Children with Autism Spectrum Disorder Exhibit Elevated Physical Activity and Reduced Sedentary Behavior
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Physical Activity Measurement
2.3. Anthropometric Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Sedentary Activity in ASD Participants
4.2. Vigorous PA in ASD Patients
4.3. Vigorous PA and Sleep Disruptions in ASD Patients
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 5th ed; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Maenner, M.J.; Warren, Z.; Williams, A.R.; Amoakohene, E.; Bakian, A.V.; Bilder, D.A.; Shaw, K.A. Prevalence and Characteristics of Autism Spectrum Disorder Among Children Aged 8 Years—Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2020. MMWR Surveill. Summ. 2023, 72, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.J.; Rosenqvist, M.A.; Larsson, H.; Gillberg, C.; D’Onofrio, B.M.; Lichtenstein, P.; Lundström, S. Etiology of Autism Spectrum Disorders and Autistic Traits Over Time. JAMA Psychiatry 2020, 77, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Manoli, D.S.; State, M.W. Autism Spectrum Disorder Genetics and the Search for Pathological Mechanisms. Am. J. Psychiatry 2021, 178, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.; Niyazov, D.M.; Rossignol, D.A.; Goldenthal, M.; Kahler, S.G.; Frye, R.E. Clinical and Molecular Characteristics of Mitochondrial Dysfunction in Autism Spectrum Disorder. Mol. Diagn. Ther. 2018, 22, 571–593. [Google Scholar] [CrossRef] [PubMed]
- Montanari, M.; Martella, G.; Bonsi, P.; Meringolo, M. Autism Spectrum Disorder: Focus on Glutamatergic Neurotransmission. Int. J. Mol. Sci. 2022, 23, 3861. [Google Scholar] [CrossRef]
- Pitzianti, M.; Fagioli, S.; Pontis, M.; Pasini, A. Attention Deficits Influence the Development of Motor Abnormalities in High Functioning Autism. Child Psychiatry Hum. Dev. 2021, 52, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Leech, K.A.; Tager-Flusberg, H.; Nelson, C.A. Development of fine motor skills is associated with expressive language outcomes in infants at high and low risk for autism spectrum disorder. J. Neurodev. Disord. 2018, 10, 14. [Google Scholar] [CrossRef] [PubMed]
- Mohd Nordin, A.; Ismail, J.; Kamal Nor, N. Motor Development in Children With Autism Spectrum Disorder. Front. Pediatr. 2021, 9, 598276. [Google Scholar] [CrossRef]
- Bandini, L.G.; Gleason, J.; Curtin, C.; Lividini, K.; Anderson, S.E.; Cermak, S.A.; Maslin, M.; Must, A. Comparison of physical activity between children with autism spectrum disorders and typically developing children. Autism 2013, 17, 44–54. [Google Scholar] [CrossRef]
- Tyler, K.; MacDonald, M.; Menear, K. Physical Activity and Physical Fitness of School-Aged Children and Youth with Autism Spectrum Disorders. Autism Res. Treat. 2014, 2014, 312163. [Google Scholar] [CrossRef]
