Effect of Meditation on Brain Activity during an Attention Task: A Comparison Study of ASL and BOLD Task fMRI
Abstract
:1. Introduction
2. Methods
2.1. Study Population
2.2. Task fMRI Experimental Design
2.3. MRI Acquisition
2.4. Data Analyses
3. Results
3.1. Basic Characteristics of Subjects
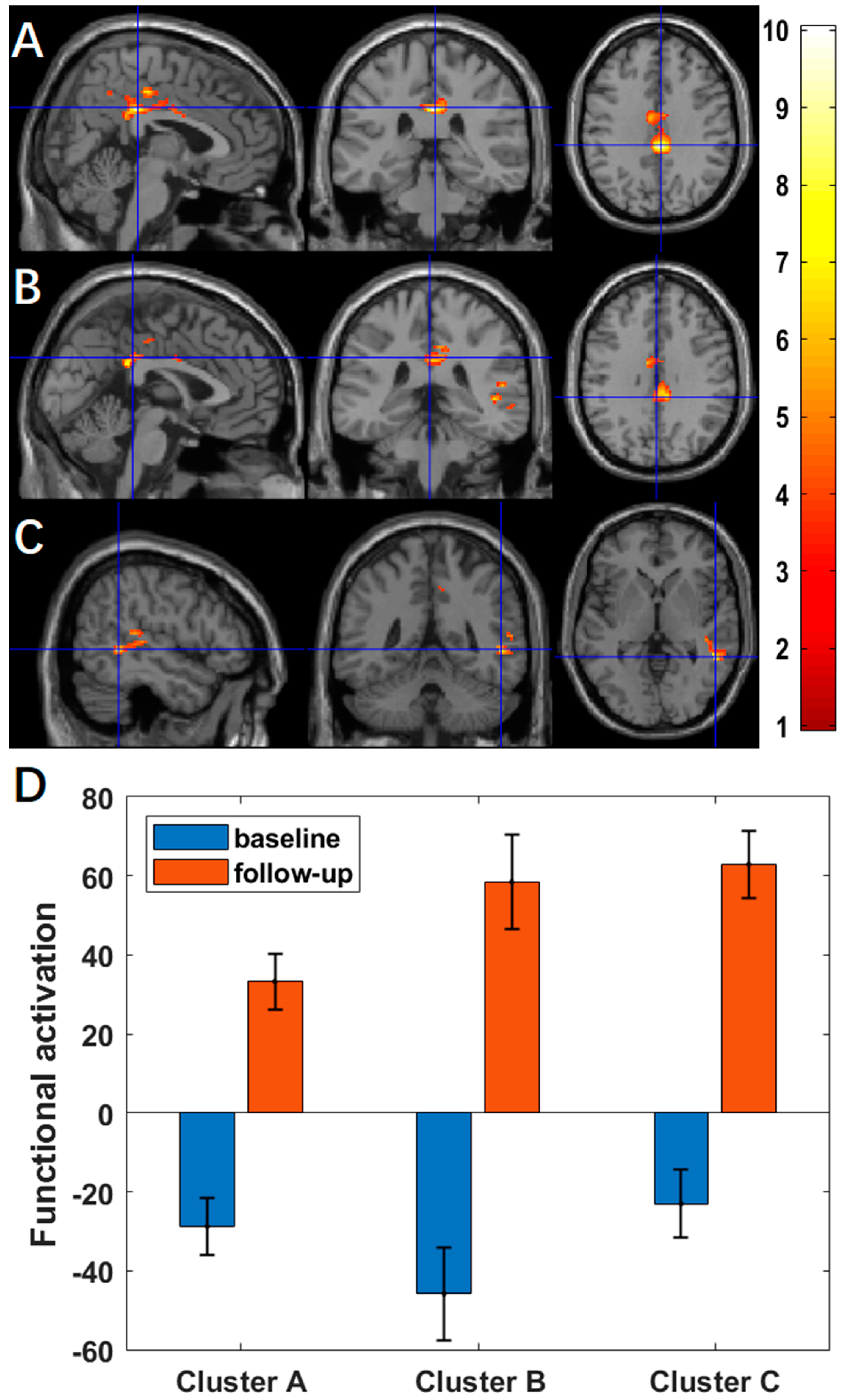
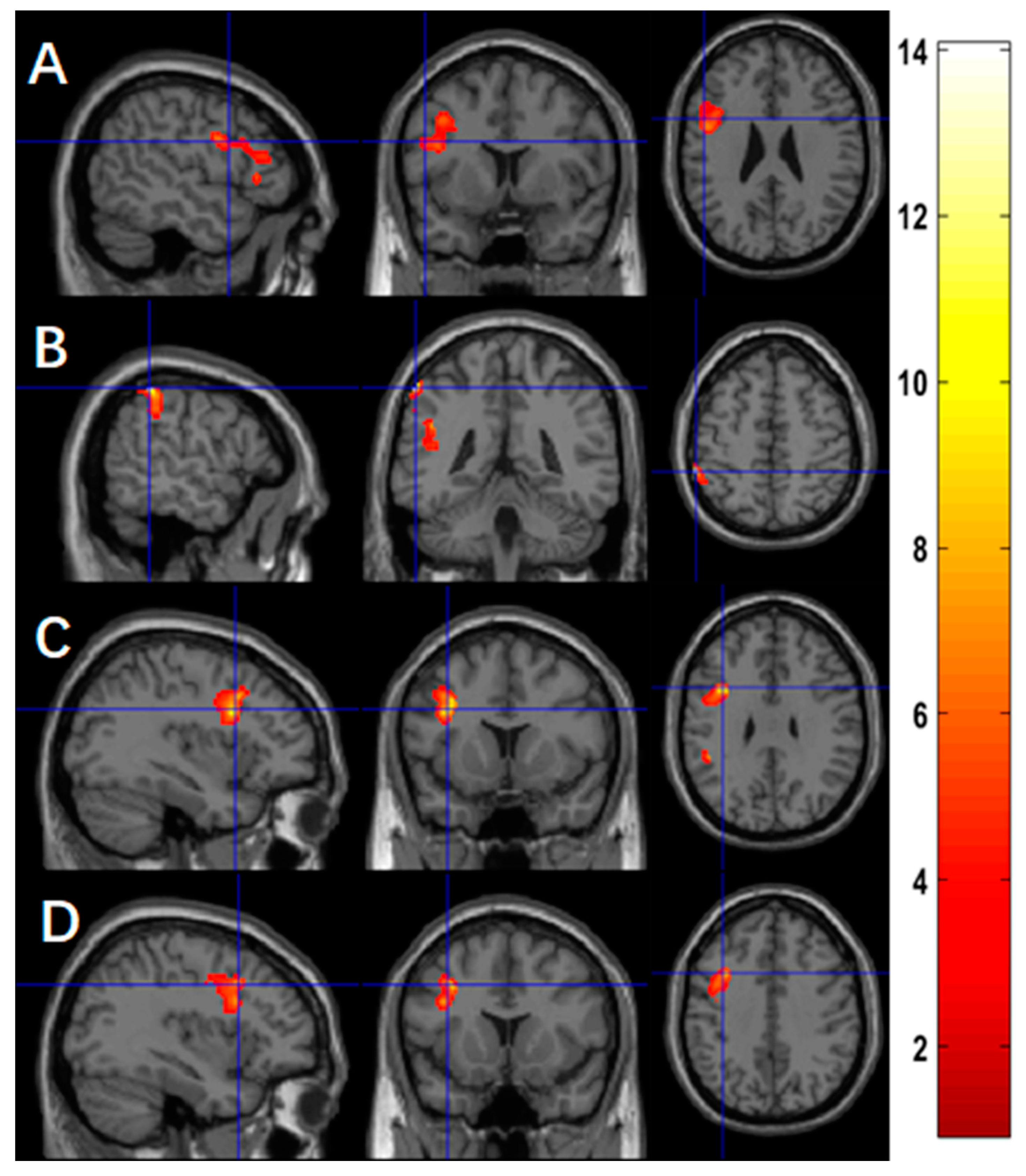
3.2. Changes of Activation between Baseline and Follow-Up
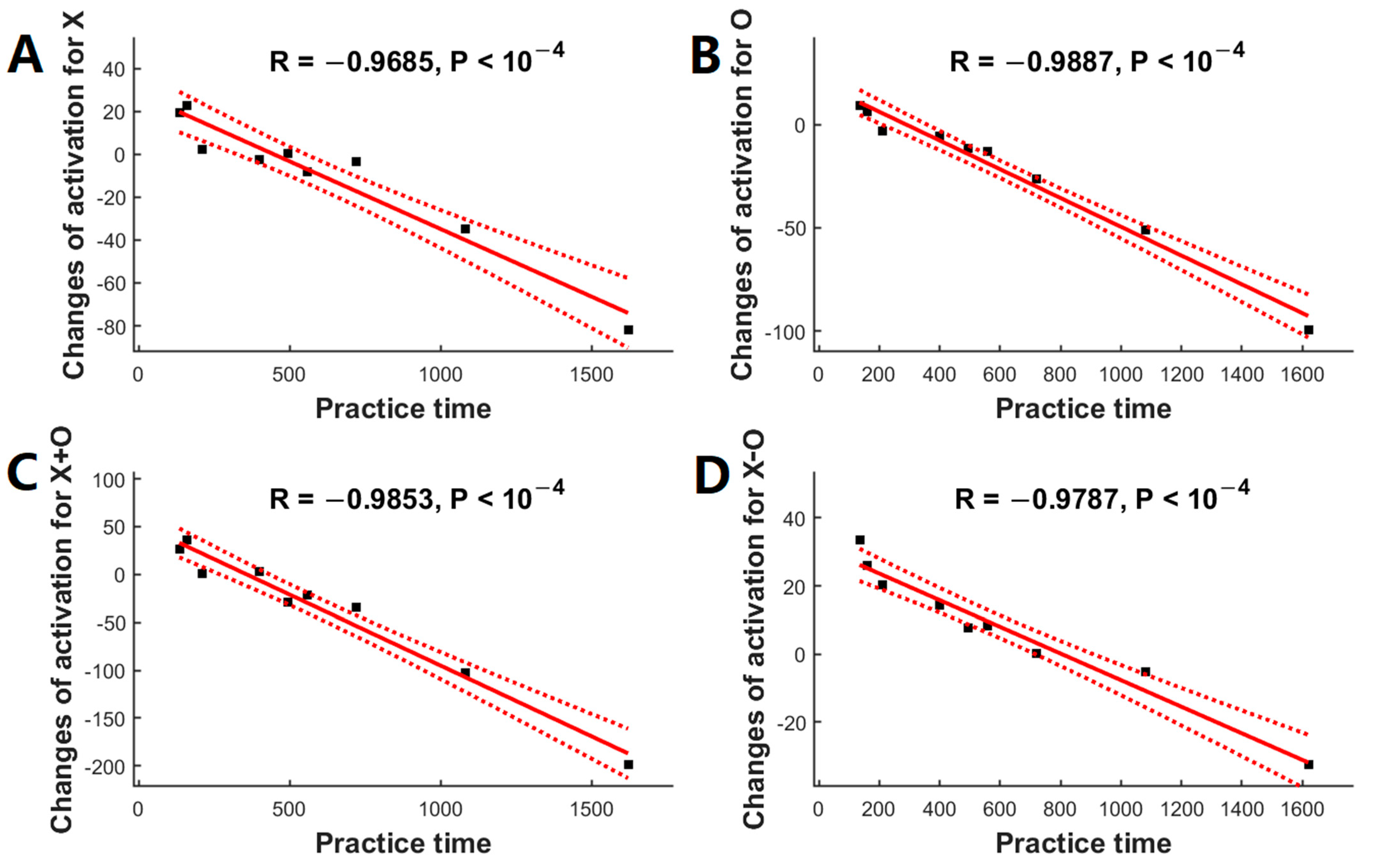
3.3. Relationship between Practice Time and Activation Changes
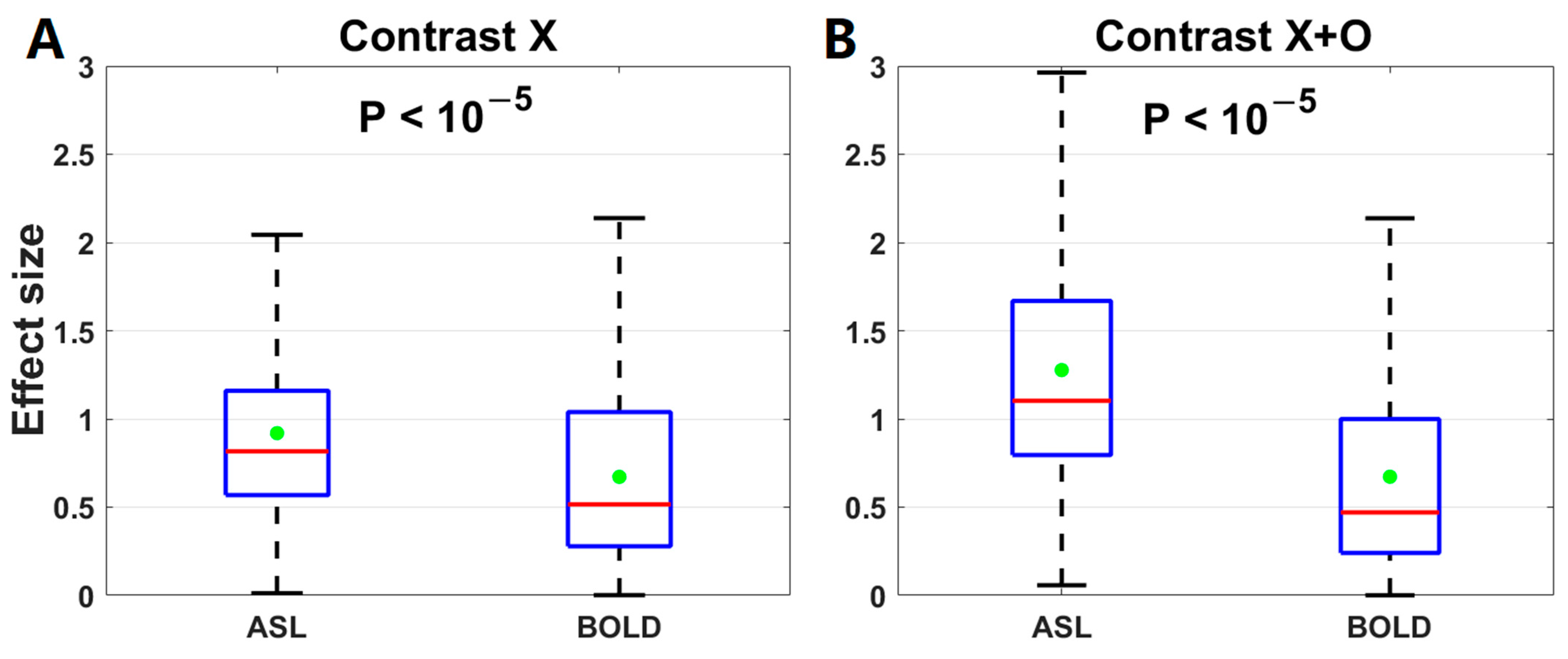
3.4. Comparison of Effect Sizes between ASL fMRI and BOLD fMRI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADHD | Attention-deficit/hyperactivity disorder |
| ANT | Attention network task |
| ASL | Arterial spin labeling |
| BOLD | blood oxygenation level-dependent |
| CSF | Cerebrospinal fluid |
| DAN | Dorsal attention network |
| DLPFC | Dorsolateral prefrontal cortex |
| DMN | Default mode network |
| EEG | Electroencephalography |
| EPI | Echo planar imaging |
| ERP | Event-related potential |
| FAM | Focused attention meditation |
| fMRI | Functional magnetic resonance imaging |
| FOV | Field of view |
| FPN | Frontoparietal control network |
| FWHM | Full width at half maximum |
| GLM | General linear model |
| HRF | Hemodynamic response function |
| ISI | Inter-stimulus interval |
| MNI | Montreal Neurologic Institute |
| mPFC | Medial prefrontal cortex |
| MPRAGE | Magnetization prepared rapid gradient echo |
| PCASL | Pseudo-continuous arterial spin labeling |
| PCC | Posterior cingulate cortex |
| RARE | Rapid Acquisition with Refocused Echoes |
| rBW | Receiver bandwidth |
| ROI | Region of interest |
| rsFC | Resting-state functional connectivity |
| SN | Salience network |
| SPM12 | Statistical parametric mapping |
| TE | Echo time |
| TI | Inversion time |
| TR | Repetition time |
| VN | Visual network |
Appendix A
| Cluster-Level p-Value | N Voxels | Peak-t | Peak-t MNI Coordinate | Anatomical Locations | %Clusters | %Region | ||
|---|---|---|---|---|---|---|---|---|
| BOLD: Decreased activation for ‘X’ (voxel-level p = 0.04) | 0.030 | 2973 | 5.15 | −12, −100, 18 | Occipital Lobe | Occipital_Sup_L | 16.01 | 34.85 |
| Lingual_L | 14.70 | 20.86 | ||||||
| Occipital_Mid_L | 12.18 | 11.07 | ||||||
| Cuneus_L | 11.40 | 22.21 | ||||||
| Fusiform_L | 7.13 | 9.18 | ||||||
| Occipital_Sup_R | 5.58 | 11.75 | ||||||
| Lingual_R | 5.58 | 7.22 | ||||||
| Cuneus_R | 5.21 | 10.88 | ||||||
