Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging-Tractography in Resective Brain Surgery: Lesion Coverage Strategies and Patient Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Anatomical MRI
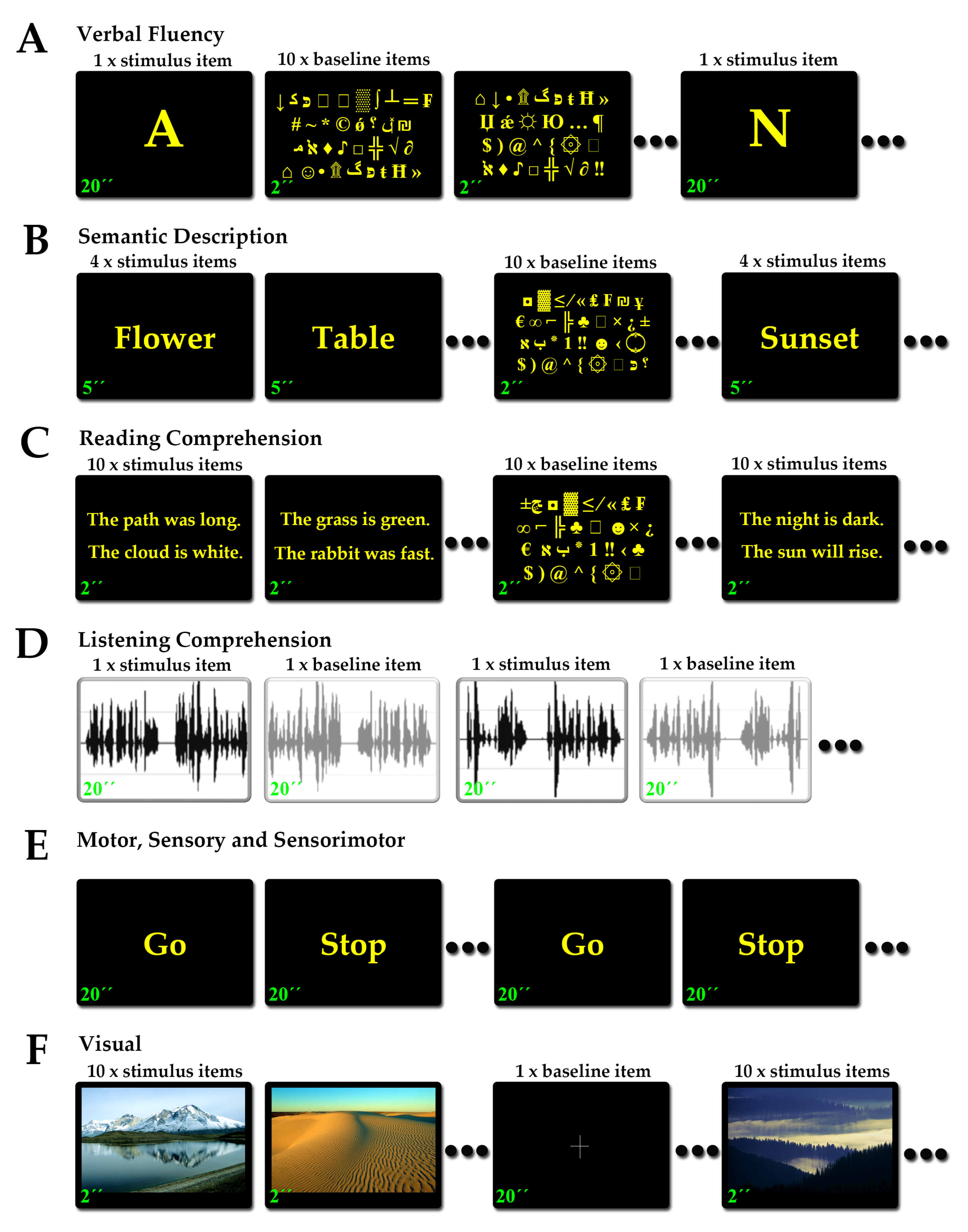
2.3. Functional MRI and Tasks
2.3.1. Language Tasks
2.3.2. Motor Tasks
2.3.3. Somatosensory Tasks
2.3.4. Sensorimotor Tasks
2.3.5. Visual Tasks
2.4. DTI-Tractography and Tracts
2.4.1. Cortico-Spinal Tract (The Motor Pathway)
2.4.2. Spino-Thalamic and Thalamo-Cortical Tracts (The Sensory Pathway)
2.4.3. Arcuate Fasciculus (The Language Network)
2.4.4. Optic Radiation (The Visual Pathway)
2.5. Strategy and Rationale of Functional and Structural Peri-Lesional Coverage
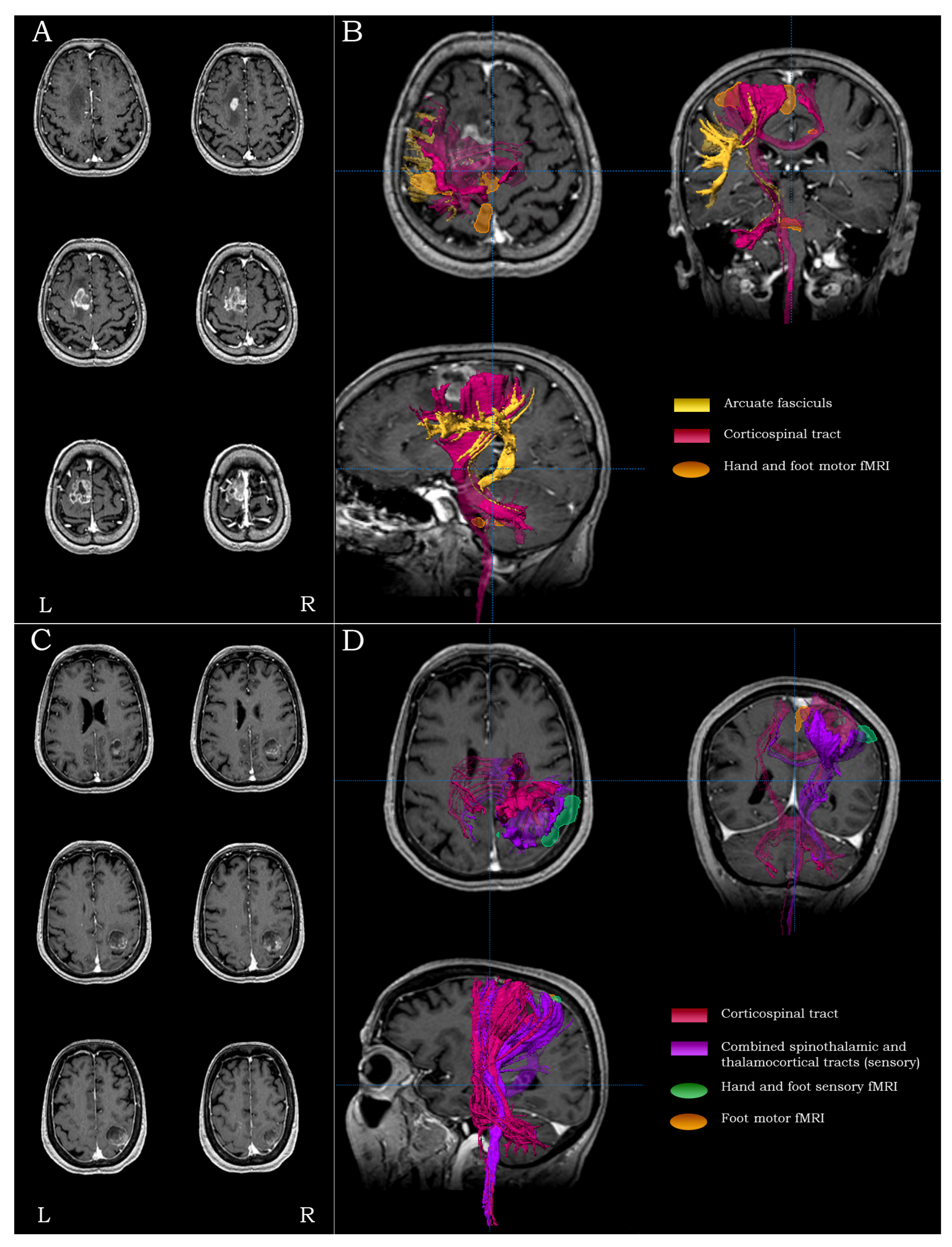
2.5.1. Precentral Lesions
2.5.2. Central Lesions
2.5.3. Inferior Frontal Lesions
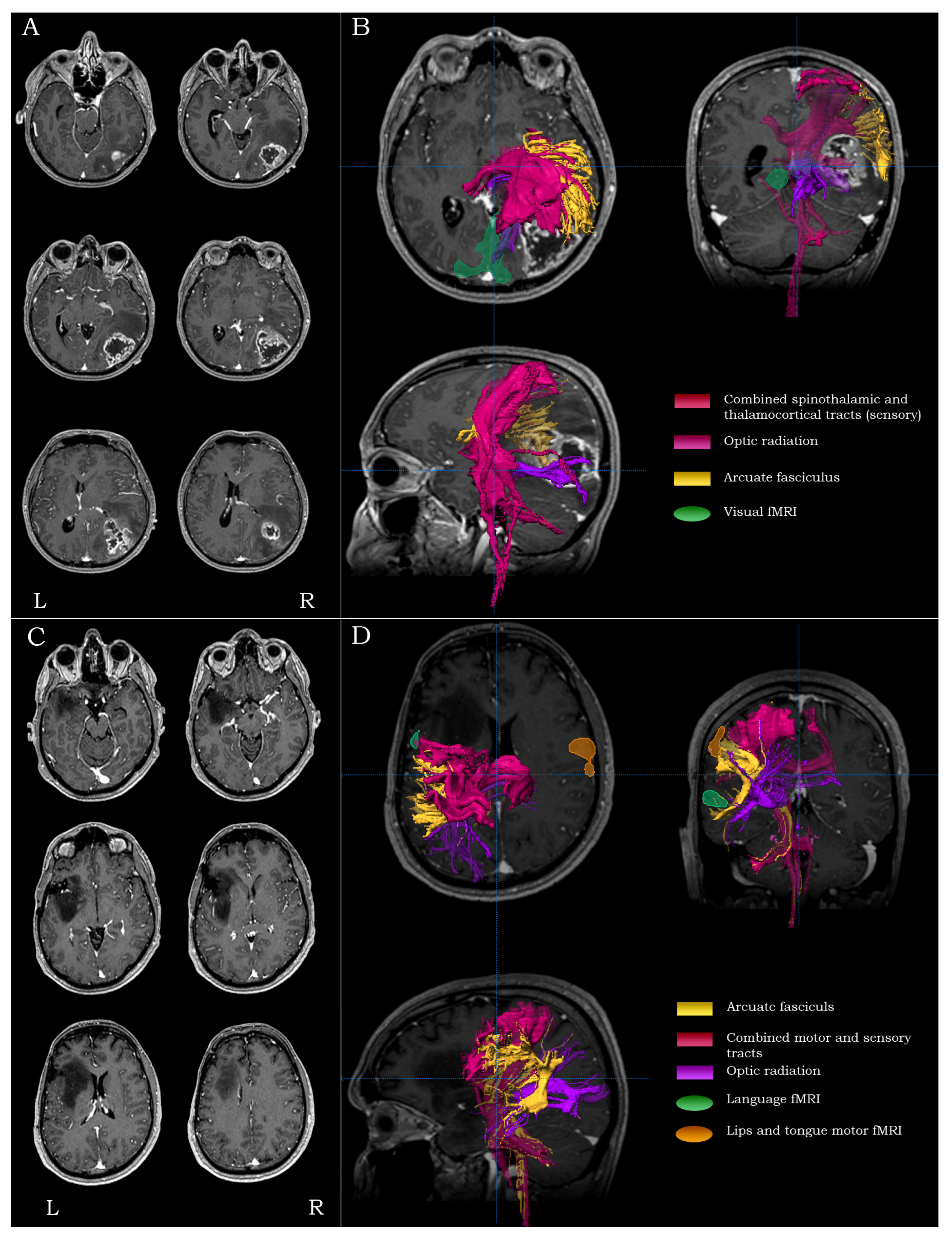
2.5.4. Inferior Parietal/Posterior Temporal Lesions
2.5.5. Temporo-Occipital Lesions
2.5.6. Anterior Temporal Lesions
2.5.7. Anterior Frontal Lesions
2.5.8. Superior Parietal Lesions
2.5.9. Occipital Lesions
2.5.10. Lesions Extending in Two Lobes
2.6. Intraoperative Procedure
2.7. Outcomes and Statistics
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berger, M.S. Glioma surgery: A century of challenge. Clin. Neurosurg. 2011, 58, 7–9. [Google Scholar] [CrossRef]
- Afra, D.; Osztie, E.; Sipos, L.; Vitanovics, D. Preoperative history and postoperative survival of supratentorial low-grade astrocytomas. Br. J. Neurosurg. 1999, 13, 299–305. [Google Scholar] [PubMed]
- Kılıç, T.; Özduman, K.; Elmacı, I.; Sav, A.; Pamir, M.N. Effect of surgery on tumor progression and malignant degeneration in hemispheric diffuse low-grade astrocytomas. J. Clin. Neurosci. 2002, 9, 549–552. [Google Scholar] [CrossRef]
- Aghi, M.K.; Nahed, B.V.; Sloan, A.E.; Ryken, T.C.; Kalkanis, S.N.; Olson, J.J. The role of surgery in the management of patients with diffuse low grade glioma: A systematic review and evidence-based clinical practice guideline. J. Neurooncol. 2015, 125, 503–530. [Google Scholar] [CrossRef] [PubMed]
- Keles, G.E.; Anderson, B.; Berger, M.S. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg. Neurol. 1999, 52, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.; Abi-Said, D.; Fourney, D.R.; Gokaslan, Z.L.; Shi, W.; DeMonte, F.; Lang, F.F.; McCutcheon, I.E.; Hassenbusch, S.J.; Holland, E.; et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001, 95, 190–198. [Google Scholar] [CrossRef]
- Vuorinen, V.; Hinkka, S.; Färkkilä, M.; Jääskeläinen, J. Debulking or biopsy of malignant glioma in elderly people—A randomised study. Acta Neurochir. 2003, 145, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Berger, M.S. Glioma extent of resection and its impact on patient outcome. Neurosurgery 2008, 62, 753–766. [Google Scholar] [CrossRef]
- Stummer, W.; Reulen, H.J.; Meinel, T.; Pichlmeier, U.; Schumacher, W.; Tonn, J.C.; Rohde, V.; Oppel, F.; Turowski, B.; Woiciechowsky, C.; et al. Extent of resection and survival in glioblastoma multiforme: Identification of and adjustment for bias. Neurosurgery 2008, 62, 564–576. [Google Scholar] [CrossRef]
- Sanai, N.; Polley, M.-Y.; McDermott, M.W.; Parsa, A.T.; Berger, M.S. An extent of resection threshold for newly diagnosed glioblastomas. J. Neurosurg. 2011, 115, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Ius, T.; Ng, S.; Young, J.S.; Tomasino, B.; Polano, M.; Ben-Israel, D.; Kelly, J.J.P.; Skrap, M.; Duffau, H.; Berger, M.S. The benefit of early surgery on overall survival in incidental low-grade glioma patients: A multicenter study. Neuro-Oncology 2022, 24, 624–638. [Google Scholar] [CrossRef]
- Jolesz, F.A. Intraoperative imaging in neurosurgery: Where will the future take us? Acta Neurochir. Suppl. 2011, 109, 21–25. [Google Scholar] [PubMed]
- Zebian, B.; Vergani, F.; Lavrador, J.P.; Mukherjee, S.; Kitchen, W.J.; Stagno, V.; Chamilos, C.; Pettorini, B.; Mallucci, C. Recent technological advances in pediatric brain tumor surgery. CNS Oncol. 2017, 6, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Duffau, H.; Mandonnet, E. The “onco-functional balance” in surgery for diffuse low-grade glioma: Integrating the extent of resection with quality of life. Acta Neurochir. 2013, 155, 951–957. [Google Scholar] [CrossRef]
- Magill, S.T.; Han, S.J.; Li, J.; Berger, M.S. Resection of primary motor cortex tumors: Feasibility and surgical outcomes. J. Neurosurg. 2018, 129, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Kow, C.Y.; Kim, B.J.; Park, T.I.-H.; Chen, J.C.; Vong, C.K.; Kim, J.Y.; Shim, V.; Dragunow, M.; Heppner, P. Extent of resection affects prognosis for patients with glioblastoma in non-eloquent regions. J. Clin. Neurosci. 2020, 80, 242–249. [Google Scholar] [CrossRef]
- Wykes, V.; Zisakis, A.; Irimia, M.; Ughratdar, I.; Sawlani, V.; Watts, C. Importance and evidence of extent of resection in glioblastoma. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2021, 82, 075–086. [Google Scholar] [CrossRef]
