.; Ebada, M.A.; Suhail, M.; Ali, A.; Uddin, M.S.; Bilgrami, A.L.; Perveen, A.; Husain, A.; Tarique, M.; Hafeez, A.; et al. Dissecting Sex-Related Cognition between Alzheimer’s Disease and Diabetes: From Molecular Mechanisms to Potential Therapeutic Strategies. Oxid. Med. Cell. Longev. 2021, 2021, 4572471. [Google Scholar] [CrossRef] [PubMed]
- Rademakers, R.; Rovelet-Lecrux, A. Recent insights into the molecular genetics of dementia. Trends Neurosci. 2009, 32, 451–461. [Google Scholar] [CrossRef]
- Yang, G.; Song, Y.; Zhou, X.; Deng, Y.; Liu, T.; Weng, G.; Yu, D.; Pan, S. DNA methyltransferase 3, a target of microRNA-29c, contributes to neuronal proliferation by regulating the expression of brain-derived neurotrophic factor. Mol. Med. Rep. 2015, 12, 1435–1442. [Google Scholar] [CrossRef]
- Fernández-Santiago, R.; Iranzo, A.; Gaig, C.; Serradell, M.; Fernández, M.; Tolosa, E.; Santamaría, J.; Ezquerra, M. MicroRNA association with synucleinopathy conversion in rapid eye movement behavior disorder. Ann. Neurol. 2015, 77, 895–901. [Google Scholar] [CrossRef]
- Zhou, Z.; Ribas, V.; Rajbhandari, P.; Drew, B.G.; Moore, T.M.; Fluitt, A.H.; Reddish, B.R.; Whitney, K.A.; Georgia, S.; Vergnes, L.; et al. Estrogen receptor α protects pancreatic β-cells from apoptosis by preserving mitochondrial function and suppressing endoplasmic reticulum stress. J. Biol. Chem. 2018, 293, 4735–4751. [Google Scholar] [CrossRef]
- Shi, G.; Liu, Y.; Liu, T.; Yan, W.; Liu, X.; Wang, Y.; Shi, J.; Jia, L. Upregulated miR-29b promotes neuronal cell death by inhibiting Bcl2L2 after ischemic brain injury. Exp. Brain Res. 2012, 216, 225–230. [Google Scholar] [CrossRef]
- Mott, J.L.; Kobayashi, S.; Bronk, S.F.; Gores, G.J. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007, 26, 6133–6140. [Google Scholar] [CrossRef]
- Ma, Y.-H.; Deng, W.-J.; Luo, Z.-Y.; Jing, J.; Pan, P.-W.; Yao, Y.-B.; Fang, Y.-B.; Teng, J.-F. Inhibition of microRNA-29b suppresses oxidative stress and reduces apoptosis in ischemic stroke. Neural Regen. Res. 2022, 17, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Ouerdane, Y.; El-Nahas, Z.S.; Ouerdane, F.; Hamam, K.M.; Ebada, M.A. Gut–Brain Axis in Alzheimer’s Disease: Interplay Between Cholecystokinin, Dysbiosis, and Brain-Derived Neurotrophic Factor. In Current Thoughts on Dementia; Springer Nature: Singapore, 2022; pp. 311–353. [Google Scholar]
- Benmelouka, A.; Sherif, A.M.; Ebada, M.A. A Review of the Relationship Between Gut Microbiota and Memory. In Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease; Springer: Singapore, 2019; pp. 151–165. [Google Scholar] [CrossRef]
- Kiko, T.; Nakagawa, K.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. MicroRNAs in plasma and cerebrospinal fluid as potential markers for Alzheimer’s disease. J. Alzheimers. Dis. 2014, 39, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Jäkel, L.; Bruinsma, I.B.; Claassen, J.A.; Kuiperij, H.B.; Verbeek, M.M. MicroRNA-29a Is a Candidate Biomarker for Alzheimer’s Disease in Cell-Free Cerebrospinal Fluid. Mol. Neurobiol. 2016, 53, 2894–2899. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Wang, L. Inflamma-MicroRNAs in Alzheimer’s Disease: From Disease Pathogenesis to Therapeutic Potentials. Front. Cell. Neurosci. 2021, 15, 785433. [Google Scholar] [CrossRef] [PubMed]
- Vest, R.S.; Pike, C.J. Gender, sex steroid hormones, and Alzheimer’s disease. Horm. Behav. 2013, 63, 301–307. [Google Scholar] [CrossRef]
- Pan, Q.; Guo, K.; Xue, M.; Tu, Q. Estradiol exerts a neuroprotective effect on SH-SY5Y cells through the miR-106b-5p/TXNIP axis. J. Biochem. Mol. Toxicol. 2021, 35, e22861. [Google Scholar] [CrossRef]
- Guglielmotto, M.; Manassero, G.; Vasciaveo, V.; Venezia, M.; Tabaton, M.; Tamagno, E. Estrogens Inhibit Amyloid-β-Mediated Paired Helical Filament-Like Conformation of Tau Through Antioxidant Activity and miRNA 218 Regulation in hTau Mice. J. Alzheimers. Dis. 2020, 77, 1339–1351. [Google Scholar] [CrossRef]
