Reduced Age-Related Gray Matter Loss in the Orbitofrontal Cortex in Long-Term Meditators
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample and Brain Images
2.2. Data Processing and Volume Extraction
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gard, T.; Holzel, B.K.; Lazar, S.W. The potential effects of meditation on age-related cognitive decline: A systematic review. Ann. N. Y. Acad. Sci. 2014, 1307, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.S.Y.; Deng, K.; Wu, J.; Yan, J.H. Effects of Meditation and Mind-Body Exercises on Older Adults’ Cognitive Performance: A Meta-analysis. Gerontologist 2019, 59, e782–e790. [Google Scholar] [CrossRef] [PubMed]
- Pagnoni, G.; Cekic, M. Age effects on gray matter volume and attentional performance in Zen meditation. Neurobiol. Aging 2007, 28, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.W.; Kerr, C.E.; Wasserman, R.H.; Gray, J.R.; Greve, D.N.; Treadway, M.T.; McGarvey, M.; Quinn, B.T.; Dusek, J.A.; Benson, H.; et al. Meditation experience is associated with increased cortical thickness. Neuroreport 2005, 16, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Cherbuin, N.; Gaser, C. Estimating brain age using high-resolution pattern recognition: Younger brains in long-term meditation practitioners. NeuroImage 2016, 134, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Laneri, D.; Schuster, V.; Dietsche, B.; Jansen, A.; Ott, U.; Sommer, J. Effects of Long-Term Mindfulness Meditation on Brain’s White Matter Microstructure and its Aging. Front. Aging Neurosci. 2016, 7, 254. [Google Scholar] [CrossRef] [PubMed]
- Chetelat, G.; Mezenge, F.; Tomadesso, C.; Landeau, B.; Arenaza-Urquijo, E.; Rauchs, G.; Andre, C.; de Flores, R.; Egret, S.; Gonneaud, J.; et al. Reduced age-associated brain changes in expert meditators: A multimodal neuroimaging pilot study. Sci. Rep. 2017, 7, 10160. [Google Scholar] [CrossRef]
- Luders, E. Exploring age-related brain degeneration in meditation practitioners. Ann. N. Y. Acad. Sci. 2014, 1307, 82–88. [Google Scholar] [CrossRef]
- Luders, E.; Cherbuin, N.; Kurth, F. Forever Young(er): Potential age-defying effects of long-term meditation on gray matter atrophy. Front. Psychol. 2014, 5, 1551. [Google Scholar] [CrossRef]
- Kurth, F.; Cherbuin, N.; Luders, E. Promising Links between Meditation and Reduced (Brain) Aging: An Attempt to Bridge Some Gaps between the Alleged Fountain of Youth and the Youth of the Field. Front. Psychol. 2017, 8, 860. [Google Scholar] [CrossRef]
- Luders, E.; Toga, A.W.; Lepore, N.; Gaser, C. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. Neuroimage 2009, 45, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Miyashiro, S.; Yamada, Y.; Muta, T.; Ishikawa, H.; Abe, T.; Hori, M.; Oka, K.; Koshikawa, F.; Ito, E. Activation of the orbitofrontal cortex by both meditation and exercise: A near-infrared spectroscopy study. PLoS ONE 2021, 16, e0247685. [Google Scholar] [CrossRef] [PubMed]