- Behling, F.; Rang, J.; Dangel, E.; Noell, S.; Renovanz, M.; Mäurer, I.; Schittenhelm, J.; Bender, B.; Paulsen, F.; Brendel, B.; et al. Complete and incomplete resection for progressive glioblastoma prolongs post-progression survival. Front. Oncol. 2022, 12, 755430. [Google Scholar] [CrossRef] [PubMed]
- Faro, S.H.; Mohammed, F.B. Functional MRI: Basic Principles and Clinical Applications; Springer: New York, NY, USA, 2006. [Google Scholar]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133–139. [Google Scholar] [CrossRef]
- Petrella, J.R.; Shah, L.M.; Harris, K.M.; Friedman, A.H.; George, T.M.; Sampson, J.H.; Pekala, J.S.; Voyvodic, J.T. Preoperative functional MR imaging localization of language and motor areas: Effect on therapeutic decision making in patients with potentially resectable brain tumors. Radiology 2006, 240, 793–802. [Google Scholar] [CrossRef]
- Detre, J.A. Clinical applicability of functional MRI. J. Magn. Reson. Imaging 2006, 23, 808–815. [Google Scholar] [CrossRef]
- Bookheimer, S. Pre-surgical language mapping with functional magnetic resonance imaging. Neuropsychol. Rev. 2007, 17, 145–155. [Google Scholar] [CrossRef]
- Chakraborty, A.; McEvoy, A.W. Presurgical functional mapping with functional MRI. Curr. Opin. Neurol. 2008, 21, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Pillai, J. The evolution of clinical functional imaging during the past 2 decades and its current impact on neurosurgical planning. AJNR Am. J. Neuroradiol. 2010, 31, 219–225. [Google Scholar] [CrossRef]
- Beers, C.A.; Federico, P. Functional MRI applications in epilepsy surgery. Can. J. Neurol. Sci./J. Can. Sci. Neurol. 2012, 39, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Stippich, C.; Rapps, N.; Dreyhaupt, J.; Durst, A.; Kress, B.; Nennig, E.; Tronnier, V.M.; Sartor, K. Localizing and lateralizing language in patients with brain tumors: Feasibility of routine preoperative functional MR imaging in 81 consecutive patients. Radiology 2007, 243, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Tyndall, A.J.; Reinhardt, J.; Tronnier, V.; Mariani, L.; Stippich, C. Presurgical motor, somatosensory and language fMRI: Technical feasibility and limitations in 491 patients over 13 years. Eur. Radiol. 2017, 27, 267–278. [Google Scholar] [CrossRef]
- Meier, J.D.; Aflalo, T.N.; Kastner, S.; Graziano, M.S.A. Complex organization of human primary motor cortex: A high-resolution fMRI study. J. Neurophysiol. 2008, 100, 1800–1812. [Google Scholar] [CrossRef] [PubMed]
- Blatow, M.; Reinhardt, J.; Riffel, K.; Nennig, E.; Wengenroth, M.; Stippich, C. Clinical functional MRI of sensorimotor cortex using passive motor and sensory stimulation at 3 tesla. J. Magn. Reson. Imaging 2011, 34, 429–437. [Google Scholar] [CrossRef]
- Tozakidou, M.; Wenz, H.; Reinhardt, J.; Nennig, E.; Riffel, K.; Blatow, M.; Stippich, C. Primary motor cortex activation and laterali-zation in patients with tumors of the central region. Neuroimage Clin. 2013, 2, 221–228. [Google Scholar] [CrossRef]
- Stippich, C.; Blatow, M.; Durst, A.; Dreyhaupt, J.; Sartor, K. Global activation of primary motor cortex during voluntary movements in man. NeuroImage 2007, 34, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Berntsen, E.M.; Samuelsen, P.; Lagopoulos, J.; Rasmussen, I.-A.; Håberg, A.K.; Haraldseth, O. Mapping the primary motor cortex in healthy subjects and patients with peri-rolandic brain lesions before neurosurgery. Neurol. Res. 2008, 30, 968–973. [Google Scholar] [CrossRef]
- Francis, S.; Kelly, E.; Bowtell, R.; Dunseath, W.; Folger, S.; McGlone, F. fMRI of the responses to vibratory stimulation of digit tips. NeuroImage 2000, 11, 188–202. [Google Scholar] [CrossRef] [PubMed]
- Gasser, T.G.; Sandalcioglu, E.I.; Wiedemayer, H.; Hans, V.; Gizewski, E.; Forsting, M.; Stolke, D. A novel passive functional MRI para-digm for preoperative identification of the somatosensory cortex. Neurosurg. Rev. 2004, 27, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Schulder, M.; Holodny, A.; Liu, W.-C.; Gray, A.; Lange, G.; Carmel, P.W. Functional magnetic resonance image-guided surgery of tumors in or near the primary visual cortex. Stereotact. Funct. Neurosurg. 1999, 73, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.G.; Zalusky, E.J.; Kirbas, C. Functional MRI mapping of visual function and selective attention for performance assessment and presurgical planning using conjunctive visual search. Brain Behav. 2014, 4, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Vikingstad, E.M.; Cao, Y.; Thomas, A.J. Language hemispheric dominance in patients with congenital lesions of eloquent brain. Neurosurgery 2000, 47, 562–570. [Google Scholar]
- Kekhia, H.; Rigolo, L.; Norton, I.; Golby, A.J. Special surgical considerations for functional brain mapping. Neurosurg. Clin. N. Am. 2011, 22, 111–132. [Google Scholar] [CrossRef]
- Kundu, B.; Penwarden, A.; Wood, J.M.; Gallagher, T.A.; Andreoli, M.J.; Voss, J.; Meier, T.; Nair, V.A.; Kuo, J.S.; Field, A.S.; et al. Association of functional magnetic resonance imaging indices with postoperative language outcomes in patients with primary brain tumors. Neurosurg. Focus 2013, 34, E6. [Google Scholar] [CrossRef]