| Occipital_Inf_L | 1.31 | 4.14 | ||||||
| Occipital_Mid_R | 0.61 | 0.86 | ||||||
| Basal Ganglia | Calcarine_L | 6.59 | 8.68 | |||||
| Calcarine_R | 6.36 | 10.16 | ||||||
| Temporal Lobe | Temporal_Inf_L | 3.26 | 3.03 | |||||
| Temporal_Mid_L | 0.67 | 0.40 | ||||||
| Cerebellum | Cerebelum_6_L | 2.72 | 4.78 | |||||
| Cerebelum_4_5_L | 0.54 | 1.42 | ||||||
| Vermis_6 | 0.07 | 0.54 | ||||||
| Parietal Lobe | Angular_L | 0.07 | 0.17 | |||||
| BOLD: Decreased activation for ‘X+O’ (voxel-level p = 0.01) | 0.048 | 692 | 6.04 | −12, −100, 16 | Occipital Lobe | Occipital_Sup_L | 48.12 | 24.38 |
| Cuneus_L | 24.13 | 10.94 | ||||||
| Occipital_Mid_L | 18.35 | 3.88 | ||||||
| Lingual_L | 1.30 | 0.43 | ||||||
| Basal Ganglia | Calcarine_L | 8.09 | 2.48 | |||||

References
- Mueller, A.; Hong, D.S.; Shepard, S.; Moore, T. Linking ADHD to the Neural Circuitry of Attention. Trends Cogn. Sci. 2017, 21, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Norris, C.J.; Creem, D.; Hendler, R.; Kober, H. Brief Mindfulness Meditation Improves Attention in Novices: Evidence from ERPs and Moderation by Neuroticism. Front. Hum. Neurosci. 2018, 12, 315. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.P.; Krompinger, J.; Baime, M.J. Mindfulness training modifies subsystems of attention. Cogn. Affect. Behav. Neurosci. 2007, 7, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Y.; Ma, Y.; Wang, J.; Fan, Y.; Feng, S.; Lu, Q.; Yu, Q.; Sui, D.; Rothbart, M.K.; Fan, M.; et al. Short-term meditation training improves attention and self-regulation. Proc. Natl. Acad. Sci. USA 2007, 104, 17152–17156. [Google Scholar] [CrossRef]
- Zeidan, F.; Johnson, S.K.; Diamond, B.J.; David, Z.; Goolkasian, P. Mindfulness meditation improves cognition: Evidence of brief mental training. Conscious. Cogn. 2010, 19, 597–605. [Google Scholar] [CrossRef]
- Lutz, A.; Slagter, H.A.; Dunne, J.D.; Davidson, R.J. Attention regulation and monitoring in meditation. Trends Cogn. Sci. 2008, 12, 163–169. [Google Scholar] [CrossRef]
- Holzel, B.K.; Ott, U.; Hempel, H.; Hackl, A.; Wolf, K.; Stark, R.; Vaitl, D. Differential engagement of anterior cingulate and adjacent medial frontal cortex in adept meditators and non-meditators. Neurosci. Lett. 2007, 421, 16–21. [Google Scholar] [CrossRef]
- Brefczynski-Lewis, J.A.; Lutz, A.; Schaefer, H.S.; Levinson, D.B.; Davidson, R.J. Neural correlates of attentional expertise in long-term meditation practitioners. Proc. Natl. Acad. Sci. USA 2007, 104, 11483–11488. [Google Scholar] [CrossRef]
