Abstract
Many studies have shown that low back pain (LBP) is associated with psychosomatic symptoms which may lead to brain changes. This study aimed to investigate the effect of the concurrent application of cognitive behavioral therapy (CBT) and transcranial direct electrical stimulation (tDCS) over the left dorsolateral prefrontal cortex (DLPFC) on fear of pain, fear of movement, and disability in patients with nonspecific LBP. This study was performed on 45 LBP patients (23 women, 22 men; mean age 33.00 ± 1.77 years) in three groups: experimental (2 mA cathodal tDCS (c-tDCS)), sham (c-tDCS turned off after 30 s), and control (only received CBT). In all groups, CBT was conducted for 20 min per session, with two sessions per week for four weeks. Fear of pain, fear of movement, and disability were evaluated using questionnaires at baseline, immediately after, and one month after completion of interventions. Results indicated that all three different types of intervention could significantly reduce fear and disability immediately after intervention (p > 0.05). However, improvement in the experimental group was significantly higher than in the other groups immediately after and at the one-month follow-up after interventions (p < 0.05). DLPFC c-tDCS can prime the immediate effects of CBT and also the lasting effects on the reduction in the fear of pain, fear of movement, and disability in LBP patients.
1. Introduction
Low back pain (LBP) is one of the most common disabling musculoskeletal disorders, which involves about 70 to 85% of individuals in different countries [1,2,3]. Evidence indicates that chronic LBP could affect the function and structure of some parts of the brain in addition to the function and structure of the lumbar spine [4]. These changes could in turn lead to abnormal changes in the processing of sensorimotor signals and psychological processes in the brain [5,6,7]. After experiencing chronic pain, several psychosocial factors, such as the attitude and beliefs of the patients about their pain, are changed. These changes may lead to increases in fear of pain and movement, stress, and depression [8,9].
Evidence indicates that patients with chronic LBP who experience high levels of pain-related anxiety have significantly higher levels of disability [10,11,12]. In addition, a high level of pain-related anxiety could reduce the function of back stabilizer muscles, which may hurt the routine treatment in chronic LBP patients [13,14]. Therefore, control of pain-related anxiety in these patients is an important step in the rehabilitation of these patients. Cognitive behavioral therapy (CBT) is one of the effective interventions for the treatment of pain-related anxiety and beliefs following chronic pain [15,16]. Aneis et al. (2021) indicated that the pain severity, disability, and pain-related anxiety were significantly reduced after CBT treatments condcuted up to three times per week for six weeks in patients with nonspecific chronic LBP [17]. However, a recent systematic review indicated that while CBT can reduce pain and disability immediately after treatment, its lasting effects in the follow-up period are not clear [18].
Transcranial direct current stimulation (tDCS), is a noninvasive neuromodulatory technique that recently gained popularity for various purposes [19,20]. In addition, a study in 2020 showed that tDCS is effective in reducing pain [21]. The literature indicates that tDCS may induce sustained neuroplastic changes in the brain in line with changes during the long-term potentiation (LTP) mechanism [22,23]. The significant effect of tDCS on the management of chronic pain is documented in patients with trigeminal neuralgia, poststroke pain syndrome, fibromyalgia, and LBP [20]. In parallel, several studies showed the significant effects of tDCS on the treatment of depression and fear of movement in patients with major depression and other psychological disorders [24,25]. Asthana et al. (2013) investigated the effects of cathodal tDCS (c-tDCS) and anodal tDCS (a-tDCS) over the dorsolateral prefrontal cortex (DLPFC) on the experimental memory of fear in a healthy individual and concluded that c-tDCS over the DLPFC could induce a significant reduction in the memory of fear, while a-tDCS of the DLPFC failed to do so [26]. Another study evaluated the effect of concurrent CBT and a-tDCS over the primary motor cortex (M1) on the reduction in pain and disability in patients with nonspecific chronic LBP [15]. The study concluded that M1 a-tDCS did not have any significant effect on the reduction in pain and disability in CLBP patients [15]. In other words, the studies showed that the c-tDCS method on the DLPFC can inhibit the generation of fear in the memory and, unlike the anodal method, it can increase fear memories [27]. It was also reported that the prefrontal cortex (DLPFC) region is involved in the formation of fear in the memory. On the other hand, the evidence showed that the inhibition of the left DLPFC causes adjustments in the level of anxiety and fear [28,29].
Therefore, in keeping with the reviewed studies, this study aimed to investigate the short-term and lasting effects of concurrent CBT and c-tDCS over the DLPFC on pain, pain-related anxiety, and fear of movements in patients with LBP with high pain-related anxiety. We hypothesized the following:
- Multiple sessions of a CBT program or sham tDCS with CBT reduces pain-related anxiety, fear of movement, and disability in LBP patients with high pain-related anxiety.
- Multiple sessions of concurrent CBT with c-tDCS over the left DLPFC will be more effective for the reduction in the pain-related anxiety, fear of movement, and disability in LBP patients with high pain-related anxiety compared to CBT alone or concurrent CBT and sham c-tDCS of the left DLPFC.
- Multiple sessions of concurrent CBT with c-tDCS over the left DLPFC will have a more lasting effect on the reduction in the pain-related anxiety, fear of movement, and disability in LBP patients with high pain-related anxiety compared to CBT alone or concurrent CBT and sham c-tDCS of the left DLPFC.
