Differences in Brain Network Topology Based on Alcohol Use History in Adolescents
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. MRI Acquisition and Processing
2.3. Statistical Analysis
3. Results
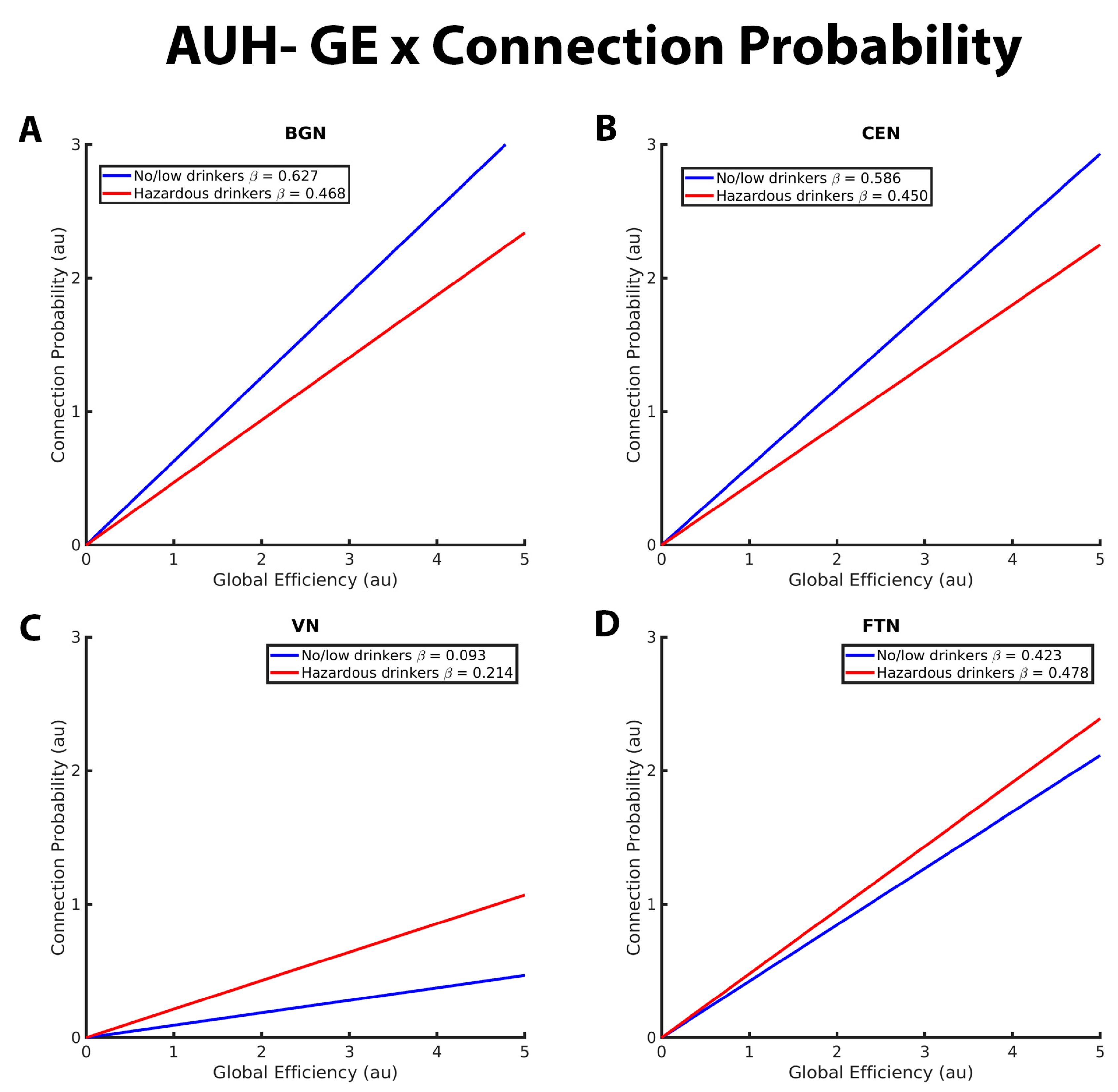
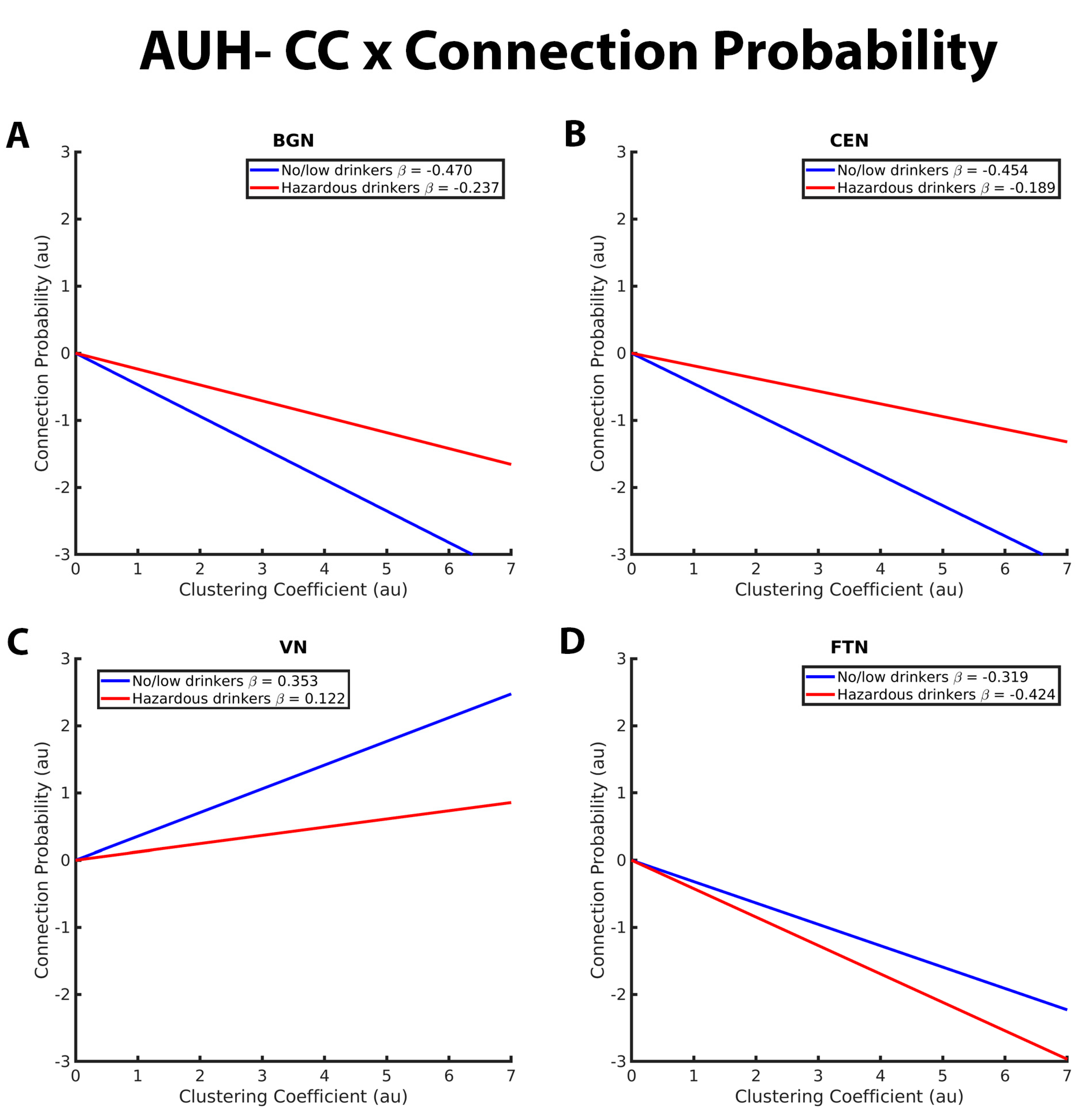
3.1. Mixed-Effects Results from Connection Probability Models
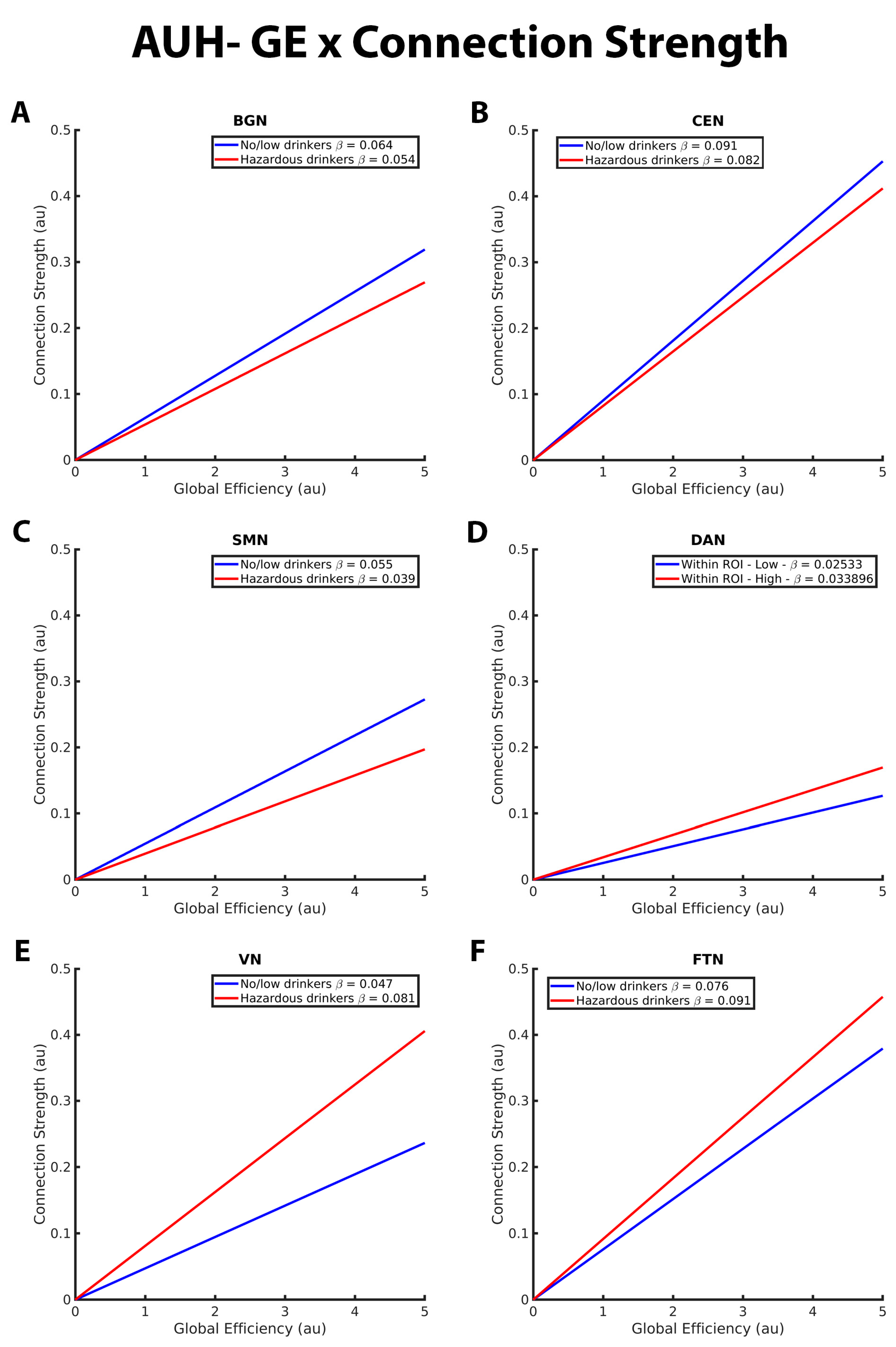
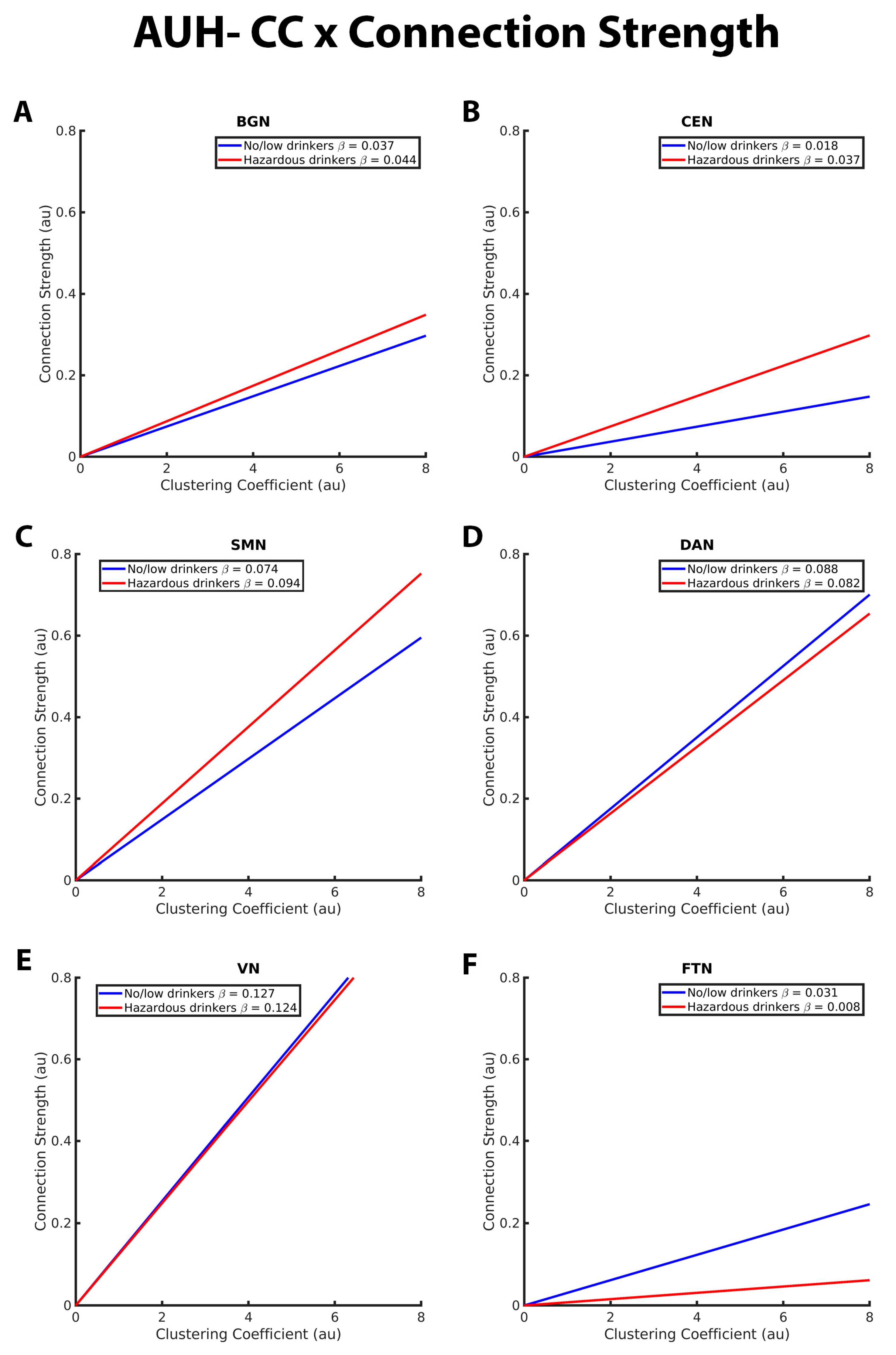
3.2. Mixed-Effects Results from Connection Strength Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giedd, J.N. Structural magnetic resonance imaging of the adolescent brain. Ann. N. Y. Acad. Sci. 2004, 1021, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Giedd, J.N.; Blumenthal, J.; Jeffries, N.O.; Castellanos, F.X.; Liu, H.; Zijdenbos, A.; Paus, T.; Evans, A.C.; Rapoport, J.L. Brain development during childhood and adolescence: A longitudinal MRI study. Nat. Neurosci. 1999, 2, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Sowell, E.R.; Thompson, P.M.; Tessner, K.D.; Toga, A.W. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J. Neurosci. 2001, 21, 8819–8829. [Google Scholar] [CrossRef] [PubMed]