- Fang, Q.; Aiken, C.A.; Fang, C.; Pan, Z. Effects of Exergaming on Physical and Cognitive Functions in Individuals with Autism Spectrum Disorder: A Systematic Review. Games Health J. 2019, 8, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Toscano, C.V.A.; Ferreira, J.P.; Quinaud, R.T.; Silva, K.M.N.; Carvalho, H.M.; Gaspar, J.M. Exercise improves the social and behavioral skills of children and adolescent with autism spectrum disorders. Front. Psychiatry 2022, 13, 1027799. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.; Lord, C.; Ulrich, D.A. The Relationship of Motor Skills and Social Communicative Skills in School-Aged Children With Autism Spectrum Disorder. Adapt. Phys. Act. Q. 2013, 30, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.N.; Galloway, J.C.; Landa, R.J. Relation between early motor delay and later communication delay in infants at risk for autism. Infant Behav. Dev. 2012, 35, 838–846. [Google Scholar] [CrossRef]
- Ohara, R.; Kanejima, Y.; Kitamura, M.; Izawa, K.P. Association between Social Skills and Motor Skills in Individuals with Autism Spectrum Disorder: A Systematic Review. Eur. J. Investig. Health Psychol. Educ. 2019, 10, 276–296. [Google Scholar] [CrossRef]
- West, K.L. Infant motor development in autism spectrum disorder: A synthesis and meta-analysis. Child Dev. 2019, 90, 2053–2070. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Huang, J.; Du, C.; Liu, J.; Tan, G. Meta-Analysis on Intervention Effects of Physical Activities on Children and Adolescents with Autism. Int. J. Environ. Res. Public Health 2020, 17, 1950. [Google Scholar] [CrossRef]
- Alhowikan, A. Benefits of physical activity for autism spectrum disorders: A systematic review. Saudi J. Sports Med. 2016, 16, 163. [Google Scholar] [CrossRef]
- Thomas, S.; Hinkley, T.; Barnett, L.M.; May, T.; Rinehart, N. Young Children with ASD Participate in the Same Level of Physical Activity as Children Without ASD: Implications for Early Intervention to Maintain Good Health. J. Autism Dev. Disord. 2019, 49, 3278–3289. [Google Scholar] [CrossRef]
- Bremer, E.; Crozier, M.; Lloyd, M. A systematic review of the behavioural outcomes following exercise interventions for children and youth with autism spectrum disorder. Autism 2016, 20, 899–915. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.-T.; Luo, H.-J. Effect of physical activity interventions on children and adolescents with autism spectrum disorder: A systematic review and meta-analysis. Physiotherapy 2015, 101, e1685–e1686. [Google Scholar]
- Liang, X.; Li, R.; Wong, S.H.S.; Sum, R.K.W.; Sit, C.H.P. Accelerometer-measured physical activity levels in children and adolescents with autism spectrum disorder: A systematic review. Prev. Med. Rep. 2020, 19, 101147. [Google Scholar] [CrossRef] [PubMed]
- Su, W.C.; Amonkar, N.; Cleffi, C.; Srinivasan, S.; Bhat, A. Neural Effects of Physical Activity and Movement Interventions in Individuals With Developmental Disabilities-A Systematic Review. Front. Psychiatry 2022, 13, 794652. [Google Scholar] [CrossRef] [PubMed]