- Farb, N.A.; Segal, Z.V.; Mayberg, H.; Bean, J.; McKeon, D.; Fatima, Z.; Anderson, A.K. Attending to the present: Mindfulness meditation reveals distinct neural modes of self-reference. Soc. Cogn. Affect. Neurosci. 2007, 2, 313–322. [Google Scholar] [CrossRef]
- Goldin, P.R.; Gross, J.J. Effects of mindfulness-based stress reduction (MBSR) on emotion regulation in social anxiety disorder. Emotion 2010, 10, 83–91. [Google Scholar] [CrossRef]
- Lomas, T.; Ivtzan, I.; Fu, C.H. A systematic review of the neurophysiology of mindfulness on EEG oscillations. Neurosci. Biobehav. Rev. 2015, 57, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, D.J.; McNaughton, N.; Flanagan, D.; Kirk, I.J. Frontal-midline theta from the perspective of hippocampal “theta”. Prog. Neurobiol. 2008, 86, 156–185. [Google Scholar] [CrossRef] [PubMed]
- DeLosAngeles, D.; Williams, G.; Burston, J.; Fitzgibbon, S.P.; Lewis, T.W.; Grummett, T.S.; Clark, C.R.; Pope, K.J.; Willoughby, J.O. Electroencephalographic correlates of states of concentrative meditation. Int. J. Psychophysiol. 2016, 110, 27–39. [Google Scholar] [CrossRef]
- Lee, D.J.; Kulubya, E.; Goldin, P.; Goodarzi, A.; Girgis, F. Review of the Neural Oscillations Underlying Meditation. Front. Neurosci. 2018, 12, 178. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.C.; Dixon, M.L.; Nijeboer, S.; Girn, M.; Floman, J.L.; Lifshitz, M.; Ellamil, M.; Sedlmeier, P.; Christoff, K. Functional neuroanatomy of meditation: A review and meta-analysis of 78 functional neuroimaging investigations. Neurosci. Biobehav. Rev. 2016, 65, 208–228. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.A.; Worhunsky, P.D.; Gray, J.R.; Tang, Y.Y.; Weber, J.; Kober, H. Meditation experience is associated with differences in default mode network activity and connectivity. Proc. Natl. Acad. Sci. USA 2011, 108, 20254–20259. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.F.; Norton, M.I.; Van Horn, J.D.; Wegner, D.M.; Grafton, S.T.; Macrae, C.N. Wandering minds: The default network and stimulus-independent thought. Science 2007, 315, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Lazar, S.W.; Kerr, C.E.; Wasserman, R.H.; Gray, J.R.; Greve, D.N.; Treadway, M.T.; McGarvey, M.; Quinn, B.T.; Dusek, J.A.; Benson, H.; et al. Meditation experience is associated with increased cortical thickness. Neuroreport 2005, 16, 1893–1897. [Google Scholar] [CrossRef]
- Grant, J.A.; Duerden, E.G.; Courtemanche, J.; Cherkasova, M.; Duncan, G.H.; Rainville, P. Cortical thickness, mental absorption and meditative practice: Possible implications for disorders of attention. Biol. Psychol. 2013, 92, 275–281. [Google Scholar] [CrossRef]