- Manna, A.; Raffone, A.; Perrucci, M.G.; Nardo, D.; Ferretti, A.; Tartaro, A.; Londei, A.; Del Gratta, C.; Belardinelli, M.O.; Romani, G.L. Neural correlates of focused attention and cognitive monitoring in meditation. Brain Res. Bull. 2010, 82, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, S.E.; Suero, J.; Barros, A.; Gonzalez-Mora, J.L.; Rubia, K. Increased Grey Matter Associated with Long-Term Sahaja Yoga Meditation: A Voxel-Based Morphometry Study. PLoS ONE 2016, 11, e0150757. [Google Scholar] [CrossRef] [PubMed]
- Denburg, N.L.; Cole, C.A.; Hernandez, M.; Yamada, T.H.; Tranel, D.; Bechara, A.; Wallace, R.B. The orbitofrontal cortex, real-world decision making, and normal aging. Ann. N. Y. Acad. Sci. 2007, 1121, 480–498. [Google Scholar] [CrossRef] [PubMed]
- Pieperhoff, P.; Homke, L.; Schneider, F.; Habel, U.; Shah, N.J.; Zilles, K.; Amunts, K. Deformation field morphometry reveals age-related structural differences between the brains of adults up to 51 years. J. Neurosci. 2008, 28, 828–842. [Google Scholar] [CrossRef] [PubMed]
- Terribilli, D.; Schaufelberger, M.S.; Duran, F.L.; Zanetti, M.V.; Curiati, P.K.; Menezes, P.R.; Scazufca, M.; Amaro, E., Jr.; Leite, C.C.; Busatto, G.F. Age-related gray matter volume changes in the brain during non-elderly adulthood. Neurobiol. Aging 2011, 32, 354–368. [Google Scholar] [CrossRef]
- Ziegler, G.; Dahnke, R.; Jancke, L.; Yotter, R.A.; May, A.; Gaser, C. Brain structural trajectories over the adult lifespan. Hum. Brain Mapp. 2012, 33, 2377–2389. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Rohlfing, T.; Rosenbloom, M.J.; Chu, W.; Colrain, I.M.; Sullivan, E.V. Variation in longitudinal trajectories of regional brain volumes of healthy men and women (ages 10 to 85 years) measured with atlas-based parcellation of MRI. NeuroImage 2013, 65, 176–193. [Google Scholar] [CrossRef]
- Fjell, A.M.; Walhovd, K.B.; Fennema-Notestine, C.; McEvoy, L.K.; Hagler, D.J.; Holland, D.; Brewer, J.B.; Dale, A.M. One-year brain atrophy evident in healthy aging. J. Neurosci. 2009, 29, 15223–15231. [Google Scholar] [CrossRef]
- Fjell, A.M.; Westlye, L.T.; Amlien, I.; Espeseth, T.; Reinvang, I.; Raz, N.; Agartz, I.; Salat, D.H.; Greve, D.N.; Fischl, B.; et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb. Cortex 2009, 19, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Henssen, A.; Zilles, K.; Palomero-Gallagher, N.; Schleicher, A.; Mohlberg, H.; Gerboga, F.; Eickhoff, S.B.; Bludau, S.; Amunts, K. Cytoarchitecture and probability maps of the human medial orbitofrontal cortex. Cortex 2016, 75, 87–112. [Google Scholar] [CrossRef] [PubMed]
- Wojtasik, M.; Bludau, S.; Eickhoff, S.B.; Mohlberg, H.; Gerboga, F.; Caspers, S.; Amunts, K. Cytoarchitectonic Characterization and Functional Decoding of Four New Areas in the Human Lateral Orbitofrontal Cortex. Front. Neuroanat. 2020, 14, 2. [Google Scholar] [CrossRef] [PubMed]
- Kurth, F.; Cherbuin, N.; Luders, E. The impact of aging on subregions of the hippocampal complex in healthy adults. NeuroImage 2017, 163, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Luders, E.; Kurth, F.; Toga, A.W.; Narr, K.L.; Gaser, C. Meditation effects within the hippocampal complex revealed by voxel-based morphometry and cytoarchitectonic probabilistic mapping. Front. Psychol. 2013, 4, 398. [Google Scholar] [CrossRef]