2. Method and Materials
2.1. Participants
Fifty-six patients with nonspecific LBP were assessed against the inclusion and exclusion criteria. Overall, 8 patients were excluded and 45 CLBP patients (23 female, 22 male) with high pain-related anxiety, aged between 18 and 45 (33.00 ± 1.74), participated in this study (Figure 1).
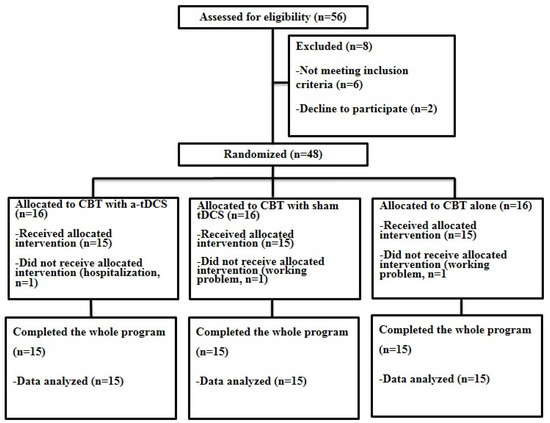
Figure 1.
Flow diagram of participants’ eligibility assessment.
Inclusion criteria were as follows: (1) suffering from LBP for more than six weeks or having recurrent LBP with at least three episodes lasting more than one week (subacute LBP) [30,31]; (2) a pain score between 1 and 3 out of 10 on a visual analog scale (VAS) on the testing day [26]; (3) a score above 30 on the Pain Anxiety Symptoms Scale (PASS) [32].
Exclusion criteria included reporting any history of neurological diseases, such as Parkinson’s disease, Alzheimer’s, and cerebellar disorders; reporting any history of psychological illnesses; the presence of any signs of radiculopathy or root lumbar spinal cord involvement; structural deformities in the spine, such as scoliosis, kyphosis, or lordosis; and any abnormalities in the vestibular system [33,34,35].
All patients provided written informed consent before inclusion in this study. This study conforms to the consort checklist criteria. Eligible individuals were randomly based on computer coding assigned into three groups: (1) concurrent left DLPFC c-tDCS and CBT (experimental group); (2) concurrent sham c-tDCS and CBT (sham stimulation group); (3) CBT alone (control group).
2.2. Study Design
This is a randomized, parallel, double-blind sham-controlled study. From a pool of 56 LBP participants who were referred from neurological clinics by a neurologist, 48 LBP participants with high pain-related anxiety met the inclusion and exclusion criteria. The participants were randomly assigned to three groups following a computer-generated list of random numbers (n = 16 in each group, Figure 1). The number of patients was estimated based on Cohen’s table. This sample size allows detection of the effect of DLPFC c-tDCS on the outcome measures in this study with the power of 85 and 95% confidence intervals (CI). All participants in the first two groups received concurrent c-tDCS or sham c-tDCS and CBT. Participants in the control group received only CBT. Fear of pain, fear of movement, and disability were evaluated using the Pain Anxiety Symptoms Scale (PASS), Tampa Scale for Kinesiophobia (TSK), and Roland–Morris questionnaire (RMDQ), respectively, before, immediately after, and one month after the interventions. Finally, 45 participants completed the whole program of study (Figure 1). Throughout this study, one of the researchers was responsible for the application of the interventions in each group, and the second researcher, blinded to the treatment groups, was responsible for the assessment of the outcome measures. All participants were also blinded to the application of sham or active c-tDCS.
This study was approved by the Human Ethics Committee of the Semnan University of Medical Sciences, Semnan, Iran (IR.SEMUMS.REC.1396.162) and was performed following the ethical standards laid down by the Declaration of Helsinki. This study was registered as a clinical trial on the Iranian Registry of Clinical Trials (IRCT20150302021294N6).
2.3. Transcranial Direct Current Stimulation
In both the experimental and sham stimulation groups, 2 mA c-tDCS (ActivaDose® II, ActivaTeK™Inc., Gilroy, CA, USA) was applied for 20 min using large electrodes (5 × 7 cm) [36,37]. In the first and the last 10 s of stimulation, the current was gradually ramped up or down [38,39] to avoid any sudden changes in the induced sensations [40]. In the experimental group, the active electrode (cathode) and the returning electrode (anode) were placed over the left DLPFC (F3, 10–20 international encephalography system) and the right supraorbital region, respectively [41] (Figure 2).
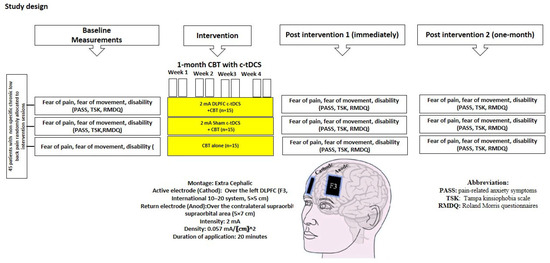
Figure 2.
Experimental design: 8 sessions of interventions in three groups. PASS, TSK, and RMDQ questionnaires were used for assessment before, immediately after, and 1 month after intervention.
In the sham c-tDCS group, the “Fade-in Short stimulation Fade-out (FiSsFo) approach” was used [37,41], which is a reliable method for maintaining the blinding integrity and thus creating the assumed initial cutaneous sensations. In this group, the electrode montage was identical to the active stimulation, and the stimulation was slowly turned off after 30 s [42].