- Jernigan, T.L.; Tallal, P. Late childhood changes in brain morphology observable with MRI. Dev. Med. Child Neurol. 1990, 32, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Lenroot, R.K.; Giedd, J.N. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neurosci. Biobehav. Rev. 2006, 30, 718–729. [Google Scholar] [CrossRef] [PubMed]
- Moorman, D.E. The role of the orbitofrontal cortex in alcohol use, abuse, and dependence. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 87 Pt A, 85–107. [Google Scholar] [CrossRef]
- Blakemore, S.-J. Imaging brain development: The adolescent brain. NeuroImage 2012, 61, 397–406. [Google Scholar] [CrossRef]
- Barnea-Goraly, N.; Menon, V.; Eckert, M.; Tamm, L.; Bammer, R.; Karchemskiy, A.; Dant, C.C.; Reiss, A.L. White matter development during childhood and adolescence: A cross-sectional diffusion tensor imaging study. Cereb. Cortex 2005, 15, 1848–1854. [Google Scholar] [CrossRef]
- Rakic, P.; Bourgeois, J.P.; Goldman-Rakic, P.S. Goldman-Rakic, Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Prog. Brain Res. 1994, 102, 227–243. [Google Scholar]
- Bick, J.; Nelson, C.A. Early Adverse Experiences and the Developing Brain. Neuropsychopharmacology 2016, 41, 177–196. [Google Scholar] [CrossRef]
- Hart, H.; Rubia, K. Neuroimaging of child abuse: A critical review. Front. Hum. Neurosci. 2012, 6, 52. [Google Scholar] [CrossRef] [PubMed]
- Casey, B.J.; Jones, R.M.; Hare, T.A. The adolescent brain. Ann. N. Y. Acad. Sci. 2008, 1124, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Winters, K.C.; Arria, A. Adolescent Brain Development and Drugs. Prev. Res. 2011, 18, 21–24. [Google Scholar] [PubMed]
- DeWitt, S.J.; Aslan, S.; Filbey, F.M. Adolescent risk-taking and resting state functional connectivity. Psychiatry Res. 2014, 222, 157–164. [Google Scholar] [CrossRef] [PubMed]
- NIAAA. Underage Drinking. October 2022. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking (accessed on 21 September 2023).