- Benjamin, C.F.A.; Dhingra, I.; Li, A.X.; Blumenfeld, H.; Alkawadri, R.; Bickel, S.; Helmstaedter, C.; Meletti, S.; Bronen, R.A.; Warfield, S.K.; et al. Presurgical language fMRI: Technical practices in epilepsy surgical planning. Hum. Brain Map. 2018, 39, 4032–4042. [Google Scholar] [CrossRef]
- Kokkinos, V.; Selviaridis, P.; Seimenis, I. Feasibility, contrast sensitivity and network specificity of language fMRI in presurgical evaluation for epilepsy and brain tumor surgery. Brain Topogr. 2021, 34, 511–524. [Google Scholar] [CrossRef]
- Wengenroth, M.; Blatow, M.; Guenther, J.; Akbar, M.; Tronnier, V.M.; Stippich, C. Diagnostic benefits of presurgical fMRI in patients with brain tumours in the primary sensorimotor cortex. Eur. Radiol. 2011, 21, 1517–1525. [Google Scholar] [CrossRef]
- Voss, J.; Meier, T.B.; Freidel, R.; Kundu, B.; Nair, V.A.; Holdsworth, R.; Kuo, J.S.; Prabhakaran, V. The role of secondary motor and language cortices in morbidity and mortality: A retrospective functional MRI study of surgical planning for patients with in-tracranial tumors. Neurosurg. Focus 2013, 34, E7. [Google Scholar] [CrossRef]
- Vysotski, S.; Madura, C.; Swan, B.; Holdsworth, R.; Lin, Y.; Del Rio, A.M.; Wood, J.; Kundu, B.; Penwarden, A.; Voss, J.; et al. Preoperative FMRI associated with decreased mortality and morbidity in brain tumor patients. Interdiscip. Neurosurg. 2018, 13, 40–45. [Google Scholar] [CrossRef]
- Mori, S.; Crain, B.J.; Chacko, V.P.; van Zijl, P.C.M. Three-dimensional tracking of axonal projections in the brain by magnetic reso-nance imaging. Ann. Neurol. 1999, 45, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Frederiksen, K.; van Zijl, P.C.M.; Stieltjes, B.; Kraut, M.A.; Solaiyappan, M.; Pomper, M.G. Brain white matter anatomy of tumor patients evaluated with diffusion tensor imaging. Ann. Neurol. 2002, 51, 377–380. [Google Scholar] [CrossRef]
- Conturo, T.E.; Lori, N.F.; Cull, T.S.; Akbudak, E.; Snyder, A.Z.; Shimony, J.S.; McKinstry, R.C.; Burton, H.; Raichle, M.E. Tracking neuronal fiber pathways in the living human brain. Proc. Natl. Acad. Sci. USA 1999, 96, 10422–10427. [Google Scholar] [CrossRef]
- Ito, R.; Mori, S.; Melhem, E.R. Diffusion tensor brain imaging and tractography. Neuroimaging Clin. N. Am. 2002, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Kizu, O.; Mori, S.; Ito, H.; Nakamura, H.; Yuen, S.; Kubota, T.; Tanaka, O.; Akada, W.; Sasajima, H.; et al. Brain fiber tracking with clinically feasible diffusion-tensor MR imaging: Initial experience. Radiology 2003, 227, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Akai, H.; Mori, H.; Aoki, S.; Masutani, Y.; Kawahara, N.; Shibahara, J.; Ohtomo, K. Diffusion tensor tractography of gliomatosis cerebri. J. Comput. Assist. Tomogr. 2005, 29, 127–129. [Google Scholar] [CrossRef]
- Nimsky, C.; Ganslandt, O.; Hastreiter, P.; Wang, R.; Benner, T.; Sorensen, A.G.; Fahlbusch, R. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurgery 2005, 56, 130–138. [Google Scholar] [CrossRef]
- Wakana, S.; Caprihan, A.; Panzenboeck, M.M.; Fallon, J.H.; Perry, M.; Gollub, R.L.; Hua, K.; Zhang, J.; Jiang, H.; Dubey, P.; et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage 2007, 36, 630–644. [Google Scholar] [CrossRef]
- Kamali, A.; Kramer, L.A.; Butler, I.J.; Hasan, K.M. Diffusion tensor tractography of the somatosensory system in the human brainstem: Initial findings using high isotropic spatial resolution at 3.0 T. Eur. Radiol. 2009, 19, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Bernal, B.; Altman, N. The connectivity of the superior longitudinal fasciculus: A tractography DTI study. Magn. Reson. Imaging 2010, 28, 217–225. [Google Scholar] [CrossRef]
- Winston, G.P.; Daga, P.; Stretton, J.; Modat, M.; Symms, M.R.; McEvoy, A.W.; Ourselin, S.; Duncan, J.S. Optic radiation tractography and vision in anterior temporal lobe resection. Ann. Neurol. 2012, 71, 334–341. [Google Scholar] [CrossRef]
- Wycoco, V.; Shroff, M.; Sudhakar, S.; Lee, W. White matter anatomy: What the radiologist needs to know. Neuroimaging Clin. N. Am. 2013, 23, 197–216. [Google Scholar] [CrossRef]
- Christidi, F.; Karavasilis, E.; Samiotis, K.; Bisdas, S.; Papanikolaou, N. Fiber tracking: A qualitative and quantitative comparison between four different software tools on the reconstruction of major white matter tracts. Eur. J. Radiol. Open 2016, 3, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wedeen, V.J.; Hagmann, P.; Tseng, W.I.; Reese, T.G.; Weisskoff, R.M. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn. Reson. Med. 2005, 54, 1377–1386. [Google Scholar] [CrossRef]
- Wedeen, V.; Wang, R.; Schmahmann, J.; Benner, T.; Tseng, W.; Dai, G.; Pandya, D.; Hagmann, P.; D’Arceuil, H.; de Crespigny, A. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage 2008, 41, 1267–1277. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.W.; Chou, M.C.; Chen, C.Y. Principles and limitations of computational algorithms in clinical diffusion tensor MR trac-tography. AJNR Am. J. Neuroradiol. 2011, 32, 3–13. [Google Scholar] [CrossRef]
- Yan, H.; Carmichael, O.; Paul, D.; Peng, J.; Alzheimer’s Disease Neuroimaging Initiative. Estimating fiber orientation distribution from diffusion MRI with spherical needlets. Med. Image Anal. 2018, 46, 57–72. [Google Scholar] [CrossRef]