2.4. Cognitive Behavioral Therapy (CBT)
All participants received 8 sessions of CBT administered by a psychologist expert in this field of work [43]. Eight different topics were covered in the 8 treatment sessions of CBT, including identification of participants’ beliefs about pain and pain treatment as well as reconceptualization of the pain experience (session 1); education about the various theories of pain and relaxation techniques such as diaphragmatic breathing, progressive muscle relaxation, and visual imagery (sessions 2–4); education regarding the importance of scheduling pleasant activities and thought, a time-based pacing technique in which individuals take breaks based on time or amount of work accomplished rather than pain level (sessions 5–6); cognitive restructuring to help participants learn to identify maladaptive thoughts and beliefs related to pain and substitute more adaptive ones (session 7); and education of additional training in techniques related to anger management and sleep hygiene (session 8).
In addition, participants were given homework assignments at the end of each session to practice the various techniques presented during the previous sessions. The therapist also collaborated with each patient to generate specific intersession goals (e.g., daily walks for 20 min) after each session. From the second session, a review of the homework assignments and intersession goal accomplishment; problem-solving discussions; a brief review of materials covered in the previous session; the presentation and practice of new skills; and the collaborative establishment of new homework/goals for the next session were performed by the therapist in each session [44].
2.5. Outcome Measures
Persian translation of the TSK, as a valid and reliable scale [45,46], was used to assess the fear avoidance belief in the movement in patients. The TSK is a 17-item scale with a four-point Likert score for each item from 1 (strongly disagree) to 4 (strongly agree). The minimum–maximum score for the kinesiophobia in the TSK is 17–68. Higher scores showed higher kinesiophobia or fear of movement [47,48].
Persian translation of the PASS, as a valid and reliable scale [32], was used to investigate the pain-related anxiety rate. This questionnaire includes 14 subscales of pain-related cognitive anxiety, escape, pain-related avoidance, pain-related fear, and pain-related physiologic anxiety symptoms. The short form of this scale has 20 items with a 5-point score for each item from 0 (never) to 5 (always). The total score is 100, and those who obtained a score higher than 30 are categorized as patients with high pain-related anxiety [24].
To measure the disability level, the Roland–Morris questionnaire, which is a valid and reliable questionnaire [49,50], was used. This questionnaire has 24 statements that describe the possibility of limiting the probable activities. Scores range from 0 (indicating no disability) to 24 (indicating severe disability) [49,50].
3. Results
Table 1 shows the demographic details and baseline data for each group. There were no significant differences in variables of age, gender, and PASS, TSK, and RMDQ scores among the groups (p > 0.05).

Table 1.
Demographic data and baseline values for the participants in experimental and sham groups (mean ± SEM). PASS (Pain Anxiety Symptoms Scale), TSK (Tampa Scale for Kinesiophobia), RMDQ (Roland–Morris Disability Questionnaire), DLPFC (dorsolateral prefrontal cortex), CBT (cognitive behavioral therapy).
RM-ANOVA shows a significant main effect of “Time” and also the “Group” × “Time” interaction effect for PASS, TSK, and RMDQ scores (p < 0.01) (Table 2). Accordingly, post hoc tests were performed using Bonferroni correction (Table 3). The findings indicated a significant reduction in the PASS, TSK, and RMDQ scores in the experimental group immediately and one month after the intervention (p < 0.001, Table 3, Figure 3, Figure 4 and Figure 5). In addition, a significant reduction in the PASS, TSK, and RMDQ scores was shown in the sham and control groups immediately after the intervention (p < 0.001, Table 3, Figure 3, Figure 4 and Figure 5), while this reduction did not remain one month after the intervention (p > 0.05, Figure 3, Figure 4 and Figure 5). Moreover, although PASS, TSK, and RMDQ scores decreased in the sham and control groups immediately after the intervention, the reduction in PASS, TSK, and RMDQ scores in the experimental group was significantly more than in the sham and control groups immediately and one month after completion of the intervention (Figure 3, Figure 4 and Figure 5, p < 0.01).

Table 2.
ANOVA results for the effects of c-tDCS on fear of pain, fear of movement, and disability. PASS (Pain Anxiety Symptoms Scale), TSK (Tampa Scale for Kinesiophobia), RMDQ (Roland–Morris Disability Questionnaire), and CBT (cognitive behavioral therapy).

Table 3.
Post hoc pair-wise comparison of PASS (Pain Anxiety Symptoms Scale), TSK (Tampa Scale for Kinesiophobia), and RMDQ (Roland–Morris Disability Questionnaire) scores before and after intervention in each group.
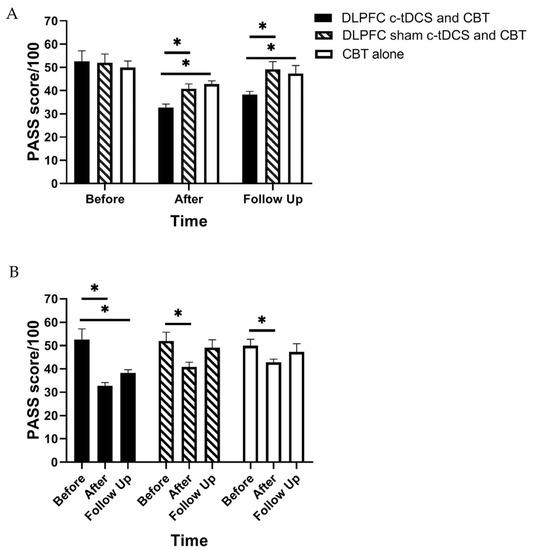
Figure 3.