- Mota, N.; Parada, M.; Crego, A.; Doallo, S.; Caamaño-Isorna, F.; Holguín, S.R.; Cadaveira, F.; Corral, M. Binge drinking trajectory and neuropsychological functioning among university students: A longitudinal study. Drug Alcohol Depend. 2013, 133, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.L.; Medina, K.L.; Padula, C.B.; Tapert, S.F.; Brown, S.A. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J. Child Adolesc. Subst. Abus. 2011, 20, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Hanson, K.L.; Cummins, K.; Tapert, S.F.; Brown, S.A. Changes in neuropsychological functioning over 10 years following adolescent substance abuse treatment. Psychol. Addict. Behav. 2011, 25, 127–142. [Google Scholar] [CrossRef]
- Winward, J.L.; Hanson, K.L.; Tapert, S.F.; Brown, S.A. Heavy alcohol use, marijuana use, and concomitant use by adolescents are associated with unique and shared cognitive decrements. J. Int. Neuropsychol. Soc. 2014, 20, 784–795. [Google Scholar] [CrossRef]
- Nguyen-Louie, T.T.; Tracas, A.; Squeglia, L.M.; Matt, G.E.; Eberson-Shumate, S.; Tapert, S.F. Learning and Memory in Adolescent Moderate, Binge, and Extreme-Binge Drinkers. Alcohol. Clin. Exp. Res. 2016, 40, 1895–1904. [Google Scholar] [CrossRef]
- Lees, B.; Meredith, L.R.; Kirkland, A.E.; Bryant, B.E.; Squeglia, L.M. Effect of alcohol use on the adolescent brain and behavior. Pharmacol. Biochem. Behav. 2020, 192, 172906. [Google Scholar] [CrossRef]
- Squeglia, L.M.; Spadoni, A.D.; Infante, M.A.; Myers, M.G.; Tapert, S.F. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol. Addict. Behav. 2009, 23, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, L.M.; Tapert, S.F.; Sullivan, E.V.; Jacobus, J.; Meloy, M.; Rohlfing, T.; Pfefferbaum, A.; Luby, J.L.; Agrawal, A.; Belden, A.; et al. Brain development in heavy-drinking adolescents. Am. J. Psychiatry 2015, 172, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Pfefferbaum, A.; Kwon, D.; Brumback, T.; Thompson, W.K.; Cummins, K.; Tapert, S.F.; Brown, S.A.; Colrain, I.M.; Baker, F.C.; Prouty, D.; et al. Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am. J. Psychiatry 2018, 175, 370–380. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D.; Clark, D.B.; Beers, S.R.; Soloff, P.H.; Boring, A.M.; Hall, J.; Kersh, A.; Keshavan, M.S. Hippocampal volume in adolescent-onset alcohol use disorders. Am. J. Psychiatry 2000, 157, 737–744. [Google Scholar] [CrossRef] [PubMed]
- De Bellis, M.D.; Narasimhan, A.; Thatcher, D.L.; Keshavan, M.S.; Soloff, P.; Clark, D.B. Prefrontal cortex, thalamus, and cerebellar volumes in adolescents and young adults with adolescent-onset alcohol use disorders and comorbid mental disorders. Alcohol. Clin. Exp. Res. 2005, 29, 1590–1600. [Google Scholar] [CrossRef] [PubMed]
- Jacobus, J.; Tapert, S.F. Neurotoxic effects of alcohol in adolescence. Annu. Rev. Clin. Psychol. 2013, 9, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Tapert, S.F.; Schweinsburg, A.D.; Barlett, V.C.; Brown, S.A.; Frank, L.R.; Brown, G.G.; Meloy, M.J. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol. Clin. Exp. Res. 2004, 28, 1577–1586. [Google Scholar] [CrossRef]
- Tapert, S.F.; Brown, G.G.; Kindermann, S.S.; Cheung, E.H.; Frank, L.R.; Brown, S.A. fMRI measurement of brain dysfunction in alcohol-dependent young women. Alcohol. Clin. Exp. Res. 2001, 25, 236–245. [Google Scholar]
- Xiao, L.; Bechara, A.; Gong, Q.; Huang, X.; Li, X.; Xue, G.; Wong, S.; Lu, Z.-L.; Palmer, P.; Wei, Y.; et al. Abnormal affective decision making revealed in adolescent binge drinkers using a functional magnetic resonance imaging study. Psychol. Addict. Behav. 2013, 27, 443–454. [Google Scholar] [CrossRef]
- Norman, A.L.; Pulido, C.; Squeglia, L.M.; Spadoni, A.D.; Paulus, M.P.; Tapert, S.F. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011, 119, 216–223. [Google Scholar] [CrossRef]
- Gonçalves, S.F.; Turpyn, C.C.; Niehaus, C.E.; Mauro, K.L.; Hinagpis, C.L.; Thompson, J.C.; Chaplin, T.M. Neural activation to loss and reward among alcohol naive adolescents who later initiate alcohol use. Dev. Cogn. Neurosci. 2021, 50, 100978. [Google Scholar] [CrossRef]
- Müller-Oehring, E.M.; Kwon, D.; Nagel, B.J.; Sullivan, E.V.; Chu, W.; Rohlfing, T.; Prouty, D.; Nichols, B.N.; Poline, J.-B.; Tapert, S.F.; et al. Influences of Age, Sex, and Moderate Alcohol Drinking on the Intrinsic Functional Architecture of Adolescent Brains. Cereb. Cortex 2018, 28, 1049–1063. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.; Yetkin, F.Z.; Haughton, V.M.; Hyde, J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn. Reson. Med. 1995, 34, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Lee, T.M.; Kay, A.R.; Tank, D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc. Natl. Acad. Sci. USA 1990, 87, 9868–9872. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Denier, N.; Magon, S.; Radue, E.-W.; Huber, C.G.; Riecher-Rossler, A.; Wiesbeck, G.A.; Lang, U.E.; Borgwardt, S.; Walter, M. Increased functional connectivity in the resting-state basal ganglia network after acute heroin substitution. Transl. Psychiatry 2015, 5, e533. [Google Scholar] [CrossRef] [PubMed]