- Mccoy, S.M.; Jakicic, J.M.; Gibbs, B.B. Comparison of Obesity, Physical Activity, and Sedentary Behaviors Between Adolescents With Autism Spectrum Disorders and Without. J. Autism Dev. Disord. 2016, 46, 2317–2726. [Google Scholar] [CrossRef] [PubMed]
- Guner, U.U.C.; İrem, B. The Relationship between Nutrition-Physical Activity Behaviors of Autistic Children with Their Families and Children’s Obesity Levels During Covid Pandemic. J. Autism Dev. Disord. 2022. [Google Scholar] [CrossRef] [PubMed]
- Memari, A.H.; Ghaheri, B.; Ziaee, V.; Kordi, R.; Hafizi, S.; Moshayedi, P. Physical activity in children and adolescents with autism assessed by triaxial accelerometry. Pediatr. Obes. 2013, 8, 150–158. [Google Scholar] [CrossRef]
- Gehricke, J.G.; Chan, J.; Farmer, J.G.; Fenning, R.M.; Steinberg-Epstein, R.; Misra, M.; Parker, R.A.; Neumeyer, A.M. Physical activity rates in children and adolescents with autism spectrum disorder compared to the general population. Res. Autism Spectr. Disord. 2020, 70, 101490. [Google Scholar] [CrossRef]
- Li, R.; Liang, X.; Zhou, Y.; Ren, Z. A Systematic Review and Meta-Analysis of Moderate-to-Vigorous Physical Activity Levels in Children and Adolescents With and Without ASD in Inclusive Schools. Front. Pediatr. 2021, 9, 726942. [Google Scholar] [CrossRef]
- Hauck, J.L.; Ketcheson, L.R. Ulrich DA. Methodology to Promote Physical Activity Monitoring Adherence in Youth with Autism Spectrum Disorder. Front. Public Health 2016, 4, 206. [Google Scholar] [CrossRef]
- Haegele, J.; Zhu, X.; Bennett, H. Accelerometer Measured Physical Activity among Youth with Autism and Age, Sex, and Body Mass Index Matched Peers: A Preliminary Study. Disabil. Health J. 2021, 14, 101102. [Google Scholar] [CrossRef]
- Sirard, J.; Trost, S.; Pfeiffer, K.; Dowda, M.; Pate, R. Calibration and Evaluation of an Objective Measure of Physical Activity in Preschool Children. J. Phys. Act. Health 2011, 3. [Google Scholar] [CrossRef]
- Moulton, E.; Bradbury, K.; Barton, M.; Fein, D. Factor Analysis of the Childhood Autism Rating Scale in a Sample of Two Year Olds with an Autism Spectrum Disorder. J. Autism Dev. Disord. 2016, 49, 2733–2746. [Google Scholar] [CrossRef] [PubMed]
- Schanding, G.T.; Nowell, K.P.; Goin-Kochel, R.P. Utility of the Social Communication Questionnaire-Current and Social Responsiveness Scale as Teacher-Report Screening Tools for Autism Spectrum Disorders. J. Autism Dev. Disord. 2011, 42, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.E.; John, D.; Freedson, P.S. Validation and comparison of ActiGraph activity monitors. J. Sci. Med. Sport/Sports Med. Aust. 2011, 14, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Malow, B.; Fawkes, D.; Weiss, S.; Reynolds, A.; Loh, A.; Adkins, K.; Wofford, D.; Wyatt, A.; Goldman, S. Actigraphy in Children with Autism Spectrum Disorders: Strategies for Success. In Proceedings of the 2014 International Meeting for Autism Research, Atlanta, GA, USA, 14–17 May 2014; Available online: https://www.researchgate.net/publication/268130972 (accessed on 8 August 2022).