(A) The comparison of the Pain Anxiety Symptoms Scale (PASS) scores (mean ± SEM) before, immediately after, and one month after the intervention in DLPFC c-tDCS concurrent with CBT, sham DLPFC c-tDCS concurrent with CBT, and CBT alone groups; * indicates the significant differences in PASS score after intervention between groups. (B) The comparison of the Pain Anxiety Symptoms Scale (PASS) scores (mean ± SEM) before, immediately after, and one month after the intervention in each group; * indicates the significant differences in PASS score after intervention rather than baseline in each group.
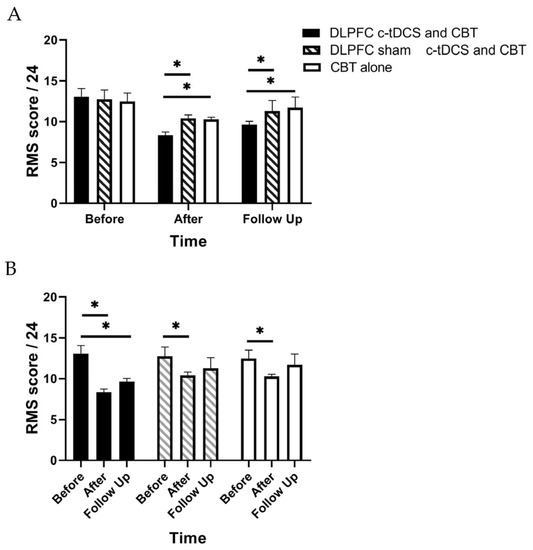
Figure 4.
(A) The comparison of the Roland–Morris Scale (RMS) scores (mean ± SEM) before, immediately after, and one month after the intervention in DLPFC c-tDCS concurrent with CBT, sham DLPFC c-tDCS concurrent with CBT, and CBT alone groups; * indicates the significant differences in RMS score after intervention between groups. (B) The comparison of the Roland–Morris Scale (RMS) scores (mean ± SEM) before, immediately after, and one month after the intervention in each group; * indicates the significant differences in RMS score after intervention rather than baseline in each group.
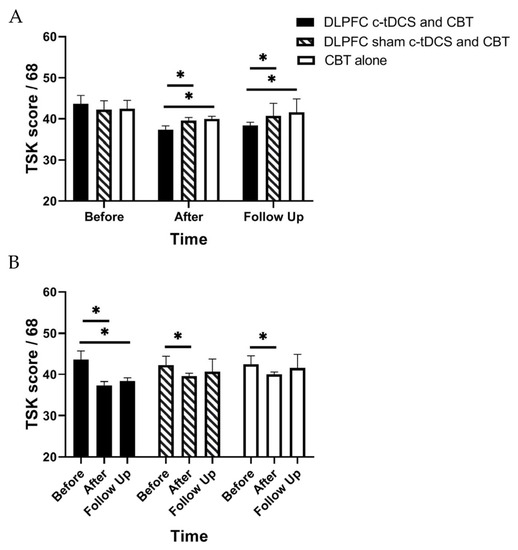
Figure 5.
(A) The comparison of the Tampa Scale for Kinesiophobia (TSK) scores (mean ± SEM) before, immediately after, and one month after the intervention in DLPFC c-tDCS concurrent with CBT, sham DLPFC c-tDCS concurrent with CBT, and CBT alone groups; * indicates the significant differences in TSK score after intervention between groups. (B) The comparison of the Tampa Scale for Kinesiophobia (TSK) scores (mean ± SEM) before, immediately after, and one month after the intervention in each group; * indicates the significant differences in TSK score after intervention rather than baseline in each group.
Safety and Side Effects of c-tDCS
Table 4 shows the side effects (mean ± SEM) under the anode and cathode that were reported by all participants. Itching was the side effect reported by the majority of participants. None of the participants reported any burning sensation or pain during or after c-tDCS. This study indicated that DLPFC c-tDCS intervention was tolerated very well with minimal adverse side effects by all participants. The presence and severity of the possible side effects were determined by a questionnaire. This questionnaire included a rating scale and numeric analog scale (NAS) (e.g., 0 = no tingling to 10 = worst tingling imaginable).

Table 4.
Evaluation of side effects during tDCS intervention in both groups (numeric sensation scores).
4. Discussion
In this study, for the first time, the short-term and lasting priming effects of multisession concurrent c-tDCS of the left DLPFC and CBT on pain-related anxiety, fear of movement, and disability level of LBP patients with high pain-related anxiety were studied. The findings in the current study indicated that there was a significant decrease in the PASS, TSK, and Roland–Morris questionnaire scores immediately after the interventions in sham and intervention groups. Also, it showed that the combination of c-tDCS and CBT programs was more effective in the reduction in pain-related anxiety, fear of movement, and disability compared to CBT programs alone. The results of the present study showed that the significant improvement in kinesiophobia, pain-related anxiety, and disability after treatment remained for one month postintervention.