- Gozdas, E.; Holland, S.K.; Altaye, M.; CMIND Authorship Consortium. Developmental changes in functional brain networks from birth through adolescence. Hum. Brain Mapp. 2019, 40, 1434–1444. [Google Scholar] [CrossRef] [PubMed]
- Fair, D.A.; Cohen, A.L.; Power, J.D.; Dosenbach, N.U.F.; Church, J.A.; Miezin, F.M.; Schlaggar, B.L.; Petersen, S.E. Functional brain networks develop from a “local to distributed” organization. PLoS Comput. Biol. 2009, 5, e1000381. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Beaulieu, C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011, 31, 10937–10947. [Google Scholar] [CrossRef] [PubMed]
- Power, J.D.; Fair, D.A.; Schlaggar, B.L.; Petersen, S.E. The Development of human functional brain networks. Neuron 2010, 67, 735–748. [Google Scholar] [CrossRef]
- Whelan, R.; Conrod, P.J.; Poline, J.-B.; Lourdusamy, A.; Banaschewski, T.; Barker, G.J.; Bellgrove, M.A.; Büchel, C.; Byrne, M.; Cummins, T.D.R.; et al. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat. Neurosci. 2012, 15, 920–925. [Google Scholar] [CrossRef]
- Castellanos, F.X.; Aoki, Y. Intrinsic Functional Connectivity in Attention-Deficit/Hyperactivity Disorder: A Science in Development. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 253–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Samea, F.; Soluki, S.; Nejati, V.; Zarei, M.; Cortese, S.; Eickhoff, S.B.; Tahmasian, M.; Eickhoff, C.R. Brain alterations in children/adolescents with ADHD revisited: A neuroimaging meta-analysis of 96 structural and functional studies. Neurosci. Biobehav. Rev. 2019, 100, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.E.; Hernandez, L.M.; Bookheimer, S.Y.; Dapretto, M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 2019, 12, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Belmonte, M.K.; Allen, G.; Beckel-Mitchener, A.; Boulanger, L.M.; Carper, R.A.; Webb, S.J. Autism and abnormal development of brain connectivity. J. Neurosci. 2004, 24, 9228–9231. [Google Scholar] [CrossRef] [PubMed]
- Uddin, L.Q.; Supekar, K.; Menon, V. Reconceptualizing functional brain connectivity in autism from a developmental perspective. Front. Hum. Neurosci. 2013, 7, 458. [Google Scholar] [CrossRef] [PubMed]
- Reese, E.D.; Yi, J.Y.; McKay, K.G.; Stein, E.A.; Ross, T.J.; Daughters, S.B. Triple Network Resting State Connectivity Predicts Distress Tolerance and Is Associated with Cocaine Use. J. Clin. Med. 2019, 8, 2135. [Google Scholar] [CrossRef]
- Zhao, Q.; Sullivan, E.V.; Műller-Oehring, E.M.; Honnorat, N.; Adeli, E.; Podhajsky, S.; Baker, F.C.; Colrain, I.M.; Prouty, D.; Tapert, S.F.; et al. Adolescent alcohol use disrupts functional neurodevelopment in sensation seeking girls. Addict. Biol. 2021, 26, e12914. [Google Scholar] [CrossRef]
- Goldfarb, E.V.; Scheinost, D.; Fogelman, N.; Seo, D.; Sinha, R. High-Risk Drinkers Engage Distinct Stress-Predictive Brain Networks. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 805–813. [Google Scholar] [CrossRef]
- Holcomb, L.A.; Huang, S.; Cruz, S.M.; Marinkovic, K. Neural oscillatory dynamics of inhibitory control in young adult binge drinkers. Biol. Psychol. 2019, 146, 107732. [Google Scholar] [CrossRef]
- Bahrami, M.; Laurienti, P.J.; Simpson, S.L. A MATLAB toolbox for multivariate analysis of brain networks. Hum. Brain Mapp. 2019, 40, 175–186. [Google Scholar] [CrossRef]
- Bahrami, M.; Laurienti, P.J.; Simpson, S.L. Analysis of brain subnetworks within the context of their whole-brain networks. Hum. Brain Mapp. 2019, 40, 5123–5141. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.L.; Laurienti, P.J. A two-part mixed-effects modeling framework for analyzing whole-brain network data. NeuroImage 2015, 113, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’ networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef] [PubMed]
- Latora, V.; Marchiori, M. Efficient behavior of small-world networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef] [PubMed]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Brown, S.A.; Brumback, T.; Tomlinson, K.; Cummins, K.; Thompson, W.K.; Nagel, B.J.; De Bellis, M.D.; Hooper, S.R.; Clark, D.B.; Chung, T.; et al. The National Consortium on Alcohol and NeuroDevelopment in Adolescence (NCANDA): A Multisite Study of Adolescent Development and Substance Use. J. Stud. Alcohol Drugs 2015, 76, 895–908. [Google Scholar] [CrossRef]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol Screening and Brief Intervention for Youth: A Practitioner’s Guide; National Institute on Alcohol Abuse and Alcoholism: Bethesda, MD, USA, 2011.