- Wu, Z.; Wu, D.; Xu, D. White matter fiber tractography using nonuniform rational b-splines curve fitting. J. Healthc. Eng. 2018, 2018, 8643871. [Google Scholar] [CrossRef]
- Abhinav, K.; Yeh, F.-C.; Mansouri, A.; Zadeh, G.; Fernandez-Miranda, J.C. High-definition fiber tractography for the evaluation of perilesional white matter tracts in high-grade glioma surgery. Neuro-Oncology 2015, 17, 1199–1209. [Google Scholar] [CrossRef]
- Yeh, F.-C.; Wedeen, V.J.; Tseng, W.-Y.I. Generalized q-sampling imaging. IEEE Trans. Med. Imaging 2010, 29, 1626–1635. [Google Scholar] [CrossRef]
- Yan, J.; van der Hoorn, A.; Larkin, T.J.; Boonzaier, N.R.; Matys, T.; Price Stephen, J. Extent of resection of peritumoral diffusion tensor imaging–detected abnormality as a predictor of survival in adult glioblastoma patients. J. Neurosurg. 2017, 126, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Costabile, J.D.; Alaswad, E.; D’Souza, S.; Thompson, J.A.; Ormond, D.R. Current applications of diffusion tensor imaging and tractography in intracranial tumor resection. Front. Oncol. 2019, 9, 426. [Google Scholar] [CrossRef]
- Brancato, V.; Nuzzo, S.; Tramontano, L.; Condorelli, G.; Salvatore, M.; Cavaliere, C. Predicting survival in glioblastoma patients using diffusion MR imaging metrics—A systematic review. Cancers 2020, 12, 2858. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.L.; Salvan, C.V.; Mueller, W.M.; Krouwer, H.G.; Stroe, G.O.; Aralasmak, A.; Prost, R.W. The role of diffusion tensor imaging in establishing the proximity of tumor borders to functional brain systems: Implications for preoperative risk assessments and postoperative outcomes. Technol. Cancer Res. Treat. 2004, 3, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Scaccianoce, E.; Laganà, M.M.; Baglio, F.; Preti, M.G.; Bergsland, N.; Cecconi, P.; Clerici, M.; Baselli, G.; Papadimitriou, G.; Makris, N. Combined DTI-fMRI analysis for a quantitative assessment of connections between WM bundles and their peripheral cortical fields in verbal fluency. Brain Topogr. 2016, 29, 814–823. [Google Scholar] [CrossRef]
- Sanvito, F.; Caverzasi, E.; Riva, M.; Jordan, K.M.; Blasi, V.; Scifo, P.; Iadanza, A.; Crespi, S.A.; Cirillo, S.; Casarotti, A.; et al. fMRI-Targeted high-angular resolution diffusion MR tractography to identify functional language tracts in healthy controls and glioma patients. Front. Neurosci. 2020, 14, 225. [Google Scholar] [CrossRef] [PubMed]
- Holodny, A.I.; Ollenschleger, M.D.; Liu, W.-C.; Schulder, M.; Kalnin, A.J. Identification of the corticospinal tracts achieved using blood-oxygen-level–dependent and diffusion functional MR imaging in patients with brain tumors. AJNR Am. J. Neuroradiol. 2001, 22, 83–88. [Google Scholar] [PubMed]
- Smits, M.; Vernooij, M.; Wielopolski, P.; Vincent, A.; Houston, G.; van der Lugt, A. Incorporating functional MR imaging into diffusion tensor tractography in the preoperative assessment of the corticospinal tract in patients with brain tumors. Am. J. Neuroradiol. 2007, 28, 1354–1361. [Google Scholar] [CrossRef]
- Schonberg, T.; Pianka, P.; Hendler, T.; Pasternak, O.; Assaf, Y. Characterization of displaced white matter by brain tumors using combined DTI and fMRI. NeuroImage 2006, 30, 1100–1111. [Google Scholar] [CrossRef]
- Bizzi, A. Presurgical mapping of verbal language in brain tumors with functional MR imaging and MR tractography. Neuroimaging Clin. N. Am. 2009, 19, 573–596. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, T.; Jiang, X.; Hu, X.; Chen, H.; Yang, N.; Lv, J.; Han, J.; Guo, L.; Liu, T. Fusing DTI and fMRI data: A survey of methods and applications. NeuroImage 2014, 102 Pt 1, 184–191. [Google Scholar] [CrossRef]
- Black, D.F.; Vachha, B.; Mian, A.; Faro, S.H.; Maheshwari, M.; Sair, H.I.; Petrella, J.R.; Pillai, J.J.; Welker, K. American society of functional neuroradiology-recommended fMRI paradigm algorithms for presurgical language assessment. Am. J. Neuroradiol. 2017, 38, E65–E73. [Google Scholar] [CrossRef]
- Kokkinos, V.; Kallifatidis, A.; Kapsalaki, E.Z.; Papanikolaou, N.; Garganis, K. Thin isotropic FLAIR MR images at 1.5T increase the yield of focal cortical dysplasia transmantle sign detection in frontal lobe epilepsy. Epilepsy Res. 2017, 132, 1–7. [Google Scholar] [CrossRef]
- Rotte, M.; Kanowski, M.; Heinze, H.-J. Functional magnetic resonance imaging for the evaluation of the motor system: Primary and secondary brain areas in different motor tasks. Stereotact. Funct. Neurosurg. 2002, 78, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wait, S.D.; Ogg, R.J.; Scoggins, M.A.; Zou, P.; Wheless, J.; Boop, F.A. Functional magnetic resonance imaging of the visual cortex performed in children under sedation to assist in presurgical planning. J. Neurosurg. Pediatr. 2013, 11, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Behrens, T.E.; Berg, H.J.; Jbabdi, S.; Rushworth, M.F.; Woolrich, M.W. Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 2007, 34, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Lebel, C.; Benner, T.; Beaulieu, C. Six is enough? Comparison of diffusion parameters measured using six or more diffusion-encoding gradient directions with deterministic tractography. Magn. Reson. Med. 2012, 68, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhang, J.; van Zijl, P.C.; Mori, S. Analysis of noise effects on DTI-based tractography using the brute-force and multi-ROI approach. Magn. Reson. Med. 2004, 52, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Mellerio, C.; Charron, S.; Lion, S.; Roca, P.; Kuchcinski, G.; Legrand, L.; Edjlali, M.; Naggara, O.; Meder, J.-F.; Pallud, J.; et al. Perioperative functional neuroimaging of gliomas in eloquent brain areas. Neurochirurgie 2017, 63, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Conti Nibali, M.; Rossi, M.; Sciortino, T.; Riva, M.; Gay, L.G.; Pessina, F.; Bello, L. Preoperative surgical planning of glioma: Limitations and reliability of fMRI and DTI tractography. J. Neurosurg. Sci. 2019, 63, 127–134. [Google Scholar] [CrossRef]
- Yang, J.Y.-M.; Yeh, C.-H.; Poupon, C.; Calamante, F. Diffusion MRI tractography for neurosurgery: The basics, current state, technical reliability and challenges. Phys. Med. Biol. 2021, 66, 15TR01. [Google Scholar] [CrossRef] [PubMed]
- Dimou, S.; Battisti, R.A.; Hermens, D.F.; Lagopoulos, J. A systematic review of functional magnetic resonance imaging and diffusion tensor imaging modalities used in presurgical planning of brain tumour resection. Neurosurg. Rev. 2013, 36, 205–214. [Google Scholar] [CrossRef]
- Jalilianhasanpour, R.; Beheshtian, E.; Ryan, D.; Luna, L.P.; Agarwal, S.; Pillai, J.J.; Sair, H.I.; Gujar, S.K. Role of functional magnetic res-onance imaging in the presurgical mapping of brain tumors. Radiol. Clin. N. Am. 2021, 59, 377–393. [Google Scholar] [CrossRef]
- Morales, H. Current and Future Challenges of Functional MRI and Diffusion Tractography in the Surgical Setting: From Elo-quent Brain Mapping to Neural Plasticity. Semin. Ultrasound CT MRI 2021, 42, 474–489. [Google Scholar] [CrossRef]
- Ulmer, J.L.; Klein, A.P.; Mueller, W.M.; DeYoe, E.A.; Mark, L.P. Preoperative diffusion tensor imaging: Improving neurosurgical out-comes in brain tumor patients. Neuroimaging Clin. N. Am. 2014, 24, 599–617. [Google Scholar] [CrossRef]
- Tamura, M.; Kurihara, H.; Saito, T.; Nitta, M.; Maruyama, T.; Tsuzuki, S.; Fukui, A.; Koriyama, S.; Kawamata, T.; Muragaki, Y. Combining Pre-operative Diffusion Tensor Images and Intraoperative Magnetic Resonance Images in the Navigation Is Useful for Detecting White Matter Tracts During Glioma Surgery. Front. Neurol. 2022, 12, 805952. [Google Scholar] [CrossRef]
- Yousem, D.M. The economics of functional magnetic resonance imaging: Clinical and research. Neuroimaging Clin. N. Am. 2014, 24, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Hancock, C.; Bernal, B.; Medina, C.; Medina, S. Cost analysis of diffusion tensor imaging and MR tractography of the brain. Open J. Radiol. 2014, 04, 260–269. [Google Scholar] [CrossRef]
- Agarwal, S.; Sair, H.I.; Gujar, S.; Pillai, J.J. Language mapping with fMRI: Current standards and reproducibility. Top. Magn. Reson. Imaging 2019, 28, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Caras, A.; Mugge, L.; Miller, W.K.; Mansour, T.R.; Schroeder, J.; Medhkour, A. Usefulness and Impact of Intraoperative Imaging for Glioma Resection on Patient Outcome and Extent of Resection: A Systematic Review and Meta-Analysis. World Neurosurg. 2020, 134, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.S.; Zhou, L.F.; Tang, W.J.; Mao, Y.; Hu, J.; Song, Y.Y.; Hong, X.N.; Du, G.H. Clinical evaluation and follow-up outcome of diffusion tensor imaging-based functional neuronavigation: A prospective, controlled study in patients with gliomas involving pyramidal tracts. Neurosurgery 2007, 61, 935–948; discussion 948–949. [Google Scholar] [CrossRef]
- Panigrahi, M.; Chandrasekhar, Y.B.; Vooturi, S.; Ram, G.A.; Rammohan, V.S. Surgical resection of insular gliomas and roles of functional magnetic resonance imaging and diffusion tensor imaging tractography—Single surgeon experience. World Neurosurg. 2017, 98, 587–593. [Google Scholar] [CrossRef]
- Bai, S.-C.; Xu, B.-N.; Wei, S.-H.; Geng, J.-F.; Wu, D.-D.; Yu, X.-G.; Chen, X.-L. Intraoperative high-field magnetic resonance imaging combined with functional neuronavigation in resection of low-grade temporal lobe tumors. World J. Surg. Oncol. 2015, 13, 286. [Google Scholar] [CrossRef][Green Version]
- Bailey, P.D.; Zacà, D.; Basha, M.M.; Agarwal, S.; Gujar, S.K.; Sair, H.I.; Eng, J.; Pillai, J.J. Presurgical fMRI and DTI for the prediction of perioperative motor and language deficits in primary or metastatic brain lesions. J. Neuroimaging 2015, 25, 776–784. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; Familiari, P.; Di Lauro, A.; Angelini, A.; Sessa, G. Safe resection of gliomas of the dominant angular gyrus availing of preoperative fMRI and intraoperative DTI: Preliminary series and surgical technique. World Neurosurg. 2016, 87, 627–639. [Google Scholar] [CrossRef] [PubMed]