- Freedson, P.S.; Melanson, E.; Sirard, J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med. Sci. Sports Exerc. 1998, 30, 777–781. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Global Recommendations on Physical Activity For health; World Health Organization (WHO): Geneva, Switzerland, 2010. [Google Scholar]
- Sandt, D.D.R.; Frey, G.C. Comparison of Physical Activity Levels between Children with and Without Autistic Spectrum Disorders. Adapt. Phys. Act. Q. 1998, 22, 146–159. [Google Scholar] [CrossRef]
- Ketcheson, L.; Hauck, J.L.; Ulrich, D. The levels of physical activity and motor skills in young children with and without autism spectrum disorder, aged 2–5 years. Autism 2017, 22, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, N. A Functional Analysis of Physical Activity in Children with Autism Spectrum Disorder. Thesis Projects. 28. 2021. Available online: https://scholarship.rollins.edu/mabacs_thesis/28 (accessed on 8 August 2022).
- Pan, C.-Y. Objectively Measured Physical Activity Between Children With Autism Spectrum Disorders and Children Without Disabilities During Inclusive Recess Settings in Taiwan. J. Autism Dev. Disord. 2008, 38, 1292–1301. [Google Scholar] [CrossRef]
- Elia, J.; Izaki, Y.; Ambrosini, A.; Hakonarson, H. Glutamatergic Neurotransmission in ADHD: Neurodevelopment and Pharmacological Implications. J. Pediatr. Neonatol. 2020, 1, 1006. [Google Scholar]
- Baskerville, R.; McGrath, T.; Castell, L. The effects of physical activity on glutamate neurotransmission in neuropsychiatric disorders. Front. Sports Act. Living 2023, 5, 1147384. [Google Scholar] [CrossRef] [PubMed]
- El-Ansary, A. GABA and Glutamate Imbalance in Autism and Their Reversal as Novel Hypothesis for Effective Treatment Strategy. Autism Dev. Disord. 2020, 18, 46–63. [Google Scholar] [CrossRef]
- Lipton, S.A.; Nicotera, P. Calcium, free radicals and excitotoxins in neuronal apoptosis. Cell Calcium 1998, 23, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Lipton, S.A. Paradigm shift in neuroprotection by NMDA receptor blockade: Memantine and beyond. Nat. Rev. Drug Discov. 2006, 5, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Lugo, J.; Fadeuilhe, C.; Gisbert, L.; Setien, I.; Delgado, M.; Corrales, M.; Richarte, V.; Ramos-Quiroga, J.A. Sleep in adults with autism spectrum disorder and attention deficit/hyperactivity disorder: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2020, 38, 1–24. [Google Scholar] [CrossRef]
- Gunes, S.; Ekinci, O.; Feyzioglu, A.; Ekinci, N.; Kalinli, M. Sleep problems in children with autism spectrum disorder: Clinical correlates and the impact of attention deficit hyperactivity disorder. Neuropsychiatr. Dis. Treat. 2019, 15, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Stutz, J.; Eiholzer, R.; Spengler, C. Effects of evening exercise on sleep in healthy participants: A systematic review and metaanalysis. Sports Med. 2019, 49, 269–287. [Google Scholar] [CrossRef]