- Substance Abuse and Mental health Services AdministrationResults from the 2011 National Survey on Drug Use and Health: Summary of National Findings; NSDUH: Rockville, MD, USA, 2012.
- Friedman, E.J.; Landsberg, A.S.; Owen, J.P.; Li, Y.-O.; Mukherjee, P. Stochastic geometric network models for groups of functional and structural connectomes. NeuroImage 2014, 101, 473–484. [Google Scholar] [CrossRef]
- Bahrami, M.; Simpson, S.L.; Burdette, J.H.; Lyday, R.G.; Quandt, S.A.; Chen, H.; Arcury, T.A.; Laurienti, P.J. Altered default mode network associated with pesticide exposure in Latinx children from rural farmworker families. NeuroImage 2022, 256, 119179. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing With Independent Statistics. J. Educ. Behav. Stat. 2000, 25, 60–83. [Google Scholar] [CrossRef]
- Menon, V. Large-scale brain networks and psychopathology: A unifying triple network model. Trends Cogn. Sci. 2011, 15, 483–506. [Google Scholar] [CrossRef]
- Fair, D.A.; Dosenbach, N.U.F.; Church, J.A.; Cohen, A.L.; Brahmbhatt, S.; Miezin, F.M.; Barch, D.M.; Raichle, M.E.; Petersen, S.E.; Schlaggar, B.L. Development of distinct control networks through segregation and integration. Proc. Natl. Acad. Sci. USA 2007, 104, 13507–13512. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. NeuroImage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. The economy of brain network organization. Nat. Rev. Neurosci. 2012, 13, 336–349. [Google Scholar] [CrossRef]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Muller, A.M.; Meyerhoff, D.J. Maladaptive brain organization at 1 month into abstinence as an indicator for future relapse in patients with alcohol use disorder. Eur. J. Neurosci. 2021, 53, 2923–2938. [Google Scholar] [CrossRef]
- Zhang, G.; Li, N.; Liu, H.; Zheng, H.; Zheng, W. Dynamic connectivity patterns of resting-state brain functional networks in healthy individuals after acute alcohol intake. Front. Neurosci. 2022, 16, 974778. [Google Scholar] [CrossRef] [PubMed]
- Squeglia, L.M.; Cservenka, A. Adolescence and Drug Use Vulnerability: Findings from Neuroimaging. Curr. Opin. Behav. Sci. 2017, 13, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Maleki, N.; Sawyer, K.S.; Levy, S.; Harris, G.J.; Oscar-Berman, M. Intrinsic brain functional connectivity patterns in alcohol use disorder. Brain Commun. 2022, 4, fcac290. [Google Scholar] [CrossRef] [PubMed]
- Lanciego, J.L.; Luquin, N.; Obeso, J.A. Functional Neuroanatomy of the Basal Ganglia. Cold Spring Harb. Perspect. Med. 2012, 2, a009621. [Google Scholar] [CrossRef] [PubMed]
- Renteria, R.; Baltz, E.T.; Gremel, C.M. Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat. Commun. 2018, 9, 211. [Google Scholar] [CrossRef]
- Sitzia, G.; Lovinger, D.M. Circuit dysfunctions of associative and sensorimotor basal ganglia loops in alcohol use disorder: Insights from animal models. Addict. Neurosci. 2023, 5, 100056. [Google Scholar] [CrossRef]
- Rzepecki-Smith, C.I.; Meda, S.A.; Calhoun, V.D.; Stevens, M.C.; Jafri, M.J.; Astur, R.S.; Pearlson, G.D. Disruptions in functional network connectivity during alcohol intoxicated driving. Alcohol. Clin. Exp. Res. 2010, 34, 479–487. [Google Scholar] [CrossRef]
- Ersche, K.D.; Meng, C.; Ziauddeen, H.; Stochl, J.; Williams, G.B.; Bullmore, E.T.; Robbins, T.W. Brain networks underlying vulnerability and resilience to drug addiction. Proc. Natl. Acad. Sci. USA 2020, 117, 15253–15261. [Google Scholar] [CrossRef] [PubMed]
- Chenji, S.; Jha, S.; Lee, D.; Brown, M.; Seres, P.; Mah, D.; Kalra, S. Investigating Default Mode and Sensorimotor Network Connectivity in Amyotrophic Lateral Sclerosis. PLoS ONE 2016, 11, e0157443. [Google Scholar] [CrossRef] [PubMed]
- Silveira, S.; Shah, R.; Nooner, K.B.; Nagel, B.J.; Tapert, S.F.; de Bellis, M.D.; Mishra, J. Impact of Childhood Trauma on Executive Function in Adolescence—Mediating Functional Brain Networks and Prediction of High-Risk Drinking. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 2010, 35, 217–238. [Google Scholar] [CrossRef] [PubMed]
- Bracht, T.; Soravia, L.; Moggi, F.; Stein, M.; Grieder, M.; Federspiel, A.; Tschümperlin, R.; Batschelet, H.M.; Wiest, R.; Denier, N. The role of the orbitofrontal cortex and the nucleus accumbens for craving in alcohol use disorder. Transl. Psychiatry 2021, 11, 1–10. [Google Scholar] [CrossRef]
- Myrick, H.; Anton, R.F.; Li, X.; Henderson, S.; Drobes, D.; Voronin, K.; George, M.S. Differential brain activity in alcoholics and social drinkers to alcohol cues: Relationship to craving. Neuropsychopharmacology 2004, 29, 393–402. [Google Scholar] [CrossRef]