- Kurth, F.; Jancke, L.; Luders, E. Integrating cytoarchitectonic tissue probabilities with MRI-based signal intensities to calculate volumes of interest. In Brain Morphometry: Methods and Clinical Applications; Spalletta, G., Gili, T., Piras, F., Eds.; Humana Press: New York, NY, USA, 2018; pp. 121–129. [Google Scholar]
- Luders, E.; Kurth, F.; Mayer, E.A.; Toga, A.W.; Narr, K.L.; Gaser, C. The unique brain anatomy of meditation practitioners: Alterations in cortical gyrification. Front. Hum. Neurosci. 2012, 6, 34. [Google Scholar] [CrossRef]
- Gaser, C.; Dahnke, R.; Thompson, P.M.; Kurth, F.; Luders, E. CAT—A Computational Anatomy Toolbox for the Analysis of Structural MRI Data. bioRxiv 2022. [Google Scholar] [CrossRef]
- Amunts, K.; Mohlberg, H.; Bludau, S.; Caspers, S.; Brandstetter, A.; Eickhoff, S.B.; Pieperhoff, P.; Dickscheid, T. Julich-Brain Atlas—Whole-brain collections of cytoarchitectonic probabilistic maps (v2.9). EBRAINS 2021. [Google Scholar] [CrossRef]
- Amunts, K.; Mohlberg, H.; Bludau, S.; Zilles, K. Julich-Brain: A 3D probabilistic atlas of the human brain’s cytoarchitecture. Science 2020, 369, 988–992. [Google Scholar] [CrossRef]
- Kurth, F.; Cherbuin, N.; Luders, E. Reduced age-related degeneration of the hippocampal subiculum in long-term meditators. Psychiatry Res. 2015, 232, 214–218. [Google Scholar] [CrossRef]
- Ashburner, J.; Friston, K.J. Diffeomorphic registration using geodesic shooting and Gauss-Newton optimisation. Neuroimage 2011, 55, 954–967. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. Neuroimage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 2001, 14, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.; Holmes, A. Non-parametric procedures. In Statistical Parametric Mapping: The Analysis of Functional Brain Images; Friston, K., Ashburner, J., Kiebel, S., Nichols, T.E., Penny, W.D., Eds.; Elsevier: London, UK, 2007; pp. 253–272. [Google Scholar]
- Winkler, A.M.; Ridgway, G.R.; Webster, M.A.; Smith, S.M.; Nichols, T.E. Permutation inference for the general linear model. Neuroimage 2014, 92, 381–397. [Google Scholar] [CrossRef]
- Benjamini, Y.; Yekutieli, D. The control of the false discovery rate in multiple testing under dependency. Ann. Stat. 2001, 29, 1165–1188. [Google Scholar] [CrossRef]
- Hochberg, Y.; Benjamini, Y. More powerful procedures for multiple significance testing. Stat. Med. 1990, 9, 811–818. [Google Scholar] [CrossRef] [PubMed]
- Schnider, A.; Treyer, V.; Buck, A. Selection of currently relevant memories by the human posterior medial orbitofrontal cortex. J. Neurosci. 2000, 20, 5880–5884. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bechara, A.; Damasio, H.; Damasio, A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex 2000, 10, 295–307. [Google Scholar] [CrossRef]
- O’Doherty, J.P.; Dayan, P.; Friston, K.; Critchley, H.; Dolan, R.J. Temporal difference models and reward-related learning in the human brain. Neuron 2003, 38, 329–337. [Google Scholar] [CrossRef]