In this study, for the first time, the short-term and lasting priming effects of multisession concurrent c-tDCS of the left DLPFC and CBT on pain-related anxiety, fear of movement, and disability level of LBP patients with high pain-related anxiety were studied. We hypothesized that CBT alone could reduce pain-related anxiety, fear of movement, and disability. The findings in the current study supported this hypothesis and indicated that there was a significant decrease in the PASS, TSK, and Roland–Morris questionnaire scores immediately after the interventions in all groups (p < 0.001). This finding is in line with the findings of other studies that concluded that CBT can significantly reduce the pain severity, fear of pain, and disability and also improve the quality of life in patients with chronic LBP [44,51]. In addition, a study on elderly patients with chronic LBP indicated that pain severity and disability were significantly reduced up to two weeks after a 10-session course of CBT [44]. Monticone et al. (2015) also investigated the effects of exercise and CBT on fear of pain, disability, and pain severity in patients with nonspecific chronic LBP [44]. The study indicated that CBT along with exercise as compared to exercise alone can significantly improve the fear of pain, disability, pain severity, and quality of life in patients with nonspecific chronic LBP [44].
The findings in the current study also supported the second hypothesis with the fact that the combination of c-tDCS and the CBT program was more effective in the reduction in pain-related anxiety, fear of movement, and disability compared to the CBT program alone (p < 0.001). In this regard, several review studies concluded that although CBT, as compared to the other interventions, is an effective intervention in the treatment of chronic pain syndromes such as chronic LBP, its effect on fear, anxiety, and pain is not long-lasting [16,47,48,52]. A recent systematic review in 2022 indicated that CBT can reduce pain and disability immediately after treatment, but its lasting effects in the follow-up period are not clear [18]. However, the results of another review study conducted by Richmond et al. (2015) showed that CBT could have positive short-term and long-term effects on pain and disability in acute LBP patients [46]. It seems that the reason behind this discrepancy lies in the differences between the stages of LBP in these studies. Richmond et al. (2015) included the studies that assessed the effect of CBT on acute LBP, while other studies, like the current study, assessed the effect of CBT on chronic LBP patients [52]. Indeed, unlike acute pain, chronic pain can lead to functional and structural changes in different areas of the brain. Chronic low back pain (CLBP) has a more complex nature compared with acute episodes, since cognitive, emotional, behavioral, and social factors directly affect the CLBP experience [53].
The results of the present study showed that the significant improvement in kinesiophobia, pain-related anxiety, and disability after treatment remained for one month postintervention (p < 0.001), while in CBT alone and CBT with sham c-tDCS groups, the results were not significant after one month (p > 0.05). Middlkoop et al. (2010), in their systematic review study, stated that the tDCS technique along with CBT can modulate the central nervous system and so increase the effects of CBT in nonspecific chronic LBP patients [54]. There is evidence that tDCS can lead to functional and structural effects on the cortex and facilitate neural plasticity by a mechanism similar to long-term potential (LPT) [22,23,47]. In addition, some studies reported the positive and significant effects of tDCS on the pain, disability, fear, and anxiety in patients with chronic pain such as chronic LBP [28,54,55]. Results of the Antal study also show that c-tDCS has significant short-term and long-term effects on reducing chronic pain and modulating neuronal activity [47]. Furthermore, the Mariano study found that 10 sessions of 2 mA c-tDCS on the left dorsal anterior cingulate cortex (dACC) could modulate CLBP’s affective symptoms such as pain intensity, acceptance, interference, disability, and anxiety, plus general anxiety and depression immediately and 6 weeks after intervention [56]. However, Kerstin Leudtke et al. (2015) examined the effect of M1 a-tDCS alone and along with CBT on reducing the pain and disability in nonspecific chronic LBP patients and showed that none of them had any effect on reducing pain and disability [15]. There is evidence that the prefrontal cortex is one of the important brain areas involved in the formation of fear and pain-related anxiety following chronic pain [26,29]. Therefore, it seems that this area has a key role in modulating the neural activity and memory of fear during chronic pain. Furthermore, this study showed that DLPFC c-tDCS could decrease the fear while a-tDCS could increase it in healthy humans [33]. The results of the current study also showed that concurrent CBT and left DLPFC c-tDCS as compared to CBT with sham DLPFC c-tDCS or CBT alone can reduce pain-related anxiety, kinesiophobia, and disability in LBP patients with high pain-related anxiety.
The findings of this study have to be seen in light of some limitations. One of the limitations of this study was the age of the participants, who were young adults. Therefore, the results cannot be generalized to middle-aged or older CLBP adults. Therefore, we recommend conducting further studies to investigate the effect of DLPFC c-tDCS on pain-related anxiety, fear of movement, and disability levels in middle-aged or older patients with LBP. In addition, the current study considered only a one-month follow-up. Longer follow-up assessments are also recommended in further studies. Moreover, the main outcome measures for anxiety, fear, and disability were not in quantity in the current study. Conducting future studies is suggested to quantify the outcome measures.
5. Conclusions
The findings in this study provided evidence for the priming effects of multiple-session c-tDCS over the left DLPFC on the effects of CBT on pain-related anxiety, fear of movement, and disability in LBP patients with high pain-related anxiety. The findings in the current study indicated significant decreases in PASS, TSK, and Roland–Morris questionnaire scores immediately after the interventions in all groups. However, these immediate effects were more in the c-tDCS and CBT group compared to the CBT alone and the sham groups. The findings in this study also confirmed the lasting effects of c-tDCS along with CBT in LBP patients with high pain-related anxiety.