- Maia, T.V.; Cooney, R.E.; Peterson, B.S. The neural bases of obsessive–compulsive disorder in children and adults. Dev. Psychopathol. 2008, 20, 1251–1283. [Google Scholar] [CrossRef]
- Peters, S.; Peper, J.S.; Van Duijvenvoorde, A.C.; Braams, B.R.; Crone, E.A. Amygdala–orbitofrontal connectivity predicts alcohol use two years later: A longitudinal neuroimaging study on alcohol use in adolescence. Dev. Sci. 2017, 20, e12448. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry 2012, 71, 443–450. [Google Scholar] [CrossRef]
- Sami, M.B.; McCutcheon, R.A.; Ettinger, U.; Williams, S.; Lythgoe, D.; McGuire, P.; Bhattacharyya, S. Cannabis Use Linked to Altered Functional Connectivity of the Visual Attentional Connectivity in Patients With Psychosis and Controls. Schizophr. Bull. Open 2020, 1, sgaa018. [Google Scholar] [CrossRef]
- Menon, V. Developmental pathways to functional brain networks: Emerging principles. Trends Cogn. Sci. 2013, 17, 627–640. [Google Scholar] [CrossRef]
- Canessa, N.; Basso, G.; Carne, I.; Poggi, P.; Gianelli, C. Increased decision latency in alcohol use disorder reflects altered resting-state synchrony in the anterior salience network. Sci. Rep. 2021, 11, 19581. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.-W.; Hwang, S.; Cheong, C. Functional and Structural Alteration of Default Mode, Executive Control, and Salience Networks in Alcohol Use Disorder. Front. Psychiatry 2021, 12, 742228. [Google Scholar] [CrossRef] [PubMed]
- Amaratunga, D.; Cabrera, J. Analysis of Data From Viral DNA Microchips. J. Am. Stat. Assoc. 2001, 96, 1161–1170. [Google Scholar] [CrossRef]
- Bolstad, B.M.; Irizarry, R.A.; Astrand, M.; Speed, T.P. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 2003, 19, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Fraiman, D.; Balenzuela, P.; Foss, J.; Chialvo, D.R. Ising-like dynamics in large-scale functional brain networks. Phys. Rev. E 2009, 79, 061922. [Google Scholar] [CrossRef]
- Laurienti, P.J.; Miller, M.E.; Lyday, R.G.; Boyd, M.C.; Tanase, A.D.; Burdette, J.H.; Hugenschmidt, C.E.; Rejeski, W.J.; Simpson, S.L.; Baker, L.D.; et al. Associations of physical function and body mass index with functional brain networks in community-dwelling older adults. Neurobiol. Aging 2023, 127, 43–53. [Google Scholar] [CrossRef]
- Parente, F.; Frascarelli, M.; Mirigliani, A.; Di Fabio, F.; Biondi, M.; Colosimo, A. Negative functional brain networks. Brain Imaging Behav. 2017, 12, 467–476. [Google Scholar] [CrossRef]
- Pruim, R.H.R.; Mennes, M.; van Rooij, D.; Llera, A.; Buitelaar, J.K.; Beckmann, C.F. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage 2015, 112, 267–277. [Google Scholar] [CrossRef]
- Shen, X.; Tokoglu, F.; Papademetris, X.; Constable, R. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. NeuroImage 2013, 82, 403–415. [Google Scholar] [CrossRef] [PubMed]
| Age | Maximum Drinks Per Occasion: Female | Maximum Drinks Per Occasion: Male | Total Days of Drinking in Lifetime |
|---|---|---|---|
| 12–13.9 | ≤3 | ≤3 | ≤5 |
| 14–15.9 | ≤3 | ≤4 | ≤5 |
| 16–16.9 | ≤3 | ≤4 | ≤11 |
| 17–17.9 | ≤3 | ≤4 | ≤23 |
| 18–19.9 | ≤3 | ≤4 | ≤51 |
| ≥20 | ≤3 | ≤5 | ≤51 |
| Hazardous Drinkers | No/low Drinkers | Difference between Matched Groups; P= | ||
|---|---|---|---|---|
| Total | 117 | 117 | ||
| Girls/Boys | 62/55 | 62/55 | ||
| Age | Girls | 18.6 ± 2 | 18.4 ± 1.9 | 0.39 |
| Boys | 18.7 ± 1.9 | 18.4 ± 1.7 | ||
| GE/Siemens | 80/37 | 72/45 | 0.27 * | |
| Pubertal Development Scale | 3.7 ± 0.4 | 3.6 ± 0.4 | 0.28 | |
| Alcohol use | # days lifetime | 50.6 ± 75.5 | 3.1 ± 7.2 | <0.001 |
| # days past year | 23.2 ± 31.8 | 1.8 ± 4.8 | <0.001 | |
| Nicotine use | # cigarettes lifetime | 11.4 ± 45.3 | 0.7 ± 4.7 | 0.012 |
| # cigarettes past year | 6 ± 28.1 | 0.3 ± 2.3 | 0.03 | |
| Marijuana use | # days lifetime | 10.8 ± 17.7 | 1 ± 3.9 | 0.004 |
| # days past year | 7.5 ± 16 | 0.6 ± 2.5 | 0.015 | |
| Parental education (years) | 17.4 ± 2 | 17 ± 2 | 0.19 | |
| Estimate | SE | t Value | p-Value | |
|---|---|---|---|---|
| GE × AUH within BGN | −0.1597 | 0.02648 | −6.03 | <0.0001 |
| CC × AUH within BGN | 0.2335 | 0.03098 | 7.54 | <0.0001 |
| GE × AUH within CEN | −0.1364 | 0.03221 | −4.24 | <0.0001 |
| CC × AUH within CEN | 0.2653 | 0.03452 | 7.69 | <0.0001 |
| GE × AUH within SMN | 0.04766 | 0.02970 | 1.60 | 0.1085 |
| CC × AUH within SMN | −0.05676 | 0.03014 | −1.88 | 0.0597 |
| GE × AUH within DAN | 0.01286 | 0.03526 | 0.36 | 0.7153 |
| CC × AUH within DAN | 0.01209 | 0.03534 | 0.34 | 0.7322 |