- Rolls, E.T. Convergence of sensory systems in the orbitofrontal cortex in primates and brain design for emotion. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 281, 1212–1225. [Google Scholar] [CrossRef]
- Rolls, E.T. The functions of the orbitofrontal cortex. Brain Cogn. 2004, 55, 11–29. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T.; Kringelbach, M.L.; de Araujo, I.E. Different representations of pleasant and unpleasant odours in the human brain. Eur. J. Neurosci. 2003, 18, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Quirk, G.J.; Beer, J.S. Prefrontal involvement in the regulation of emotion: Convergence of rat and human studies. Curr. Opin. Neurobiol. 2006, 16, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Rudebeck, P.H.; Rich, E.L. Orbitofrontal cortex. Curr. Biol. 2018, 28, R1083–R1088. [Google Scholar] [CrossRef] [PubMed]
- Rolls, E.T. The orbitofrontal cortex and emotion in health and disease, including depression. Neuropsychologia 2019, 128, 14–43. [Google Scholar] [CrossRef]
- Farb, N.A.; Anderson, A.K.; Segal, Z.V. The mindful brain and emotion regulation in mood disorders. Can. J. Psychiatry 2012, 57, 70–77. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Z.; Wang, X.; Liu, L.; Zhang, J.; Zhou, R. The Effects of Different Stages of Mindfulness Meditation Training on Emotion Regulation. Front. Hum. Neurosci. 2019, 13, 208. [Google Scholar] [CrossRef]
- Luberto, C.M.; Shinday, N.; Song, R.; Philpotts, L.L.; Park, E.R.; Fricchione, G.L.; Yeh, G.Y. A Systematic Review and Meta-analysis of the Effects of Meditation on Empathy, Compassion, and Prosocial Behaviors. Mindfulness 2018, 9, 708–724. [Google Scholar] [CrossRef]
- Fabio, R.A.; Towey, G.E. Long-term meditation: The relationship between cognitive processes, thinking styles and mindfulness. Cogn. Process 2018, 19, 73–85. [Google Scholar] [CrossRef]
- van Vugt, M.K.; Jha, A.P. Investigating the impact of mindfulness meditation training on working memory: A mathematical modeling approach. Cogn. Affect. Behav. Neurosci. 2011, 11, 344–353. [Google Scholar] [CrossRef]
- Boyke, J.; Driemeyer, J.; Gaser, C.; Buchel, C.; May, A. Training-induced brain structure changes in the elderly. J. Neurosci. 2008, 28, 7031–7035. [Google Scholar] [CrossRef] [PubMed]
- Draganski, B.; Gaser, C.; Busch, V.; Schuierer, G.; Bogdahn, U.; May, A. Neuroplasticity: Changes in grey matter induced by training. Nature 2004, 427, 311–312. [Google Scholar] [CrossRef] [PubMed]
- Draganski, B.; Gaser, C.; Kempermann, G.; Kuhn, H.G.; Winkler, J.; Buchel, C.; May, A. Temporal and spatial dynamics of brain structure changes during extensive learning. J. Neurosci. 2006, 26, 6314–6317. [Google Scholar] [CrossRef] [PubMed]
- Ansell, E.B.; Rando, K.; Tuit, K.; Guarnaccia, J.; Sinha, R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol. Psychiatry 2012, 72, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Csabai, D.; Miseta, A.; Wiborg, O.; Czeh, B. Chronic stress affects the number of GABAergic neurons in the orbitofrontal cortex of rats. Behav. Brain Res. 2017, 316, 104–114. [Google Scholar] [CrossRef] [PubMed]