- Bonetti, L.; Bruzzone, S.E.P.; Sedghi, N.A.; Haumann, N.T.; Paunio, T.; Kantojarvi, K.; Kliuchko, M.; Vuust, P.; Brattico, E. Brain predictive coding processes are associated to COMT gene Val158Met polymorphism. Neuroimage 2021, 233, 117954. [Google Scholar] [CrossRef]
- Tatura, R.; Kraus, T.; Giese, A.; Arzberger, T.; Buchholz, M.; Höglinger, G.; Müller, U. Parkinson’s disease: SNCA-, PARK2-, and LRRK2- targeting microRNAs elevated in cingulate gyrus. Park. Relat. Disord. 2016, 33, 115–121. [Google Scholar] [CrossRef]
- Chatterjee, P.; Roy, D. Comparative analysis of RNA-Seq data from brain and blood samples of Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017, 484, 557–564. [Google Scholar] [CrossRef]
- Goh, S.Y.; Chao, Y.X.; Dheen, S.T.; Tan, E.-K.; Tay, S.S.-W. Role of MicroRNAs in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 5649. [Google Scholar] [CrossRef]
- Steiner, D.F.; Thomas, M.F.; Hu, J.K.; Yang, Z.; Babiarz, J.E.; Allen, C.D.C.; Matloubian, M.; Blelloch, R.; Ansel, K.M. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells. Immunity 2011, 35, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tang, Y.; Yu, M.; Wu, L.; Liu, F.; Ni, J.; Wang, Z.; Wang, J.; Fei, J.; Wang, W.; et al. Downregulation of blood serum microRNA 29 family in patients with Parkinson’s disease. Sci. Rep. 2017, 7, 5411. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, J.; Guan, T.; Yao, H.; Zhang, W.; Guan, Z.; Wang, Y. miRNAs and target genes in the blood as biomarkers for the early diagnosis of Parkinson’s disease. BMC Syst. Biol. 2019, 13, 10. [Google Scholar] [CrossRef]
- Martinez, B.; Peplow, P. V Altered microRNA expression in animal models of Huntington’s disease and potential therapeutic strategies. Neural Regen. Res. 2021, 16, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, S.S.; Fayaz, S.M.; Rajanikant, G.K. A Novel Five-Node Feed-Forward Loop Unravels miRNA-Gene-TF Regulatory Relationships in Ischemic Stroke. Mol. Neurobiol. 2018, 55, 8251–8262. [Google Scholar] [CrossRef]
- Coolen, M.; Katz, S.; Bally-Cuif, L. miR-9: A versatile regulator of neurogenesis. Front. Cell. Neurosci. 2013, 7, 220. [Google Scholar] [CrossRef]
- Wei, N.; Xiao, L.; Xue, R.; Zhang, D.; Zhou, J.; Ren, H.; Guo, S.; Xu, J. MicroRNA-9 Mediates the Cell Apoptosis by Targeting Bcl2l11 in Ischemic Stroke. Mol. Neurobiol. 2016, 53, 6809–6817. [Google Scholar] [CrossRef]
- Chen, S.; Wang, M.; Yang, H.; Mao, L.; He, Q.; Jin, H.; Ye, Z.-m.; Luo, X.-y.; Xia, Y.-p.; Hu, B. LncRNA TUG1 sponges microRNA-9 to promote neurons apoptosis by up-regulated Bcl2l11 under ischemia. Biochem. Biophys. Res. Commun. 2017, 485, 167–173. [Google Scholar] [CrossRef]
- Nampoothiri, S.S.; Rajanikant, G.K. miR-9 Upregulation Integrates Post-ischemic Neuronal Survival and Regeneration In Vitro. Cell. Mol. Neurobiol. 2019, 39, 223–240. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Fu, F.; Liu, J.; Sun, W.; Chen, Y. Diagnostic and prognostic value of serum miR-9-5p and miR-128-3p levels in early-stage acute ischemic stroke. Clinics 2021, 76, e2958. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, L.; Li, G.; Liu, H.; Fan, F.; Li, Z.; Li, Y.; Gao, G. Increased expression of microRNA-9 predicts an unfavorable prognosis in human glioma. Mol. Cell. Biochem. 2013, 384, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Wang, S.; Yang, B.; Zhu, L.; Yin, B.; Chao, T.; Zhao, J.; Yuan, J.; Qiang, B.; Peng, X. The CREB-miR-9 Negative Feedback Minicircuitry Coordinates the Migration and Proliferation of Glioma Cells. PLoS ONE 2012, 7, e49570. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, F.; Zhang, T.; Wang, W.; Xi, W.; Li, Y.; Zhang, D.; Huo, Y.; Zhang, J.; Yang, A.; et al. MiR-9 promotes tumorigenesis and angiogenesis and is activated by MYC and OCT4 in human glioma. J. Exp. Clin. Cancer Res. 2019, 38, 99. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Wei, W.; Zhang, Z.; He, C.; Yang, R.; Zhang, J.; Wu, Z.; Huang, Q.; Jiang, Q. Identification of MicroRNAs Associated with Glioma Diagnosis and Prognosis. Oncotarget 2017, 8, 26394–26403. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, L. MicroRNA-9 regulates the proliferation, migration and invasion of human glioma cells by targeting CDH1. J. B.U.ON. 2020, 25, 1091–1097. [Google Scholar]