| GE × AUH within VN | 0.1205 | 0.05416 | 2.23 | 0.0261 |
| CC × AUH within VN | −0.2312 | 0.05165 | −4.48 | <0.0001 |
| GE × AUH within FTN | 0.05511 | 0.03888 | 1.42 | 0.1564 |
| CC × AUH within FTN | −0.1051 | 0.04506 | −2.33 | 0.0196 |
| GE × AUH within DMN | 0.04298 | 0.02827 | 1.52 | 0.1285 |
| CC × AUH within DMN | −0.02400 | 0.03110 | −0.77 | 0.4403 |
| GE × AUH within SN | −0.02897 | 0.04502 | −0.64 | 0.5199 |
| CC × AUH within SN | 0.06577 | 0.04454 | 1.48 | 0.1398 |
| Estimate | SE | t Value | p-Value | |
|---|---|---|---|---|
| GE × AUH within BGN | −0.00994 | 0.002110 | −4.71 | <0.0001 |
| CC × AUH within BGN | 0.006438 | 0.002511 | 2.56 | 0.0104 |
| GE × AUH within CEN | −0.00821 | 0.002697 | −3.04 | 0.0023 |
| CC × AUH within CEN | 0.01878 | 0.002807 | 6.69 | <0.0001 |
| GE × AUH within SMN | −0.01514 | 0.002187 | −6.92 | <0.0001 |
| CC × AUH within SMN | 0.01967 | 0.002180 | 9.02 | <0.0001 |
| GE × AUH within DAN | 0.008566 | 0.003145 | 2.72 | 0.0065 |
| CC × AUH within DAN | −0.00582 | 0.003017 | −1.93 | 0.0537 |
| GE × AUH within VN | 0.03387 | 0.003597 | 9.42 | <0.0001 |
| CC × AUH within VN | −0.00249 | 0.003226 | −0.77 | 0.4400 |
| GE × AUH within FTN | 0.01567 | 0.003474 | 4.51 | <0.0001 |
| CC × AUH within FTN | −0.02309 | 0.004042 | −5.71 | <0.0001 |
| GE × AUH within DMN | 0.003482 | 0.002007 | 1.73 | 0.0828 |
| CC × AUH within DMN | −0.00071 | 0.002040 | −0.35 | 0.7281 |
| GE × AUH within SN | −0.00136 | 0.003514 | −0.39 | 0.6998 |
| CC × AUH within SN | 0.003587 | 0.003371 | 1.06 | 0.2873 |
| Connection Probability/Strength | No/Low vs. Hazardous Drinkers | |
|---|---|---|
| BGN | CP- Efficiency relation in BGN | * more positive for no/low than hazardous |
| CP- Clustering relation in BGN | * more negative for no/low than hazardous | |
| CS- Efficiency relation in BGN | * more positive for no/low than hazardous | |
| CS- Clustering relation in BGN | * more positive for hazardous than no/low | |
| CEN | CP- Efficiency relation in CEN | * more positive for no/low than hazardous |
| CP- Clustering relation in CEN | * more negative for no/low than hazardous | |
| CS- Efficiency relation in CEN | * more positive for no/low than hazardous | |
| CS- Clustering relation in CEN | * more positive for hazardous than no/low | |
| SMN | CP- Efficiency relation in SMN | no difference |
| CP- Clustering relation in SMN | no difference | |
| CS- Efficiency relation in SMN | * more positive for no/low than hazardous | |
| CS- Clustering relation in SMN | * more positive for hazardous than no/low | |
| DAN | CP- Efficiency relation in DAN | no difference |
| CP- Clustering relation in DAN | no difference | |
| CS- Efficiency relation in DAN | * more positive for hazardous than no/low | |
| CS- Clustering relation in DAN | no difference | |
| VN | CP- Efficiency relation in VN | * more positive for hazardous than no/low |
| CP- Clustering relation in VN | * more positive for no/low than hazardous | |
| CS- Efficiency relation in VN | * more positive for hazardous than no/low | |
| CS- Clustering relation in VN | no difference | |
| FTN | CP- Efficiency relation in FTN | no difference |
| CP- Clustering relation in FTN | * more positive for no/low than hazardous | |
| CS- Efficiency relation in FTN | * more positive for hazardous than no/low | |
| CS- Clustering relation inFTN | * more positive for no/low than hazardous | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirse, H.A.; Bahrami, M.; Lyday, R.G.; Simpson, S.L.; Peterson-Sockwell, H.; Burdette, J.H.; Laurienti, P.J. Differences in Brain Network Topology Based on Alcohol Use History in Adolescents. Brain Sci. 2023, 13, 1676. https://doi.org/10.3390/brainsci13121676
Kirse HA, Bahrami M, Lyday RG, Simpson SL, Peterson-Sockwell H, Burdette JH, Laurienti PJ. Differences in Brain Network Topology Based on Alcohol Use History in Adolescents. Brain Sciences. 2023; 13(12):1676. https://doi.org/10.3390/brainsci13121676
Chicago/Turabian StyleKirse, Haley A., Mohsen Bahrami, Robert G. Lyday, Sean L. Simpson, Hope Peterson-Sockwell, Jonathan H. Burdette, and Paul J. Laurienti. 2023. "Differences in Brain Network Topology Based on Alcohol Use History in Adolescents" Brain Sciences 13, no. 12: 1676. https://doi.org/10.3390/brainsci13121676
APA StyleKirse, H. A., Bahrami, M., Lyday, R. G., Simpson, S. L., Peterson-Sockwell, H., Burdette, J. H., & Laurienti, P. J. (2023). Differences in Brain Network Topology Based on Alcohol Use History in Adolescents. Brain Sciences, 13(12), 1676. https://doi.org/10.3390/brainsci13121676







