The Biomechanical Characteristics of Swallowing in Tracheostomized Patients with Aspiration following Acquired Brain Injury: A Cross-Sectional Study
Abstract
1. Introduction
2. Methods
2.1. Subjects
2.2. HRM Procedure
2.3. VFSS Procedure
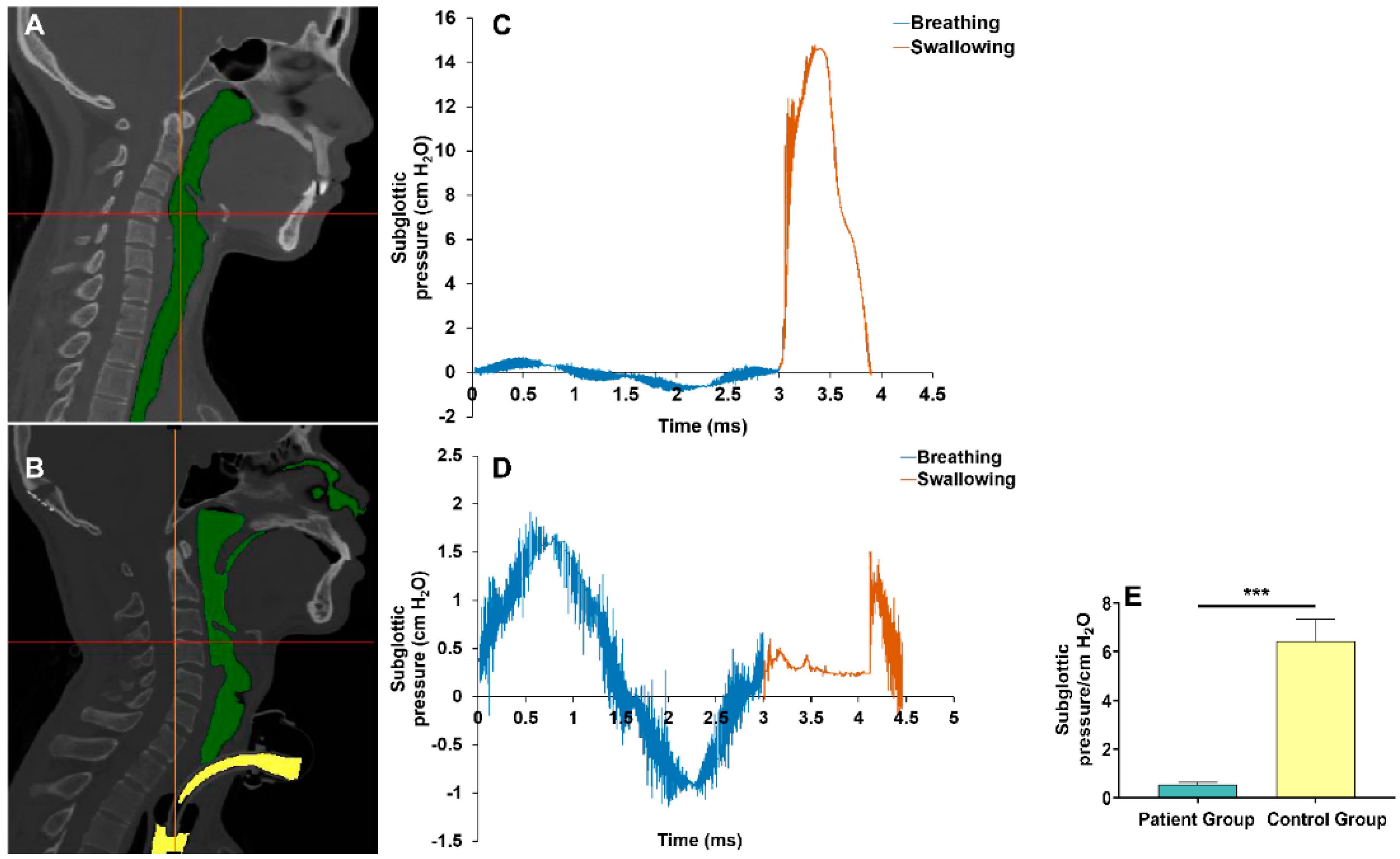
2.4. Reconstruction of the Upper Airway Model
2.5. CFD Procedure to Assess the Subglottic Pressure
2.6. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Comparison of the Biomechanics Characteristics during Swallowing between Groups
3.3. Correlation between VP Maximal Pressure, TB Maximal Pressure, UES Residual Pressure, UES Relaxation Duration, Subglottic Pressure, and PAS Score in the Patient Group
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tran, T.T.A.; Harris, B.M.; Pearson, W.G., Jr. Improvements resulting from respiratory-swallow phase training visualized in patient-specific computational analysis of swallowing mechanics. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2018, 6, 532–538. [Google Scholar] [CrossRef] [PubMed]
- AYan, N.; Jiang, J.; Liu, H.; Deng, L.; Hu, Q.; Sun, J.; Lv, M. Evidence-based bundled care for patients with dysphagia after severe traumatic brain injury: A randomized controlled trial. Am. J. Transl. Res. 2021, 13, 7819–7828. [Google Scholar]
- Westendorp, W.F.; Dames, C.; Nederkoorn, P.J.; Meisel, A. Immunodepression, Infections, and Functional Outcome in Ischemic Stroke. Stroke 2022, 53, 1438–1448. [Google Scholar] [CrossRef]
- Son, Y.G.; Shin, J.; Ryu, H.G. Pneumonitis and pneumonia after aspiration. J. Dent. Anesth. Pain Med. 2017, 17, 1–12. [Google Scholar] [CrossRef]
- Eskildsen, S.J.; Poulsen, I.; Jakobsen, D.; Riberholt, C.G.; Curtis, D.J. Scoping review to identify and map non-pharmacological, non-surgical treatments for dysphagia following moderate-to-severe acquired brain injury. BMJ Open 2021, 11, e053244. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.S.; Fortune, D.G.; Gallagher, S.; Muldoon, O.T. Acquired brain injury: Combining social psychological and neuropsychological perspectives. Health Psychol. Rev. 2014, 8, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zheng, F.; Zhang, L.; Wang, W.; Yu, C.; Shao, J.; Wu, Y. Research progress of clinical intervention and nursing for patients with post-stroke dysphagia. Neurol. Sci. 2022, 43, 5875–5884. [Google Scholar] [CrossRef]
- Dexter, A.M.; Scott, J.B. Airway Management and Ventilator-Associated Events. Respir. Care 2019, 64, 986–993. [Google Scholar] [CrossRef]
- Marvin, S.; Thibeault, S.L. Predictors of Aspiration and Silent Aspiration in Patients with New Tracheostomy. Am. J. Speech Lang. Pathol. 2021, 30, 2554–2560. [Google Scholar] [CrossRef]
- Fernandez-Carmona, A.; Penas-Maldonado, L.; Yuste-Osorio, E.; Díaz-Redondo, A. Exploration and approach to artificial airway dysphagia. Med. Intensiv. 2012, 36, 423–433. [Google Scholar] [CrossRef]