- Ulmer, J.L.; Berman, J.I.; Mueller, W.M.; Gaggl, W.; DeYoe, E.A.; Klein, A.P. Issues in translating imaging technology and pre-surgical diffusion tensor imaging. In Functional Neuroradiology; Springer: Boston, MA, USA, 2011; pp. 731–765. [Google Scholar]
- Deveaux, B.C.; O’Fallon, J.R.; Kelly, P.R. Resection, biopsy, and survival in malignant glial neoplasms: A retrospective study of clinical parameters, therapy, and outcome. J. Neurosurg. 1993, 78, 767–775. [Google Scholar] [CrossRef]
- Brell, M.; Ibanez, J.; Caral, L.; Ferrer, E. Factors influencing surgical complications of intra-axial brain tumors. Acta Neurochir. 2000, 142, 739–750. [Google Scholar] [CrossRef]
- Sawaya, R.; Hammoud, M.; Schoppa, D.; Hess, K.R.; Wu, S.Z.; Shi, W.-M.; WiIdrick, D.M. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment parenchymal tumors. Neurosurgery 1998, 42, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Oberheim Bush, N.A.; Chang, S. Treatment Strategies for Low-Grade Glioma in Adults. J. Oncol. Pract. 2016, 12, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Page, M.; Solheim, K.; Fox, S.; Chang, S.M. Quality of life in adults with brain tumors: Current knowledge and future directions. Neuro Oncol. 2009, 11, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Sizoo, E.M.; Braam, L.; Postma, T.J.; Pasman, H.R.W.; Heimans, J.J.; Klein, M.; Reijneveld, J.C.; Taphoorn, M.J.B. Symptoms and problems in the end-of-life phase of high-grade glioma patients. Neuro-Oncology 2010, 12, 1162–1166. [Google Scholar] [CrossRef]
- Gulati, S.; Berntsen, E.; Solheim, O.; Kvistad, K.; Håberg, A.; Selbekk, T.; Torp, S.; Unsgaard, G. Surgical resection of high-grade gliomas in eloquent regions guided by blood oxygenation level dependent functional magnetic resonance imaging, diffusion tensor tractography, and intraoperative navigated 3D ultrasound. MIN—Minim. Invasive Neurosurg. 2009, 52, 17–24. [Google Scholar] [CrossRef] [PubMed]
- DeYoe, E.A.; Raut, R.V. Visual mapping using blood oxygen level dependent functional magnetic resonance imaging. Neuroimaging Clin. N. Am. 2014, 24, 573–584. [Google Scholar] [CrossRef]
- DeYoe, E.A.; Ulmer, J.L.; Mueller, W.M.; Sabsevitz, D.S.; Reitsma, D.C.; Pillai, J.J. Imaging of the Functional and Dysfunctional Visual System. Semin. Ultrasound CT MRI 2015, 36, 234–248. [Google Scholar] [CrossRef][Green Version]
- D’Andrea, G.; Trillo’, G.; Picotti, V.; Raco, A. Functional Magnetic Resonance Imaging (fMRI), Pre-intraoperative Tractography in Neurosurgery: The Experience of Sant’ Andrea Rome University Hospital. Acta Neurochir. Suppl. 2017, 124, 241–250. [Google Scholar] [PubMed]
- Lorenzen, A.; Groeschel, S.; Ernemann, U.; Wilke, M.; Schuhmann, M.U. Role of presurgical functional MRI and diffusion MR tractography in pediatric low-grade brain tumor surgery: A single-center study. Child’s Nerv. Syst. 2018, 34, 2241–2248. [Google Scholar] [CrossRef] [PubMed]
- Behling, F.; Hempel, J.-M.; Schittenhelm, J. Brain Invasion in Meningioma—A Prognostic Potential Worth Exploring. Cancers 2021, 13, 3259. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Huang, M.; Pan, Y.; Li, Y.; Long, W.; Liu, Q. Brain-invasive meningiomas: Molecular mechanisms and potential therapeutic options. Brain Tumor Pathol. 2021, 38, 156–172. [Google Scholar] [CrossRef]
- Gousias, K.; Trakolis, L.; Simon, M. Meningiomas with CNS invasion. Front. Neurosci. 2023, 17, 1189606. [Google Scholar] [CrossRef] [PubMed]
- Campanella, M.; Ius, T.; Skrap, M.; Fadiga, L. Alterations in fiber pathways reveal brain tumor typology: A diffusion tractography study. PeerJ 2014, 2, e497. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilkins, B.; Lee, N.; Gajawelli, N.; Law, M.; Leporé, N. Fiber estimation and tractography in diffusion MRI: Development of simulated brain images and comparison of multi-fiber analysis methods at clinical b-values. NeuroImage 2015, 109, 341–356. [Google Scholar] [CrossRef]
- Toselli, B.; Tortora, D.; Severino, M.; Arnulfo, G.; Canessa, A.; Morana, G.; Rossi, A.; Fato, M.M. Improvement in White Matter Tract Reconstruction with Constrained Spherical Deconvolution and Track Density Mapping in Low Angular Resolution Data: A Pediatric Study and Literature Review. Front. Pediatr. 2017, 5, 182. [Google Scholar] [CrossRef] [PubMed]
- Calamuneri, A.; Arrigo, A.; Mormina, E.; Milardi, D.; Cacciola, A.; Chillemi, G.; Marino, S.; Gaeta, M.; Quartarone, A. White Matter Tissue Quantification at Low b-Values Within Constrained Spherical Deconvolution Framework. Front. Neurol. 2018, 9, 716. [Google Scholar] [CrossRef]
- Celtikci, P.; Fernandes-Cabral, D.T.; Yeh, F.C.; Panesar, S.S.; Fernandez-Miranda, J.C. Generalized q-sampling imaging fiber tractography reveals displacement and infiltration of fiber tracts in low-grade gliomas. Neuroradiology 2018, 60, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Corbetta, M.; Van Essen, D.C.; Raichle, M.E. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. USA 2005, 102, 9673–9678. [Google Scholar] [CrossRef]
- Zhang, D.; Johnston, J.M.; Fox, M.D.; Leuthardt, E.C.; Grubb, R.L.; Chicoine, M.R.; Smyth, M.D.; Snyder, A.Z.; Raichle, M.E.; Shimony, J.S. Preoperative sensorimotor mapping in brain tumor patients using spontaneous fluctuations in neuronal activity imaged with fMRI: Initial experience. Neurosurgery 2009, 65, 226–236. [Google Scholar]