Author Contributions
Conceptualization, F.E. and M.S.H.Y.; methodology, F.E. and S.J.; formal analysis, F.E. and M.S.H.Y., software, F.E.; investigation, M.S.H.Y.; data curation, M.S.H.Y., A.J. and F.E.; writing—original draft preparation, M.S.H.Y., A.J., F.E., S.J. and M.Z.; writing—review and editing, F.E., S.J., M.Z. and M.S.H.Y.; visualization, S.J. and M.Z.; supervision, S.J. and F.E. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was approved by the Human Ethics Committee of the Semnan University of Medical Sciences, Semnan, Iran, and was performed following the ethical standards laid down by the Declaration of Helsinki. This study was registered as a clinical trial on the Iranian Registry of Clinical Trials (the registration number is IRCT20150302021294N6).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank the Neuromuscular Rehabilitation Centre of Semnan University of Medical Sciences for its cooperation and for providing the necessary preconditions for this study.
Conflicts of Interest
The authors declare that there are no conflict of interest.
References
- O’Sullivan, P. Diagnosis and classification of chronic low back pain disorders: Maladaptive movement and motor control impairments as underlying mechanism. Man. Ther. 2005, 10, 242–255. [Google Scholar] [CrossRef]
- Twomey, L.T.; Taylor, J.R. Physical Therapy of the Low Back; Churchill Livingstone: London, UK, 2000. [Google Scholar]
- Carey, T.S.; Garrett, J.M.; Jackman, A.M. Beyond the good prognosis: Examination of an inception cohort of patients with chronic low back pain. Spine 2000, 25, 115. [Google Scholar] [CrossRef] [PubMed]
- Izzo, R.; Popolizio, T.; D’Aprile, P.; Muto, M. Spinal pain. Eur. J. Radiol. 2015, 84, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Higgins, J.; Bourbonnais, D. Is neuroplasticity in the central nervous system the missing link to our understanding of chronic musculoskeletal disorders? BMC Musculoskelet. Disord. 2015, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, R.; Higgins, J.; Bourbonnais, D. Addressing neuroplastic changes in distributed areas of the nervous system associated with chronic musculoskeletal disorders. Phys. Ther. 2015, 95, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Apkarian, A.V.; Hashmi, J.A.; Baliki, M.N. Pain and the brain: Specificity and plasticity of the brain in clinical chronic pain. Pain 2011, 152, S49–S64. [Google Scholar] [CrossRef] [PubMed]
- Gatchel, R.J.; Polatin, P.B.; Mayer, T.G. The dominant role of psychosocial risk factors in the development of chronic low back pain disability. Spine 1995, 20, 2702–2709. [Google Scholar] [CrossRef]
- Linton, S.J. A review of psychological risk factors in back and neck pain. Spine 2000, 25, 1148–1156. [Google Scholar] [CrossRef]
- Cox, M.E.; Asselin, S.; Gracovetsky, S.A.; Richards, M.P.; Newman, N.M.; Karakusevic, V.; Zhong, L.; Fogel, J.N. Relationship between functional evaluation measures and self-assessment in nonacute low back pain. Spine 2000, 25, 1817–1826. [Google Scholar] [CrossRef]
- ML, P. The joint contribution of physical pathology, pain-related fear and catastrophizing to chronic back pain disability. Pain 2005, 113, 40–45. [Google Scholar]
- Waddell, G.; Newton, M.; Henderson, I.; Somerville, D.; Main, C.J. A Fear-Avoidance Beliefs Questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain 1993, 52, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Karayannis, N.V.; Smeets, R.J.; van den Hoorn, W.; Hodges, P.W. Fear of movement is related to trunk stiffness in low back pain. PLoS ONE 2013, 8, e67779. [Google Scholar] [CrossRef]
- Massé-Alarie, H.; Beaulieu, L.-D.; Preuss, R.; Schneider, C. Influence of chronic low back pain and fear of movement on the activation of the transversely oriented abdominal muscles during forward bending. J. Electromyogr. Kinesiol. 2016, 27, 87–94. [Google Scholar] [PubMed]
- Luedtke, K.; Rushton, A.; Wright, C.; Jürgens, T.; Polzer, A.; Mueller, G.; May, A. Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: Sham controlled double blinded randomised controlled trial. BMJ 2015, 350, h1640. [Google Scholar] [CrossRef] [PubMed]
- Vakili, N.; Neshat Doost, H.; Asgari, K.; Rezaee, F.; Najafi, M. The effect of cognitive-behavioral group pain management therapy on depression of the female with chronic low back pain. J. Clin. Psychol. 2010, 1, 11–19. [Google Scholar]
- Aneis, M.; Shaker, H.A.; Fahmy, E.M.; Yousef, K.H. Efect of cognitive behavior therapy in patients with chronic nonspecific low back pain. Turk. J. Physiother. Rehabil. 2021, 32, 3. [Google Scholar]