- Krakowiak, P.; Goodlin-Jones, B.; Hertz-Picciotto, I. Sleep problems in children with autism spectrum disorders, developmental delays, and typical development: A population based study. J. Sleep Res. 2008, 17, 197–206. [Google Scholar] [CrossRef]
- Wang, F.; Boros, S. The effect of physical activity on sleep quality: A systematic review. Eur. J. Physiother. 2019, 23, 1–8. [Google Scholar] [CrossRef]
- Bricout, V.A.; Pace, M.; Guinot, M. Sleep and Physical Activity in Children with Autism Spectrum: About 3 Clinical Cases. Austin J. Autism Relat. Disabil. 2018, 4, 1049–1052. [Google Scholar]
- Bell, B.J.; Hollinger, K.R.; Deme, P.; Sakamoto, S.; Hasegawa, Y.; Volsky, D.; Kamiya, A.; Haughey, N.; Zhu, X.; Slusher, B.S. Glutamine antagonist JHU083 improves psychosocial behavior and sleep deficits in EcoHIV-infected mice. Brain Behav. Immun. Health 2022, 9, 100478. [Google Scholar] [CrossRef]
| Patient ID | CARS | SRS |
|---|---|---|
| 31 | 37 | 98 |
| 32 | 36 | 99 |
| 33 | 38 | 61 |
| 34 | 36 | 66 |
| 35 | 34 | 66 |
| 36 | 35 | 65 |
| 37 | 40 | 77 |
| 38 | 39 | 80 |
| 39 | 38 | 36 |
| 40 | 36 | 44 |
| 41 | 35 | 47 |
| 42 | 36 | 56 |
| 43 | 38 | 55 |
| 44 | 40 | 35 |
| 45 | 41 | 33 |
| 46 | 37 | 79 |
| 47 | 35 | 77 |
| 48 | 35 | 43 |
| 49 | 36 | 44 |
| 50 | 34 | 46 |
| 51 | 34 | 89 |
| Variable | Autism (N = 21) | Controls (N = 30) | p-Value | Power |
|---|---|---|---|---|
| Age (years) | 6.43 ± 2.29 | 7.20 ± 3.14 | 0.342 | 0.59 |
| Weight (kg) | 27.24 ± 11.45 | 27.81 ± 11.04 | 0.861 | 0.50 |
| Height (cm) | 125.19 ± 15.73 | 126.96 ± 16.28 | 0.706 | 0.48 |
| BMI (kg/m2) | 16.75 ± 3.68 | 16.60 ± 2.37 | 0.870 | 0.50 |
| Percentile | 48.91 ± 39.60 | 51.48 ± 31.64 | 0.804 | 0.51 |
| Waist (cm) | 57.20 ± 11.17 | 58.26 ± 9.40 | 0.726 | 0.51 |
| Hip (cm) | 68.40 ± 10.59 | 69.78 ± 9.72 | 0.646 | 0.52 |
| Waist-to-hip ratio | 0.83 ± 0.08 | 0.83 ± 0.04 | 0.981 | 0.50 |
| Skin fold—triceps (mm) | 12.01 ± 7.97 | 10.32 ± 5.24 | 0.396 | 0.58 |
| Skin fold—subscapular (mm) | 11.04 ± 8.06 | 7.44 ± 4.24 | 0.059 | 0.77 |
| Hand grip strength KG | 6.59 ± 0.82 | 9.09 ± 3.71 | 0.069 | 0.92 |
| METs | 1.99 ± 0.32 | 1.63 ± 0.22 | 0.001 * | 0.98 |
| Variable | Autism (N = 21) | Controls (N = 30) | p-Value | Power |
|---|---|---|---|---|
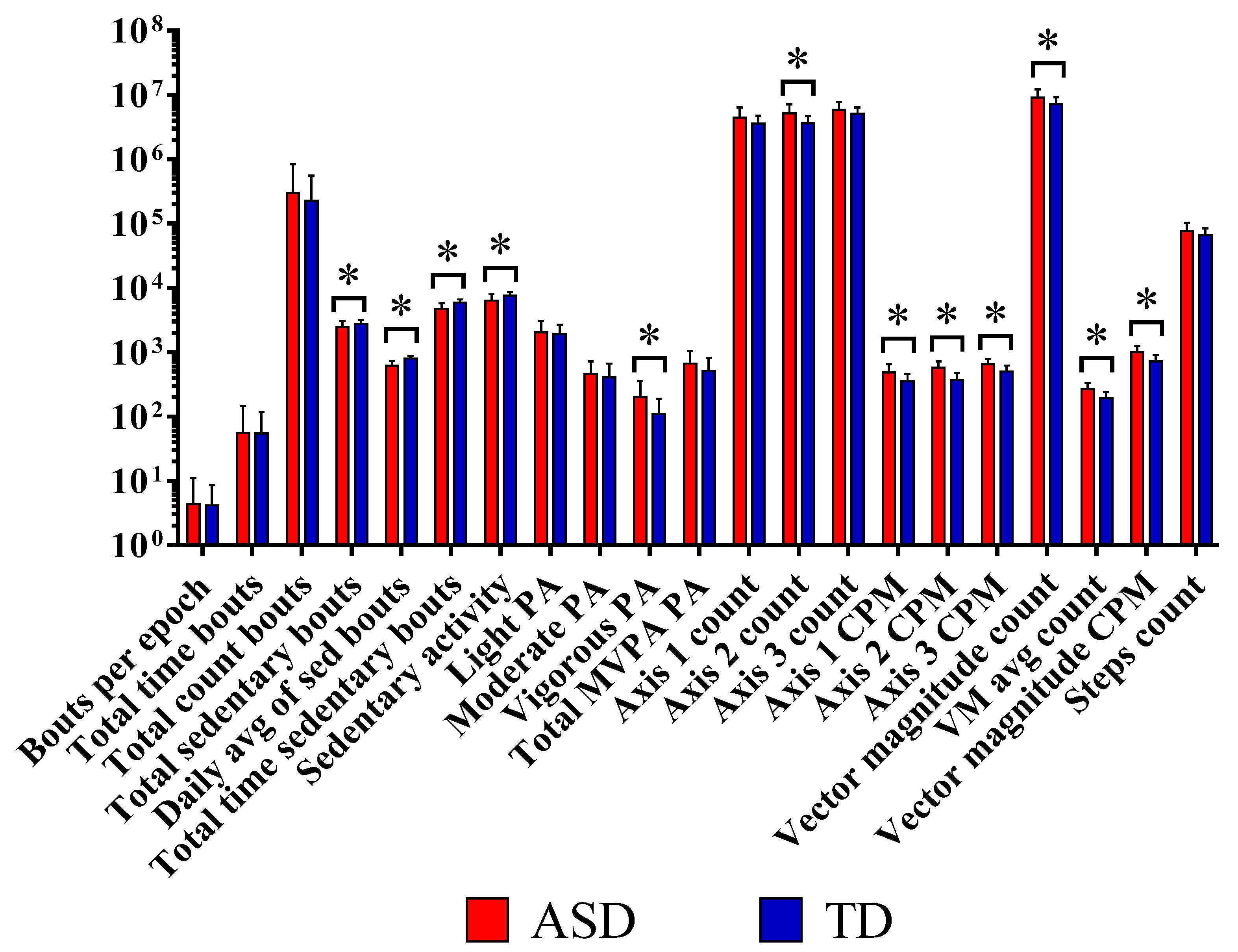
| Bouts per epoch | 4.33 ±6.71 | 4.13 ± 4.54 | 0.899 | 0.50 |
| Total time bouts | 55.49 ± 90.23 | 54.32 ± 63.42 | 0.957 | 0.50 |
| Total counts bouts | 298,698.14 ± 536,651.88 | 226,350.43 ± 329,120.57 | 0.554 | 0.53 |
| Total sedentary bouts | 2476.95 ± 598.25 | 2752.23 ± 365.64 | 0.047 * | 0.77 |
| Daily avg. sed. bouts | 615.53 ± 121.37 | 790.63 ± 90.16 | <0.001 * | 1.00 |
| Total time sedentary bouts | 4703.20 ± 1099.91 | 5934.34 ± 702.18 | <0.001 * | 0.98 |
| Sedentary activity | 6307.04 ± 1622.23 | 7570.66 ± 1003.51 | 0.001 * | 0.93 |
| Light PA | 2039.48 ± 1014.75 | 1925.73 ± 754.36 | 0.648 | 0.52 |