- Shi, C.; Rao, C.; Sun, C.; Yu, L.; Zhou, X.; Hua, D.; Wang, R.; Luo, W.; Jiang, Z.; Zhou, J.; et al. miR-29s function as tumor suppressors in gliomas by targeting TRAF4 and predict patient prognosis. Cell Death Dis. 2018, 9, 1078. [Google Scholar] [CrossRef]
- Madeddu, P. Therapeutic angiogenesis and vasculogenesis for tissue regeneration. Exp. Physiol. 2005, 90, 315–326. [Google Scholar] [CrossRef]
- Ning, R.; Xiong, Y.; Mahmood, A.; Zhang, Y.; Meng, Y.; Qu, C.; Chopp, M. Erythropoietin promotes neurovascular remodeling and long-term functional recovery in rats following traumatic brain injury. Brain Res. 2011, 1384, 140–150. [Google Scholar] [CrossRef][Green Version]
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.C.; Wu, C.C.; Chuang, D.M.; Chiang, Y.H.; Chen, K.Y. Transplantation of mesenchymal stem cells overexpressing fibroblast growth factor 21 facilitates cognitive recovery and enhances neurogenesis in a mouse model of traumatic brain injury. J. Neurotrauma 2020, 37, 14–26. [Google Scholar] [CrossRef]
- Wu, J.; He, J.; Tian, X.; Li, H.; Wen, Y.; Shao, Q.; Cheng, C.; Wang, G.; Sun, X. Upregulation of miRNA-9-5p Promotes Angiogenesis after Traumatic Brain Injury by Inhibiting Ptch-1. Neuroscience 2020, 440, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Lu, Y.; Yuan, L.; Zhang, P.; Zou, D.; Wei, F.; Chen, X. MiR-29a-5p Alleviates Traumatic Brain Injury-(TBI-) Induced Permeability Disruption via Regulating NLRP3 Pathway. Dis. Markers 2021, 2021, 9556513. [Google Scholar] [CrossRef] [PubMed]
- Recabarren, D.; Alarcón, M. Gene networks in neurodegenerative disorders. Life Sci. 2017, 183, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Recabarren-Leiva, D.; Alarcón, M. New insights into the gene expression associated to amyotrophic lateral sclerosis. Life Sci. 2018, 193, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Nebel, R.A.; Aggarwal, N.T.; Barnes, L.L.; Gallagher, A.; Goldstein, J.M.; Kantarci, K.; Mallampalli, M.P.; Mormino, E.C.; Scott, L.; Yu, W.H.; et al. Understanding the impact of sex and gender in Alzheimer’s disease: A call to action. Alzheimers. Dement. 2018, 14, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, N.; Arif, T.B.; Shafaut, R.; Zakaria, F.; Fatima, S.Z.; Haque, I.U. Association between sex and Huntington’s disease: An updated review on symptomatology and prognosis of neurodegenerative disorders. Wien. Med. Wochenschr. 2022. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Parkinsons. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef]
- Henderson, V.W. Alzheimer’s disease: Review of hormone therapy trials and implications for treatment and prevention after menopause. J. Steroid Biochem. Mol. Biol. 2014, 142, 99–106. [Google Scholar] [CrossRef]
- A randomized pilot trial of estrogen replacement therapy in post-menopausal women with Parkinson’s disease. Parkinsonism Relat. Disord. 2011, 17, 757–760. [CrossRef]
- Nicoletti, A.; Arabia, G.; Pugliese, P.; Nicoletti, G.; Torchia, G.; Condino, F.; Morgante, L.; Quattrone, A.; Zappia, M. Hormonal replacement therapy in women with Parkinson disease and levodopa-induced dyskinesia: A crossover trial. Clin. Neuropharmacol. 2007, 30, 276–280. [Google Scholar] [CrossRef]
- Tsang, K.L.; Ho, S.L.; Lo, S.K. Estrogen improves motor disability in parkinsonian postmenopausal women with motor fluctuations. Neurology 2000, 54, 2292–2298. [Google Scholar] [CrossRef] [PubMed]
- Blanchet, P.J.; Fang, J.; Hyland, K.; Arnold, L.A.; Mouradian, M.M.; Chase, T.N. Short-term effects of high-dose 17beta-estradiol in postmenopausal PD patients: A crossover study. Neurology 1999, 53, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Koller, W.C.; Barr, A.; Biary, N. Estrogen treatment of dyskinetic disorders. Neurology 1982, 32, 547–549. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).