- Kang, D.H.; Jo, H.J.; Jung, W.H.; Kim, S.H.; Jung, Y.H.; Choi, C.H.; Lee, U.S.; An, S.C.; Jang, J.H.; Kwon, J.S. The effect of meditation on brain structure: Cortical thickness mapping and diffusion tensor imaging. Soc. Cogn. Affect. Neurosci. 2013, 8, 27–33. [Google Scholar] [CrossRef]
- Holzel, B.K.; Carmody, J.; Vangel, M.; Congleton, C.; Yerramsetti, S.M.; Gard, T.; Lazar, S.W. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011, 191, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Cotier, F.A.; Zhang, R.; Lee, T.M.C. A longitudinal study of the effect of short-term meditation training on functional network organization of the aging brain. Sci. Rep. 2017, 7, 598. [Google Scholar] [CrossRef]
- Froeliger, B.; Garland, E.L.; Kozink, R.V.; Modlin, L.A.; Chen, N.K.; McClernon, F.J.; Greeson, J.M.; Sobin, P. Meditation-State Functional Connectivity (msFC): Strengthening of the Dorsal Attention Network and Beyond. Evid. Based Complement. Alternat Med. 2012, 2012, 680407. [Google Scholar] [CrossRef] [PubMed]
- Hasenkamp, W.; Barsalou, L.W. Effects of meditation experience on functional connectivity of distributed brain networks. Front. Hum. Neurosci. 2012, 6, 38. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.H.; Jung, W.H.; Kang, D.H.; Byun, M.S.; Kwon, S.J.; Choi, C.H.; Kwon, J.S. Increased default mode network connectivity associated with meditation. Neurosci. Lett. 2011, 487, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Taren, A.A.; Gianaros, P.J.; Greco, C.M.; Lindsay, E.K.; Fairgrieve, A.; Brown, K.W.; Rosen, R.K.; Ferris, J.L.; Julson, E.; Marsland, A.L.; et al. Mindfulness Meditation Training and Executive Control Network Resting State Functional Connectivity: A Randomized Controlled Trial. Psychosom. Med. 2017, 79, 674–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luh, W.M.; Duan, W.; Zhou, G.D.; Weinschenk, G.; Anderson, A.K.; Dai, W. Longitudinal effects of meditation on brain resting-state functional connectivity. Sci. Rep. 2021, 11, 11361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Luh, W.M.; Duan, W.; Zhou, T.D.; Zhao, L.; Weinschenk, G.; Anderson, A.K.; Dai, W. The longitudinal effect of meditation on resting-state functional connectivity using dynamic arterial spin labeling: A feasibility study. Brain Sci. 2021, 11, 1263. [Google Scholar] [CrossRef]