| Moderate PA | 462.85 ± 257.34 | 406.98 ± 257.54 | 0.449 | 0.56 |
| Vigorous PA | 201.55 ± 152.70 | 109.23 ± 79.08 | 0.017 * | 0.87 |
| Total MVPA PA | 664.39 ± 384.90 | 516.20 ± 304.78 | 0.132 | 0.69 |
| Axis 1 counts | 4,435,262.71 ± 1,901,170.01 | 3,545,304 ± 1,192,223 | 0.067 | 0.77 |
| Axis 2 counts | 5,192,411.81 ± 1,866,204.11 | 3,646,306 ± 1,029,070 | 0.001 * | 0.95 |
| Axis 3 counts | 5,873,091.76 ± 1,898,634.97 | 5,033,960 ± 1,362,121 | 0.092 | 0.74 |
| Axis 1 CPM | 484.20 ± 166.20 | 352.66 ± 107.74 | 0.007 * | 0.93 |
| Axis 2 CPM | 570.22 ± 144.02 | 366.19 ± 104.44 | <0.001 * | 1.00 |
| Axis 3 CPM | 645.21 ± 140.03 | 501.40 ± 120.83 | <0.001 * | 1.00 |
| Vector magnitude counts | 9,040,543 ± 3,172,723 | 7,184,405 ± 1,977,732 | 0.024 * | 0.85 |
| VM avg. counts | 264.72 ± 65.30 | 191.96 ± 47.96 | <0.001 * | 0.98 |
| Vector magnitude CPM | 991.62 ± 245.30 | 716.92 ± 181.32 | <0.001 * | 0.98 |
| Steps counts | 75,742 ± 27,182 | 65,762 ± 18,197 | 0.152 | 0.69 |
| Variable | ASD | TD | Statistical Significance of the Differences between ra and rt | |||
|---|---|---|---|---|---|---|
| ra | p-Value | rt | p-Value | Test Statistic (z) | p-Value | |
| Time spent in light-intensity activity | 0.552 | 0.009 | 0.040 | 0.8 | n/a | n/a |
| Time spent in moderate-intensity activity | 0.549 | 0.01 | 0.633 | 0.001 | −0.4254 | 0.6705 |
| Time spent in vigorous-intensity activity | 0.759 | 0.001 | 0.497 | 0.005 | 1.4740 | 0.1405 |
| Time spent in MVPA | 0.668 | 0.001 | 0.664 | 0.001 | 0.0236 | 0.9812 |
| METs | 0.768 | 0.001 | 0.773 | 0.001 | −0.0404 | 0.9677 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhowikan, A.M.; Elamin, N.E.; Aldayel, S.S.; AlSiddiqi, S.A.; Alrowais, F.S.; Hassan, W.M.; El-Ansary, A.; Alghamdi, F.A.; AL-Ayadhi, L.Y. Children with Autism Spectrum Disorder Exhibit Elevated Physical Activity and Reduced Sedentary Behavior. Brain Sci. 2023, 13, 1575. https://doi.org/10.3390/brainsci13111575
Alhowikan AM, Elamin NE, Aldayel SS, AlSiddiqi SA, Alrowais FS, Hassan WM, El-Ansary A, Alghamdi FA, AL-Ayadhi LY. Children with Autism Spectrum Disorder Exhibit Elevated Physical Activity and Reduced Sedentary Behavior. Brain Sciences. 2023; 13(11):1575. https://doi.org/10.3390/brainsci13111575
Chicago/Turabian StyleAlhowikan, Abdulrahman M., Nadra E. Elamin, Sarah S. Aldayel, Sara A. AlSiddiqi, Fai S. Alrowais, Wail M. Hassan, Afaf El-Ansary, Farah Ali Alghamdi, and Laila Y. AL-Ayadhi. 2023. "Children with Autism Spectrum Disorder Exhibit Elevated Physical Activity and Reduced Sedentary Behavior" Brain Sciences 13, no. 11: 1575. https://doi.org/10.3390/brainsci13111575
APA StyleAlhowikan, A. M., Elamin, N. E., Aldayel, S. S., AlSiddiqi, S. A., Alrowais, F. S., Hassan, W. M., El-Ansary, A., Alghamdi, F. A., & AL-Ayadhi, L. Y. (2023). Children with Autism Spectrum Disorder Exhibit Elevated Physical Activity and Reduced Sedentary Behavior. Brain Sciences, 13(11), 1575. https://doi.org/10.3390/brainsci13111575