- Yang, J.; Lo, W.L.A.; Zheng, F.; Cheng, X.; Yu, Q.; Wang, C. Evaluation of cognitive behavioral therapy on improving pain, fear avoidance, and self-efficacy in patients with chronic low back pain: A systematic review and meta-analysis. Pain Res. Manag. 2022, 2022, 4276175. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mahmoudi, Z.; Mahmoodian, N. The Effects of Cerebellar Transcranial Direct Current Stimulation (tDCS) on Timed Up and Go Test with Foot Placement in Chronic Stroke Patients. Middle East J. Rehabil. Health Stud. 2021, 8, 730–738. [Google Scholar] [CrossRef]
- Jamebozorgi, A.; Rahimi, A.; Daryabar, A.; Kazemi, M.; Jamebozorgi, F. The Effects of Transcranial Direct Current Stimulation (tDCS) and Biofeedback on Proprioception and Functional Balance in Athletes with ACL-deficiency. East J. Rehabil. Health Stud. 2023, 10, e130364. [Google Scholar] [CrossRef]
- Pacheco-Barrios, K.; Cardenas-Rojas, A.; Tibout, A.; Frengi, F. Methods and strategies of tDCS for the treatment of pain: Current status and future directions. Expert Rev. Med. Devices 2020, 19, 879–898. [Google Scholar] [CrossRef]
- DaSilva, A.F.; Mendonca, M.E.; Zaghi, S.; Lopes, M.; DosSantos, M.F.; Spierings, E.L.; Bajwa, Z.; Datta, A.; Bikson, M.; Fregni, F. tDCS-induced analgesia and electrical fields in pain-related neural networks in chronic migraine. Headache J. Head Face Pain 2012, 52, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Kimberley, T.J.; Lewis, S.M. Understanding neuroimaging. Phys. Ther. 2007, 87, 670–683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herrmann, M.J.; Beier, J.S.; Simons, B.; Polak, T. Transcranial Direct Current Stimulation (tDCS) of the right inferior frontal gyrus attenuates skin conductance responses to unpredictable threat conditions. Front. Hum. Neurosci. 2016, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- van ‘t Wout, M.; Longo, S.M.; Reddy, M.K.; Philip, N.S.; Bowker, M.T.; Greenberg, B.D. Transcranial direct current stimulation may modulate extinction memory in posttraumatic stress disorder. Brain Behav. 2017, 7, e00681. [Google Scholar] [CrossRef] [PubMed]
- Asthana, M.; Nueckel, K.; Mühlberger, A.; Neueder, D.; Polak, T.; Domschke, K.; Deckert, J.; Herrmann, M.J. Effects of transcranial direct current stimulation on consolidation of fear memory. Front. Psychiatry 2013, 4, 107. [Google Scholar] [CrossRef] [PubMed]
- Villamar, M.F.; Volz, M.S.; Bikson, M.; Datta, A.; DaSilva, A.F.; Fregni, F. Technique and considerations in the use of 4x1 ring high-definition transcranial direct current stimulation (HD-tDCS). JoVE (J. Vis. Exp.) 2013, 14, e50309. [Google Scholar]
- Hawco, C.; Berlim, M.T.; Lepage, M. The dorsolateral prefrontal cortex plays a role in self-initiated elaborative cognitive processing during episodic memory encoding: rTMS evidence. PLoS ONE 2013, 8, e73789. [Google Scholar] [CrossRef] [PubMed]
- Petrides, M. Lateral prefrontal cortex: Architectonic and functional organization. Philos. Trans. R. Soc. B Biol. Sci. 2005, 360, 781–795. [Google Scholar] [CrossRef]
- Apkarian, A.V.; Baliki, M.N.; Farmer, M.A. Predicting transition to chronic pain. Curr. Opin. Neurol. 2013, 26, 360. [Google Scholar] [CrossRef]
- Medrano-Escalada, Y.; Plaza-Manzano, G.; Fernández-de-Las-Peñas, C.; Valera-Calero, J.A. Structural, functional and neurochemical cortical brain changes associated with chronic low back pain. Tomography 2022, 8, 2153–2163. [Google Scholar] [CrossRef]
- Shanbehzadeh, S.; Salavati, M.; Tavahomi, M.; Khatibi, A.; Talebian, S.; Khademi-Kalantari, K. Reliability and validity of the pain anxiety symptom scale in Persian speaking chronic low back pain patients. Spine 2017, 42, E1238–E1244. [Google Scholar] [CrossRef]
- Airaksinen, O.; Brox, J.I.; Cedraschi, C.; Hildebrandt, J.; Klaber-Moffett, J.; Kovacs, F.; Mannion, A.F.; Reis, S.; Staal, J.; Ursin, H. European guidelines for the management of chronic nonspecific low back pain. Eur. Spine J. 2006, 15, s192. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Qaseem, A.; Snow, V.; Casey, D.; Cross, J.T., Jr.; Shekelle, P.; Owens, D.K.; Clinical Efficacy Assessment Subcommittee of the American College of Physicians and the American College of Physicians/American Pain Society Low Back Pain Guidelines Panel*. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann. Intern. Med. 2007, 147, 478–491. [Google Scholar] [CrossRef] [PubMed]
- Koes, B.W. Evidence-based management of acute low back pain. Lancet 2007, 370, 1595–1596. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, R.; Brunoni, A.R.; Parazzini, M.; Vergari, M.; Rossi, E.; Fumagalli, M.; Mameli, F.; Rosa, M.; Giannicola, G.; Zago, S. Modulating human procedural learning by cerebellar transcranial direct current stimulation. Cerebellum 2013, 12, 485–492. [Google Scholar] [CrossRef]