- Creswell, J.D.; Taren, A.A.; Lindsay, E.K.; Greco, C.M.; Gianaros, P.J.; Fairgrieve, A.; Marsland, A.L.; Brown, K.W.; Way, B.M.; Rosen, R.K.; et al. Alterations in Resting-State Functional Connectivity Link Mindfulness Meditation with Reduced Interleukin-6: A Randomized Controlled Trial. Biol. Psychiatry 2016, 80, 53–61. [Google Scholar] [CrossRef]
- Sezer, I.; Pizzagalli, D.A.; Sacchet, M.D. Resting-state fMRI functional connectivity and mindfulness in clinical and non-clinical contexts: A review and synthesis. Neurosci. Biobehav. Rev. 2022, 135, 104583. [Google Scholar] [CrossRef]
- Stevens, M.C.; Calhoun, V.D.; Kiehl, K.A. fMRI in an oddball task: Effects of target-to-target interval. Psychophysiology 2005, 42, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Rusiniak, M.; Lewandowska, M.; Wolak, T.; Pluta, A.; Milner, R.; Ganc, M.; Wlodarczyk, A.; Senderski, A.; Sliwa, L.; Skarzynski, H. A modified oddball paradigm for investigation of neural correlates of attention: A simultaneous ERP-fMRI study. MAGMA 2013, 26, 511–526. [Google Scholar] [CrossRef] [PubMed]
- Ardekani, B.A.; Choi, S.J.; Hossein-Zadeh, G.A.; Porjesz, B.; Tanabe, J.L.; Lim, K.O.; Bilder, R.; Helpern, J.A.; Begleiter, H. Functional magnetic resonance imaging of brain activity in the visual oddball task. Brain Res. Cogn. Brain Res. 2002, 14, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Stevens, A.A.; Skudlarski, P.; Gatenby, J.C.; Gore, J.C. Event-related fMRI of auditory and visual oddball tasks. Magn. Reson. Imaging 2000, 18, 495–502. [Google Scholar] [CrossRef]
- Devaney, K.J.; Levin, E.J.; Tripathi, V.; Higgins, J.P.; Lazar, S.W.; Somers, D.C. Attention and Default Mode Network Assessments of Meditation Experience during Active Cognition and Rest. Brain Sci. 2021, 11, 566. [Google Scholar] [CrossRef] [PubMed]
- Kozasa, E.H.; Balardin, J.B.; Sato, J.R.; Chaim, K.T.; Lacerda, S.S.; Radvany, J.; Mello, L.; Amaro, E., Jr. Effects of a 7-Day Meditation Retreat on the Brain Function of Meditators and Non-Meditators During an Attention Task. Front. Hum. Neurosci. 2018, 12, 222. [Google Scholar] [CrossRef] [PubMed]
- Rischka, L.; Godbersen, G.M.; Pichler, V.; Michenthaler, P.; Klug, S.; Klobl, M.; Ritter, V.; Wadsak, W.; Hacker, M.; Kasper, S.; et al. Reliability of task-specific neuronal activation assessed with functional PET, ASL and BOLD imaging. J. Cereb. Blood Flow. Metab. 2021, 41, 2986–2999. [Google Scholar] [CrossRef]
- Dai, W.; Varma, G.; Scheidegger, R.; Alsop, D.C. Quantifying fluctuations of resting state networks using arterial spin labeling perfusion MRI. J. Cereb. Blood Flow. Metab. 2016, 36, 463–473. [Google Scholar] [CrossRef]