- Gross, R.D.; Mahlmann, J.; Grayhack, J.P. Physiologic effects of open and closed tracheostomy tubes on the pharyngeal swallow. Ann. Otol. Rhinol. Laryngol. 2003, 112, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Eibling, D.E.; Gross, R.D. Subglottic air pressure: A key component of swallowing efficiency. Ann. Otol. Rhinol. Laryngol. 1996, 105, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Winiker, K.; Gillman, A.; Hernandez, E.G.; Huckabee, M.L.; Gozdzikowska, K. A systematic review of current methodology of high resolution pharyngeal manometry with and without impedance. Eur. Arch. Oto-Rhino-Laryngol. 2019, 276, 631–645. [Google Scholar] [CrossRef] [PubMed]
- Armán, J.A.; Lorente-Ramos, R.M.; García, P.G.; Lorduy, T.C. Videofluoroscopic Evaluation of Normal and Impaired Oropharyngeal Swallowing. Radiographics 2019, 39, 78–79. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.Z.; Espinoza, V.M.; Marks, K.L.; Zañartu, M.; Mehta, D.D. Improved subglottal pressure estimation from neck-surface vibration in healthy speakers producing non-modal phonation. IEEE J. Sel. Top. Signal Process. 2020, 14, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Petekkaya, E.; Yücel, A.H.; Sürmelioğlu, Ö. Evaluation of the supraglottic and subglottic activities including acoustic assessment of the opera-chant singers. J. Voice 2019, 33, 255.E1–255.E7. [Google Scholar] [CrossRef]
- Gross, R.D.; Atwood, C.W., Jr.; Grayhack, J.P.; Shaiman, S. Lung volume effects on pharyngeal swallowing physiology. J. Appl. Physiol. 2003, 95, 2211–2217. [Google Scholar] [CrossRef]
- Gross, R.D.; Carrau, R.L.; Slivka, W.A.; Gisser, R.G.; Smith, L.J.; Zajac, D.J.; Sciurba, F.C. Deglutitive subglottic air pressure and respiratory system recoil. Dysphagia 2012, 27, 452–459. [Google Scholar] [CrossRef]
- Gross, R.D.; Steinhauer, K.M.; Zajac, D.J.; Weissler, M.C. Direct measurement of subglottic air pressure while swallowing. Laryngoscope 2006, 116, 753–761. [Google Scholar] [CrossRef]
- Lin, E.L.; Bock, J.M.; Zdanski, C.J.; Kimbell, J.S.; Garcia, G.J.M. Relationship between degree of obstruction and airflow limitation in subglottic stenosis. Laryngoscope 2018, 128, 1551–1557. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, H.; Xu, Q.; Wu, W.; Du, L.; Chen, H.; Zhang, Y.; Liu, D. Computational fluid dynamics simulation of the upper airway response to large incisor retraction in adult class I bimaxillary protrusion patients. Sci. Rep. 2017, 7, 45706. [Google Scholar] [CrossRef] [PubMed]
- Diver, E.M.; Regan, J. Use of Pharyngeal High-Resolution Manometry to Evaluate Dysphagia in Adults with Motor Neurone Disease: A Scoping Review. Dysphagia 2022, 37, 1697–1714. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, T.M.; Hoffman, M.R.; Ciucci, M.R. High-resolution manometry of pharyngeal swallow pressure events associated with head turn and chin tuck. Ann. Otol. Rhinol. Laryngol. 2010, 119, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.M.; Grace-Martin, K. Reflections on Clinical and Statistical Use of the Penetration-Aspiration Scale. Dysphagia 2017, 32, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Jungheim, M.; Busche, A.; Miller, S.; Schilling, N.; Schmidt-Thieme, L.; Ptok, M. Calculation of upper esophageal sphincter restitution time from high resolution manometry data using machine learning. Physiol. Behav. 2016, 165, 413–424. [Google Scholar] [CrossRef]