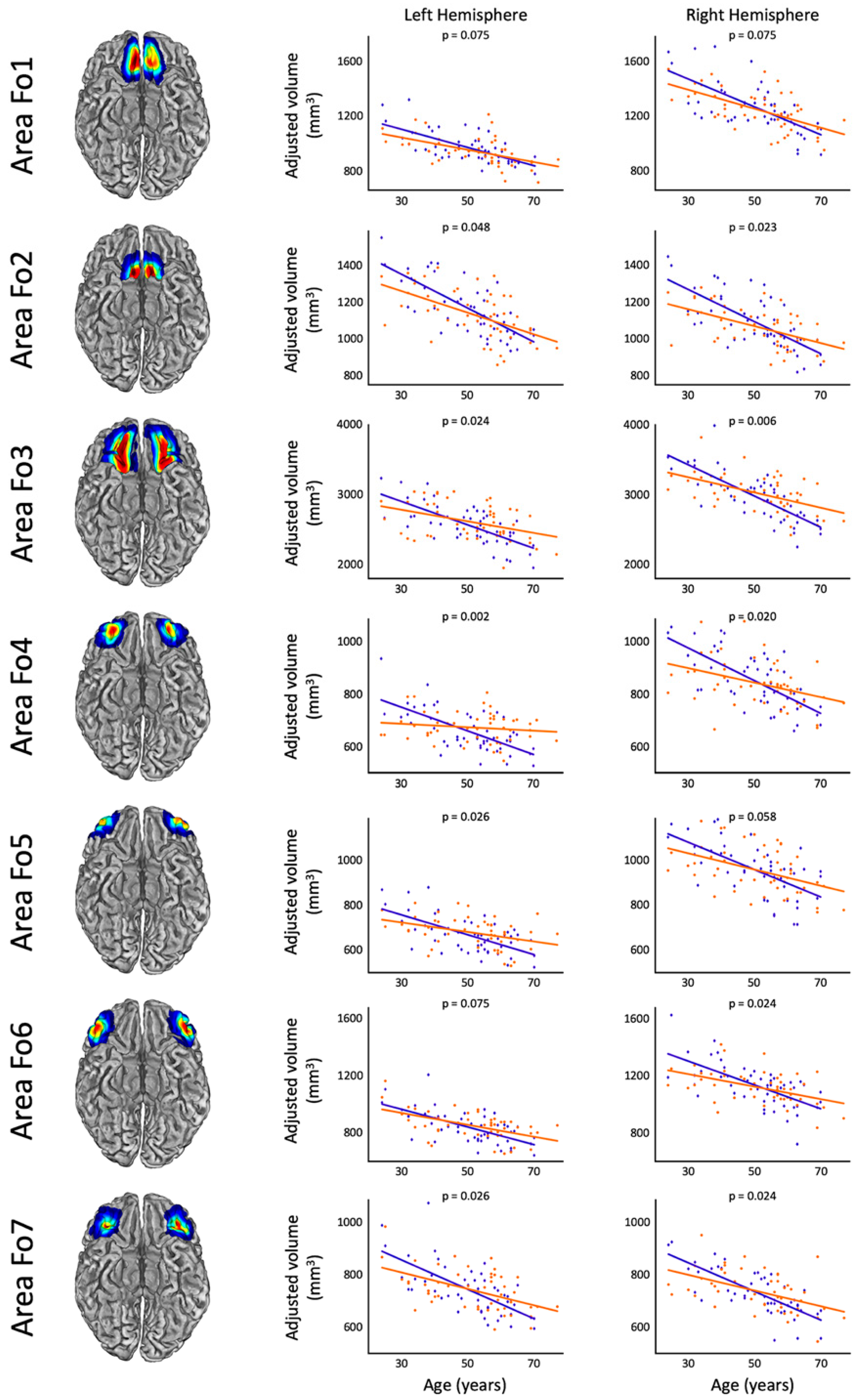
| Area | Left Hemisphere | Right Hemisphere | ||||
|---|---|---|---|---|---|---|
| Effect Size (Cohen’s d) | Significance (t) | Significance (p, FDR-Corrected) | Effect Size (Cohen’s d) | Significance (t) | Significance, (p, FDR-Corrected) | |
| OFC | 0.644 | 3.123 | 0.004 * | 0.663 | 3.212 | 0.004 * |
| Fo1 | 0.304 | 1.473 | 0.075 | 0.319 | 1.545 | 0.075 |
| Fo2 | 0.374 | 1.814 | 0.048 * | 0.513 | 2.488 | 0.023 * |
| Fo3 | 0.470 | 2.276 | 0.024 * | 0.628 | 3.044 | 0.006 * |
| Fo4 | 0.806 | 3.909 | 0.002 * | 0.525 | 2.547 | 0.020 * |
| Fo5 | 0.439 | 2.130 | 0.026 * | 0.331 | 1.603 | 0.058 T |
| Fo6 | 0.284 | 1.376 | 0.075 | 0.441 | 2.138 | 0.024 * |
| Fo7 | 0.433 | 2.097 | 0.026 * | 0.480 | 2.325 | 0.024 * |
| Area | Meditators | Controls | |||||
|---|---|---|---|---|---|---|---|
| Correlation Coefficient (r) | Significance (p, FDR-Corrected) | Volume Loss (%) | Correlation Coefficient (r) | Significance (p, FDR-Corrected) | Volume Loss (%) | ||
| Left | OFC | −0.498 | <0.001 * | −0.376 | −0.674 | <0.001 * | −0.704 |
| Fo1 | −0.434 | <0.001 * | −0.477 | −0.522 | <0.001 * | −0.696 | |
| Fo2 | −0.451 | <0.001 * | −0.514 | −0.561 | <0.001 * | −0.787 | |
| Fo3 | −0.330 | 0.001 * | −0.318 | −0.519 | <0.001 * | −0.656 | |
| Fo4 | −0.105 | 0.153 | −0.098 | −0.526 | <0.001 * | −0.686 | |
| Fo5 | −0.294 | 0.003 * | −0.313 | −0.483 | <0.001 * | −0.667 | |
| Fo6 | −0.409 | <0.001 * | −0.489 | −0.495 | <0.001 * | −0.740 | |
| Fo7 | −0.386 | 0.001 * | −0.421 | −0.540 | <0.001 * | −0.756 | |
| Right | OFC | −0.506 | <0.001 * | −0.403 | −0.683 | <0.001 * | −0.751 |
| Fo1 | −0.461 | <0.001 * | −0.559 | −0.547 | <0.001 * | −0.812 | |
| Fo2 | −0.399 | <0.001 * | −0.431 | −0.578 | <0.001 * | −0.801 | |
| Fo3 | −0.416 | <0.001 * | −0.362 | −0.626 | <0.001 * | −0.754 | |
| Fo4 | −0.305 | 0.003 * | −0.329 | −0.526 | <0.001 * | −0.734 | |
| Fo5 | −0.345 | 0.001 * | −0.379 | −0.469 | <0.001 * | −0.640 | |
| Fo6 | −0.347 | 0.001 * | −0.391 | −0.518 | <0.001 * | −0.739 | |
| Fo7 | −0.407 | <0.001 * | −0.407 | −0.571 | <0.001 * | −0.745 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurth, F.; Strohmaier, S.; Luders, E. Reduced Age-Related Gray Matter Loss in the Orbitofrontal Cortex in Long-Term Meditators. Brain Sci. 2023, 13, 1677. https://doi.org/10.3390/brainsci13121677
Kurth F, Strohmaier S, Luders E. Reduced Age-Related Gray Matter Loss in the Orbitofrontal Cortex in Long-Term Meditators. Brain Sciences. 2023; 13(12):1677. https://doi.org/10.3390/brainsci13121677
Chicago/Turabian StyleKurth, Florian, Sarah Strohmaier, and Eileen Luders. 2023. "Reduced Age-Related Gray Matter Loss in the Orbitofrontal Cortex in Long-Term Meditators" Brain Sciences 13, no. 12: 1677. https://doi.org/10.3390/brainsci13121677
APA StyleKurth, F., Strohmaier, S., & Luders, E. (2023). Reduced Age-Related Gray Matter Loss in the Orbitofrontal Cortex in Long-Term Meditators. Brain Sciences, 13(12), 1677. https://doi.org/10.3390/brainsci13121677