- Sair, H.I.; Yahyavi-Firouz-Abadi, N.; Calhoun, V.D.; Airan, R.D.; Agarwal, S.; Intrapiromkul, J.; Choe, A.S.; Gujar, S.K.; Caffo, B.; Lindquist, M.A.; et al. Presurgical brain mapping of the language network in patients with brain tumors using resting-state fMRI: Comparison with task fMRI. Hum. Brain Mapp. 2016, 37, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Incekara, F.; Olubiyi, O.; Ozdemir, A.; Lee, T.; Rigolo, L.; Golby, A. The value of pre- and intraoperative adjuncts on the extent of resection of hemispheric low-grade gliomas: A retrospective analysis. J. Neurol. Surg. Part A Cent. Eur. Neurosurg. 2016, 77, 79–87. [Google Scholar] [CrossRef]
- Huang, M.; Baskin, D.S.; Fung, S. Glioblastoma presenting with pure alexia and palinopsia involving the left inferior occipital gyrus and visual word form area evaluated with functional magnetic resonance imaging and diffusion tensor imaging tractography. World Neurosurg. 2016, 89, 725.e5–725.e10. [Google Scholar] [CrossRef] [PubMed]

| Lesion Location | fMRI | DTI-Tractography | ||||||
|---|---|---|---|---|---|---|---|---|
| Language | Motor | Sensory | Sensorimotor | Motor Pathway | Sensory Pathway | Arcuate Fasciculus | Optic Radiation | |
| Anterior frontal | √ * | √ | ||||||
| Inferior frontal | √ | √ | √ | |||||
| Pre-central | √ * | √ | √ | √ | ||||
| Central | √ | √ | √ | √ | ||||
| Superior parietal | √ | √ * | √ | √ * | ||||
| Inferior parietal | √ | √ | √ | √ | √ * | |||
| Occipital | √ | √ | ||||||
| Posterior temporal | √ | √ | √ | √ | √ * | √ | ||
| Anterior temporal | √ | |||||||
| Neuroimaging Modalities Used During Neuronavigation | Statistics (Fisher’s Exact Test) | ||||||
|---|---|---|---|---|---|---|---|
| Patient Population = 252 | Anatomical MRI (N = 128) | Anatomical MRI + DTI-Tractography (N = 68) | Anatomical MRI + DTI-Tractography + fMRI (N = 56) | PMRIvsMRI/DTI | PMRIvsMRI/DTI-fMRI | PMRI/DTIvs MRI/DTI-fMRI | PGROUP |
| Outcomes at 1 month postoperative (N; %) | |||||||
| Resolution of PNDs | 24; 18.8 | 10; 14.7 | 20; 35.7 | 0.552 | 0.015 | 0.011 | 0.011 |
| Improvement of PNDs | 3; 2.3 | 9; 13.2 | 6; 10.7 | 0.004 * | 0.024 | 0.785 | 0.005 * |
| Preservation of asymptomatic status | 52; 40.6 | 9; 13.2 | 5; 8.9 | <0.001 * | <0.001 * | 0.572 | <0.001 * |
| Preservation of PNDs | 38; 29.6 | 34; 50.0 | 21; 37.5 | 0.007 * | 0.307 | 0.204 | 0.019 |
| Worsening of PNDs | 11; 8.5 | 6; 8.8 | 4; 7.1 | 1 | 1 | 1 | 0.999 |
| Outcomes at 6 months postoperative (N; %) | |||||||
| Resolution of PNDs | 24; 18.7 | 16; 23.5 | 27; 48.2 | 0.459 | <0.001 * | 0.004 * | <0.001 * |
| Improvement of PNDs | 21; 16.4 | 22; 32.3 | 10; 17.8 | 0.017 | 0.832 | 0.098 | 0.034 |
| Preservation of asymptomatic status | 52; 40.6 | 9; 13.2 | 8; 14.2 | <0.001 * | <0.001 * | 1 | <0.001 * |
| Preservation of PNDs | 21; 16.4 | 18; 26.4 | 11; 19.6 | 0.131 | 0.673 | 0.401 | 0.246 |
| Worsening of PNDs | 10; 7.8 | 3; 4.4% | 0; 0.0 | 0.548 | 0.033 | 0.251 | 0.069 |
| Neuroimaging Modalities Used During Neuronavigation | Statistics (Fisher’s Exact Test) | ||||||
|---|---|---|---|---|---|---|---|
| Grade I and II Lesions Patient Population = 102 | Anatomical MRI (N = 68) | Anatomical MRI + DTI-Tractography (N = 18) | Anatomical MRI + DTI-Tractography + fMRI (N = 16) | PMRIvsMRI/DTI | PMRIvsMRI/DTI-fMRI | PMRI/DTIvs MRI/DTI-fMRI | PGROUP |
| Outcomes at 1 month postoperative (N; %) | |||||||
| Resolution of PNDs | 15; 22.0 | 5; 27.7 | 11; 68.7 | 0.754 | <0.001 * | 0.003 | 0.001 * |
| Improvement of PNDs | 1; 1.4 | 2; 11.1 | 3; 18.7 | 0.109 | 0.020 | 0.648 | 0.013 |
| Preservation of asymptomatic status | 33; 48.5 | 6; 33.3 | 2; 12.5 | 0.295 | 0.011 | 0.405 | 0.020 |
| Preservation of PNDs | 14; 20.5 | 4; 22.2 | 0; 0.0 | 1 | 0.061 | 0.105 | 0.114 |
| Worsening of PNDs | 5; 7.3 | 1; 5.5 | 0; 0.0 | 1 | 0.577 | 1 | 0.825 |
| Outcomes at 6 months postoperative (N; %) | |||||||
| Resolution of PNDs | 15; 22.0 | 7; 38.8 | 14; 87.5 | 0.222 | <0.001 * | 0.005 * | <0.001 * |
| Improvement of PNDs | 10; 14.7 | 4; 22.2 | 0; 0.0 | 0.478 | 0.195 | 0.105 | 0.160 |
| Preservation of asymptomatic status | 33; 48.5 | 6; 33.3 | 2; 12.5 | 0.295 | 0.011 | 0.232 | 0.020 |
| Preservation of PNDs | 5; 7.3 | 0; 0.0 | 0; 0.0 | 0.579 | 0.577 | 1 | 0.494 |
| Worsening of PNDs | 5; 7.3 | 1; 5.5 | 0; 0.0 | 1 | 0.577 | 1 | 0.830 |
| Neuroimaging Modalities Used During Neuronavigation | Statistics (Fisher’s Exact Test) | ||||||
|---|---|---|---|---|---|---|---|
| Grade III and IV Lesions Patient Population = 140 | Anatomical MRI (N = 54) | Anatomical MRI + DTI-Tractography (N = 46) | Anatomical MRI + DTI-Tractography + fMRI (N = 40) | PMRIvsMRI/DTI | PMRIvsMRI/DTI-fMRI | PMRI/DTIvs MRI/DTI-fMRI | PGROUP |
| Outcomes at 1 month postoperative (N; %) | |||||||
| Resolution of PNDs | 8; 14.8 | 5; 10.8 | 9; 22.5 | 0.766 | 0.419 | 0.393 | 0.343 |
| Improvement of PNDs | 2; 3.7 | 5; 10.8 | 3; 7.5 | 0.242 | 0.647 | 0.718 | 0.387 |
| Preservation of asymptomatic status | 15; 27.7 | 2; 4.3 | 3; 7.5 | 0.002 * | 0.016 | 0.660 | 0.001 * |