- Zhao, L.; Alsop, D.C.; Detre, J.A.; Dai, W. Global Fluctuations of Cerebral Blood Flow Indicate a Global Brain Network Independent of Systemic Factors. J. Cereb. Blood Flow. Metab. 2019, 39, 302–312. [Google Scholar] [CrossRef]
- Hasenkamp, W.; Wilson-Mendenhall, C.D.; Duncan, E.; Barsalou, L.W. Mind wandering and attention during focused meditation: A fine-grained temporal analysis of fluctuating cognitive states. Neuroimage 2012, 59, 750–760. [Google Scholar] [CrossRef]
- Di Cesare, G.; Marchi, M.; Lombardi, G.; Gerbella, M.; Sciutti, A.; Rizzolatti, G. The middle cingulate cortex and dorso-central insula: A mirror circuit encoding observation and execution of vitality forms. Proc. Natl. Acad. Sci. USA 2021, 118, e2111358118. [Google Scholar] [CrossRef]
- Craig, A.D. How do you feel—now? The anterior insula and human awareness. Nat. Rev. Neurosci. 2009, 10, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Garrison, K.A.; Zeffiro, T.A.; Scheinost, D.; Constable, R.T.; Brewer, J.A. Meditation leads to reduced default mode network activity beyond an active task. Cogn. Affect. Behav. Neurosci. 2015, 15, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Vincent, J.L.; Kahn, I.; Snyder, A.Z.; Raichle, M.E.; Buckner, R.L. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008, 100, 3328–3342. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.L.; Christoff, K. The decision to engage cognitive control is driven by expected reward-value: Neural and behavioral evidence. PLoS ONE 2012, 7, e51637. [Google Scholar] [CrossRef] [PubMed]
- Dixon, M.L.; Fox, K.C.; Christoff, K. Evidence for rostro-caudal functional organization in multiple brain areas related to goal-directed behavior. Brain Res. 2014, 1572, 26–39. [Google Scholar] [CrossRef]
- Guidotti, R.; Del Gratta, C.; Perrucci, M.G.; Romani, G.L.; Raffone, A. Neuroplasticity within and between Functional Brain Networks in Mental Training Based on Long-Term Meditation. Brain Sci. 2021, 11, 1086. [Google Scholar] [CrossRef]
- Raffone, A.; Marzetti, L.; Del Gratta, C.; Perrucci, M.G.; Romani, G.L.; Pizzella, V. Toward a brain theory of meditation. Prog. Brain Res. 2019, 244, 207–232. [Google Scholar]
- Vago, D.R.; Zeidan, F. The brain on silent: Mind wandering, mindful awareness, and states of mental tranquility. Ann. N. Y. Acad. Sci. 2016, 1373, 96–113. [Google Scholar] [CrossRef]



| Subject ID | Age (Years) | Gender | Practice Time (Minutes) |
|---|---|---|---|
| 1 | 19 | Female | 135 |