- Ferrucci, R.; Cortese, F.; Priori, A. Cerebellar tDCS: How to do it. Cerebellum 2015, 14, 27–30. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633. [Google Scholar] [CrossRef]
- Samaei, A.; Ehsani, F.; Zoghi, M.; Hafez Yosephi, M.; Jaberzadeh, S. Online and offline effects of cerebellar transcranial direct current stimulation on motor learning in healthy older adults: A randomized double-blind sham-controlled study. Eur. J. Neurosci. 2017, 45, 1177–1185. [Google Scholar] [CrossRef]
- Brunoni, A.R.; Amadera, J.; Berbel, B.; Volz, M.S.; Rizzerio, B.G.; Fregni, F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011, 14, 1133–1145. [Google Scholar] [CrossRef]
- Zuchowski, M.L.; Timmann, D.; Gerwig, M. Acquisition of conditioned eyeblink responses is modulated by cerebellar tDCS. Brain Stimul. 2014, 7, 525–531. [Google Scholar] [CrossRef]
- Gandiga, P.C.; Hummel, F.C.; Cohen, L.G. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin. Neurophysiol. 2006, 117, 845–850. [Google Scholar] [CrossRef] [PubMed]
- Mariano, T.Y.; Urman, R.D.; Hutchison, C.A.; Jamison, R.N.; Edwards, R.R. Cognitive behavioral therapy (CBT) for subacute low back pain: A systematic review. Curr. Pain Headache Rep. 2018, 22, 15. [Google Scholar] [CrossRef] [PubMed]
- Monticone, M.; Ferrante, S.; Rocca, B.; Baiardi, P.; Dal Farra, F.; Foti, C. Effect of a long-lasting multidisciplinary program on disability and fear-avoidance behaviors in patients with chronic low back pain: Results of a randomized controlled trial. Clin. J. Pain 2013, 29, 929–938. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Ebrahimi, I.; Salavati, M.; Kamali, M.; Fata, L. Psychometric properties of Iranian version of Tampa Scale for Kinesiophobia in low back pain patients. Arch. Rehabil. 2010, 11. [Google Scholar]
- Rusu, A.C.; Kreddig, N.; Hallner, D.; Hülsebusch, J.; Hasenbring, M.I. Fear of movement/(Re) injury in low back pain: Confirmatory validation of a German version of the Tampa Scale for Kinesiophobia. BMC Musculoskelet. Disord. 2014, 15, 280. [Google Scholar] [CrossRef] [PubMed]
- Sveinsdottir, V.; Eriksen, H.R.; Reme, S.E. Assessing the role of cognitive behavioral therapy in the management of chronic nonspecific back pain. J. Pain Res. 2012, 5, 371–380. [Google Scholar] [PubMed]
- Ampiah, P.K.; Hendrick, P.; Macias, E.G. Comparative effectiveness of cognitive behavioural therapy combined with exercise versus exercise in the management of non-specific chronic low back pain: A systematic review with meta-analysis. Edorium J. Disabil. Rehabil. 2018, 4, 100041D05PA2018. [Google Scholar]
- Roland, M.; Fairbank, J. The Roland–Morris disability questionnaire and the Oswestry disability questionnaire. Spine 2000, 25, 3115–3124. [Google Scholar] [CrossRef]
- Mousavi, S.J.; Parnianpour, M.; Mehdian, H.; Montazeri, A.; Mobini, B. The Oswestry disability index, the Roland-Morris disability questionnaire, and the Quebec back pain disability scale: Translation and validation studies of the Iranian versions. Spine 2006, 31, E454–E459. [Google Scholar] [CrossRef]
- Reid, M.C.; Otis, J.; Barry, L.C.; Kerns, R.D. Cognitive–behavioral therapy for chronic low back pain in older persons: A preliminary study. Pain Med. 2003, 4, 223–230. [Google Scholar] [CrossRef]
- Richmond, H.; Hall, A.M.; Copsey, B.; Hansen, Z.; Williamson, E.; Hoxey-Thomas, N.; Cooper, Z.; Lamb, S.E. The effectiveness of cognitive behavioural treatment for non-specific low back pain: A systematic review and meta-analysis. PLoS ONE 2015, 10, e0134192. [Google Scholar] [CrossRef]
- Parreira, P.; Maher, C.G.; Steffens, D.; Hancock, M.J.; Ferreira, M.L. Risk factors for low back pain and sciatica: An umbrella review. Spine J. 2018, 18, 1715–1721. [Google Scholar] [CrossRef]
- Van Middelkoop, M.; Rubinstein, S.M.; Kuijpers, T.; Verhagen, A.P.; Ostelo, R.; Koes, B.W.; van Tulder, M.W. A systematic review on the effectiveness of physical and rehabilitation interventions for chronic non-specific low back pain. Eur. Spine J. 2011, 20, 19–39. [Google Scholar] [CrossRef]
- Weigand, A.; Richtermeier, A.; Feeser, M.; Guo, J.S.; Briesemeister, B.B.; Grimm, S.; Bajbouj, M. State-dependent effects of prefrontal repetitive transcranial magnetic stimulation on emotional working memory. Brain Stimul. 2013, 6, 905–912. [Google Scholar] [CrossRef]
- Mariano, T.Y.; Burgess, F.W.; Bowker, M.; Kirschner, J.; van’t Wout-Frank, M.; Jones, R.N.; Halladay, C.W.; Stein, M.; Greenberg, B.D. Transcranial direct current stimulation for affective symptoms and functioning in chronic low back pain: A pilot double-blinded, randomized, placebo-controlled trial. Pain Med. 2019, 20, 1166–1177. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).