| Preservation of PNDs | 23; 42.5 | 30; 65.2 | 21; 52.5 | 0.028 | 0.405 | 0.274 | 0.085 |
| Worsening of PNDs | 6; 11.1 | 4; 8.6 | 4; 0.1 | 0.749 | 1 | 1 | 0.939 |
| Outcomes at 6 months postoperative (N; %) | |||||||
| Resolution of PNDs | 8; 14.8 | 8; 17.3 | 17; 42.5 | 0.788 | 0.004 * | 0.016 | 0.005 * |
| Improvement of PNDs | 10; 18.5 | 17; 36.9 | 9; 22.5 | 0.044 | 0.795 | 0.165 | 0.933 |
| Preservation of asymptomatic status | 15; 27.7 | 2; 4.3 | 3; 7.5 | 0.002 * | 0.016 | 0.655 | 0.001 * |
| Preservation of PNDs | 16; 29.6 | 18; 39.1 | 11; 27.5 | 0.397 | 1 | 0.360 | 0.483 |
| Worsening of PNDs | 5; 9.2 | 1; 2.1 | 0; 0.0 | 0.213 | 0.069 | 1 | 0.091 |
| Neuroimaging Modalities Used During Neuronavigation | Statistics (Fisher’s Exact Test) | ||||||
|---|---|---|---|---|---|---|---|
| Preoperative Motor Symptoms Patient Population = 80 | Anatomical MRI (N = 32) | Anatomical MRI + DTI-Tractography (N = 27) | Anatomical MRI + DTI-Tractography + fMRI (N = 21) | PMRIvsMRI/DTI | PMRIvsMRI/DTI-fMRI | PMRI/DTIvs MRI/DTI-fMRI | PGROUP |
| Outcomes at 1 month postoperative (N; %) | |||||||
| Resolution of PNDs | 1; 3.1 | 0; 0.0 | 0; 0.0 | 1 | 1 | 1 | 0.999 |
| Improvement of PNDs | 1; 3.1 | 7; 25.9 | 5; 23.8 | 0.018 | 0.030 | 1 | 0.024 |
| Preservation of PNDs | 27; 84.3 | 17; 62.9 | 15; 71.4 | 0.076 | 0.310 | 0.758 | 0.093 |
| Worsening of PNDs | 3; 9.3 | 3; 11.1 | 1; 4.7 | 1 | 1 | 0.621 | 0.884 |
| Outcomes at 6 months postoperative (N, %) | |||||||
| Resolution of PNDs | 1; 3.1 | 8; 29.6 | 7; 33.3 | 0.008 * | 0.004 * | 1 | 0.005 * |
| Improvement of PNDs | 16; 50.0 | 10; 37.0 | 8; 38.0 | 0.430 | 0.416 | 1 | 0.659 |
| Preservation of PNDs | 13; 40.6 | 8; 29.6 | 6; 28.5 | 0.424 | 0.399 | 1 | 0.605 |
| Worsening of PNDs | 2; 6.2 | 0; 0.0 | 0; 0.0 | 0.495 | 0.512 | 1 | 0.334 |
| Neuroimaging Modalities Used During Neuronavigation | Statistics (Fisher’s Exact Test) | ||||||
|---|---|---|---|---|---|---|---|
| Preoperative Sensory SymptomsPatient Population = 44 | Anatomical MRI (N = 15) | Anatomical MRI + DTI-Tractography (N = 21) | Anatomical MRI + DTI-Tractography + fMRI (N = 8) | PMRIvsMRI/DTI | PMRIvsMRI/DTI-fMRI | PMRI/DTIvs MRI/DTI-fMRI | PGROUP |
| Outcomes at 1 month postoperative (N; %) | |||||||
| Resolution of PNDs | 0; 0.0 | 0; 0.0 | 0; 0.0 | 1 | 1 | 1 | 1 |
| Improvement of PNDs | 1; 6.6 | 3; 14.2 | 2; 25.0 | 0.625 | 0.268 | 0.596 | 0.369 |
| Preservation of PNDs | 11; 73.3 | 18; 85.7 | 6; 75.0 | 0.417 | 1 | 0.596 | 0.611 |
| Worsening of PNDs | 3; 20.0 | 0; 0.0 | 0; 0.0 | 0.063 | 0.525 | 1 | 0.070 |
| Outcomes at 6 months postoperative (N, %) | |||||||
| Resolution of PNDs | 0; 0.0 | 2; 9.5 | 4; 50.0 | 0.500 | 0.007 * | 0.033 | 0.004 * |
| Improvement of PNDs | 5; 33.3 | 9; 42.8 | 1; 12.5 | 0.731 | 0.369 | 0.200 | 0.315 |
| Preservation of PNDs | 7; 46.6 | 10; 47.6 | 3; 37.5 | 1 | 1 | 0.696 | 0.924 |
| Worsening of PNDs | 3; 20.0 | 0; 0.0 | 0; 0.0 | 0.063 | 0.525 | 1 | 0.070 |
| Neuroimaging Modalities Used During Neuronavigation | Statistics (Fisher’s Exact Test) | ||||||
|---|---|---|---|---|---|---|---|
| Preoperative Seizures Patient Population = 47 | Anatomical MRI (N = 21) | Anatomical MRI + DTI-Tractography (N = 10) | Anatomical MRI + DTI-Tractography + fMRI (N = 16) | PMRIvsMRI/DTI | PMRIvsMRI/DTI-fMRI | PMRI/DTIvs MRI/DTI-fMRI | PGROUP |
| Outcomes at 1 month postoperative (N; %) | |||||||
| Resolution of PNDs | 19; 90.4 | 10; 100 | 16; 100 | 0.548 | 0.495 | 1 | 0.689 |
| Improvement of PNDs | 0; 0.0 | 0; 0.0 | 0; 0.0 | 1 | 1 | 1 | 1 |
| Preservation of PNDs | 1; 4.76 | 0; 0.0 | 0; 0.0 | 1 | 1 | 1 | 0.999 |
| Worsening of PNDs | 1; 4.76 | 0; 0.0 | 0; 0.0 | 1 | 1 | 1 | 0.999 |
| Outcomes at 6 months postoperative (N, %) | |||||||
| Resolution of PNDs | 19; 90.4 | 10; 100 | 16; 100 | 0.548 | 0.495 | 1 | 0.689 |
| Improvement of PNDs | 1; 4.76 | 0; 0.0 | 0; 0.0 | 1 | 1 | 1 | 0.999 |
| Preservation of PNDs | 0; 0.0 | 0; 0.0 | 0; 0.0 | 1 | 1 | 1 | 1 |
| Worsening of PNDs | 1; 4.76 | 0; 0.0 | 0; 0.0 | 1 | 1 | 1 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokkinos, V.; Chatzisotiriou, A.; Seimenis, I. Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging-Tractography in Resective Brain Surgery: Lesion Coverage Strategies and Patient Outcomes. Brain Sci. 2023, 13, 1574. https://doi.org/10.3390/brainsci13111574
Kokkinos V, Chatzisotiriou A, Seimenis I. Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging-Tractography in Resective Brain Surgery: Lesion Coverage Strategies and Patient Outcomes. Brain Sciences. 2023; 13(11):1574. https://doi.org/10.3390/brainsci13111574
Chicago/Turabian StyleKokkinos, Vasileios, Athanasios Chatzisotiriou, and Ioannis Seimenis. 2023. "Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging-Tractography in Resective Brain Surgery: Lesion Coverage Strategies and Patient Outcomes" Brain Sciences 13, no. 11: 1574. https://doi.org/10.3390/brainsci13111574
APA StyleKokkinos, V., Chatzisotiriou, A., & Seimenis, I. (2023). Functional Magnetic Resonance Imaging and Diffusion Tensor Imaging-Tractography in Resective Brain Surgery: Lesion Coverage Strategies and Patient Outcomes. Brain Sciences, 13(11), 1574. https://doi.org/10.3390/brainsci13111574