| 2 | 20 | Male | 1080 |
| 3 | 19 | Male | 1620 |
| 4 | 19 | Male | 400 |
| 5 a | 19 | Male | 360 |
| 6 b | 19 | Female | 720 |
| 7 | 18 | Male | 160 |
| 8 | 20 | Male | 495 |
| 9 ab | 19 | Female | N/A |
| 10 | 19 | Female | 210 |
| 11 | 19 | Female | 560 |
| Cluster-Level p-Value | N Voxels | Peak-t | Peak-t MNI Coordinate | Anatomical Locations | %Clusters | %Region | ||
|---|---|---|---|---|---|---|---|---|
| ASL: Increased activation for ‘X’ | <10−3 | 760 | 9.54 | 4, −32, 34 | Limbic System | Cingulum_Mid_R | 61.18 | 21.11 |
| Cingulum_Mid_L | 22.24 | 8.71 | ||||||
| Cingulum_Post_L | 4.08 | 6.70 | ||||||
| Cingulum_Post_R | 3.68 | 8.36 | ||||||
| Cingulum_Ant_L | 0.79 | 0.43 | ||||||
| Cingulum_Ant_R | 0.13 | 0.08 | ||||||
| Parietal Lobe | Precuneus_R | 7.11 | 1.65 | |||||
| Paracentral_Lobule_L | 0.26 | 0.15 | ||||||
| Frontal Lobe | Supp_Motor_Area_R | 0.39 | 0.13 | |||||
| Supp_Motor_Area_L | 0.13 | 0.05 | ||||||
| ASL: Increased activation for ‘X+O’ | 0.004 | 444 | 7.07 | 0, −38, 28 | Limbic System | Cingulum_Mid_R | 67.12 | 13.53 |
| Cingulum_Mid_L | 12.84 | 2.94 | ||||||
| Cingulum_Post_L | 7.88 | 7.56 | ||||||
| Cingulum_Post_R | 4.28 | 5.67 | ||||||
| Cingulum_Ant_L | 1.35 | 0.43 | ||||||
| Parietal Lobe | Precuneus_R | 6.53 | 0.89 | |||||
| 0.031 | 304 | 8.06 | 52, −46, 0 | Temporal Lobe | Temporal_Sup_R | 54.28 | 5.25 | |
| Temporal_Mid_R | 45.39 | 3.13 | ||||||
| Parietal Lobe | SupraMarginal_R | 0.33 | 0.05 | |||||
| Cluster-Level p-Value | N Voxels | Peak-t | Peak-t MNI Coordinate | Anatomical Locations | %Clusters | %Region | ||
|---|---|---|---|---|---|---|---|---|
| ASL: Association with practice time for ‘X’ | 0.027 | 292 | 9.74 | −44, −52, −24 | Occipital Lobe | Fusiform_L | 50.68 | 6.41 |
| Temporal Lobe | Temporal_Inf_L | 23.63 | 2.16 | |||||
| Cerebellum | Cerebelum_Crus1_L | 22.60 | 2.54 | |||||
| Cerebelum_6_L | 3.08 | 0.53 | ||||||
| ASL: Association with practice time for ‘O’ | 0.008 | 332 | 10.02 | −14, 52, 20 | Frontal Lobe | Frontal_Sup_L | 55.42 | 5.11 |
| Frontal_Sup_Medial_L | 31.33 | 3.48 | ||||||
| Frontal_Mid_L | 6.02 | 0.41 | ||||||
| Frontal_Sup_Medial_R | 5.12 | 0.80 | ||||||
| Limbic System | Cingulum_Ant_R | 2.11 | 0.53 | |||||
| ASL: Association with practice time for ‘X+O’ | 0.012 | 321 | 8.62 | −4, 64, 24 | Frontal Lobe | Frontal_Sup_L | 54.52 | 4.86 |
| Frontal_Sup_Medial_L | 31.46 | 3.38 | ||||||
| Frontal_Sup_Medial_R | 5.61 | 0.84 | ||||||
| Frontal_Mid_L | 2.80 | 0.19 | ||||||
| Limbic System | Cingulum_Ant_R | 5.61 | 1.37 | |||||
| ASL: Association with practice time for ‘X−O’ | 0.044 | 250 | 16.43 | 42, 18, 48 | Frontal Lobe | Frontal_Mid_R | 100.00 | 4.90 |
| BOLD: Association with practice time for ‘X’ | <10−4 | 912 | 8.93 | −44, 32, 12 | Frontal Lobe | Precentral_L | 42.21 | 10.92 |
| Frontal_Inf_Tri_L | 29.71 | 10.72 | ||||||
| Frontal_Inf_Oper_L | 14.91 | 13.10 | ||||||
| Frontal_Mid_L | 12.72 | 2.39 | ||||||
| Rolandic_Oper_L | 0.44 | 0.40 | ||||||
| BOLD: Association with practice time for ‘O’ | 0.019 | 452 | 13.31 | −56, −42, 52 | Parietal Lobe | Parietal_Inf_L | 39.82 | 7.36 |
| SupraMaginal_L | 25.44 | 9.16 | ||||||
| Temporal Lobe | Temporal_Sup_L | 24.34 | 4.79 | |||||
| Frontal Lobe | Rolandic_Oper_L | 10.40 | 4.75 | |||||
| 0.001 | 711 | 10.43 | −36, 12, 30 | Frontal Lobe | Precentral_L | 48.10 | 9.70 | |
| Frontal_Inf_Oper_L | 24.19 | 16.57 | ||||||
| Frontal_Mid_L | 22.78 | 3.33 | ||||||
| Frontal_Inf_Tri_L | 4.36 | 1.23 | ||||||
| Rolandic_Oper_L | 0.56 | 0.40 | ||||||
| BOLD: Association with practice time for ‘X+O’ | <10−4 | 783 | 8.97 | −32, 14, 38 | Frontal Lobe | Precentral_L | 53.77 | 11.94 |
| Frontal_Inf_Oper_L | 21.71 | 16.38 | ||||||
| Frontal_Mid_L | 18.39 | 2.96 | ||||||
| Frontal_Inf_Tri_L | 5.49 | 1.70 | ||||||
| Rolandic_Oper_L | 0.64 | 0.51 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, S.; Zhang, Z.; Duan, W.; Zhao, L.; Weinschenk, G.; Luh, W.-M.; Anderson, A.K.; Dai, W. Effect of Meditation on Brain Activity during an Attention Task: A Comparison Study of ASL and BOLD Task fMRI. Brain Sci. 2023, 13, 1653. https://doi.org/10.3390/brainsci13121653
Zhang Y, Chen S, Zhang Z, Duan W, Zhao L, Weinschenk G, Luh W-M, Anderson AK, Dai W. Effect of Meditation on Brain Activity during an Attention Task: A Comparison Study of ASL and BOLD Task fMRI. Brain Sciences. 2023; 13(12):1653. https://doi.org/10.3390/brainsci13121653
Chicago/Turabian StyleZhang, Yakun, Shichun Chen, Zongpai Zhang, Wenna Duan, Li Zhao, George Weinschenk, Wen-Ming Luh, Adam K. Anderson, and Weiying Dai. 2023. "Effect of Meditation on Brain Activity during an Attention Task: A Comparison Study of ASL and BOLD Task fMRI" Brain Sciences 13, no. 12: 1653. https://doi.org/10.3390/brainsci13121653
APA StyleZhang, Y., Chen, S., Zhang, Z., Duan, W., Zhao, L., Weinschenk, G., Luh, W.-M., Anderson, A. K., & Dai, W. (2023). Effect of Meditation on Brain Activity during an Attention Task: A Comparison Study of ASL and BOLD Task fMRI. Brain Sciences, 13(12), 1653. https://doi.org/10.3390/brainsci13121653