- Lippert, D.; Hoffman, M.R.; Britt, C.J.; Jones, C.A.; Hernandez, J.; Ciucci, M.R.; McCulloch, T.M. Preliminary evaluation of functional swallow after total laryngectomy using high-resolution manometry. Ann. Otol. Rhinol. Laryngol. 2016, 125, 541–549. [Google Scholar] [CrossRef]
- Raol, N.; Hartnick, C.J. Anatomy and physiology of velopharyngeal closure and insufficiency. Adv. Otorhinolaryngol. 2015, 76, 1–6. [Google Scholar]
- Peña-Chávez, R.E.; Schaen-Heacock, N.E.; Hitchcock, M.E.; Kurosu, A.; Suzuki, R.; Hartel, R.W.; Ciucci, M.R.; Rogus-Pulia, N.M. Effects of Food and Liquid Properties on Swallowing Physiology and Function in Adults. Dysphagia 2022. ahead of print. [Google Scholar] [CrossRef]
- Danescu, A.; Mattson, M.; Dool, C.; Diewert, V.M.; Richman, J.M. Analysis of human soft palate morphogenesis supports regional regulation of palatal fusion. J. Anat. 2015, 227, 474–486. [Google Scholar] [CrossRef]
- Dziewas, R.; Stellato, R.; van der Tweel, I.; Walther, E.; Werner, C.J.; Braun, T.; Citerio, G.; Jandl, M.; Friedrichs, M.; Nötzel, K.; et al. PHAST-TRAC investigators. Pharyngeal electrical stimulation for early decannulation in tracheotomised patients with neurogenic dysphagia after stroke (PHAST-TRAC): A prospective, single-blinded, randomised trial. Lancet Neurol. 2018, 17, 849–859. [Google Scholar] [CrossRef]
- Ponfick, M.; Linden, R.; Nowak, D.A. Dysphagia—A common, transient symptom in critical illness polyneuropathy: A fiberoptic endoscopic evaluation of swallowing study. Crit. Care Med. 2015, 43, 365–372. [Google Scholar] [CrossRef]
- Hanscom, M.; Loane, D.J.; Shea-Donohue, T. Brain-gut axis dysfunction in the pathogenesis of traumatic brain injury. J. Clin. Investig. 2021, 131, e143777. [Google Scholar] [CrossRef] [PubMed]
- Krakau, K.; Omne-Pontén, M.; Karlsson, T.; Borg, J. Metabolism and nutrition in patients with moderate and severe traumatic brain injury: A systematic review. Brain Inj. 2006, 20, 345–367. [Google Scholar] [CrossRef] [PubMed]
- Park, C.H.; Lee, Y.T.; Yi, Y.; Lee, J.S.; Park, J.H.; Yoon, K.J. Ability of high-resolution manometry to determine feeding method and to predict aspiration pneumonia in patients with dysphagia. Am. J. Gastroenterol. 2017, 112, 1074–1083. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, S.; Shingai, T.; Kitagawa, J.; Takahashi, Y.; Taguchi, Y.; Noda, T.; Yamada, Y. Role of the pharyngeal branch of the vagus nerve in laryngeal elevation and UES pressure during swallowing in rabbits. Dysphagia 2003, 18, 58–63. [Google Scholar] [CrossRef]
- Karaho, T.; Nakajima, J.; Satoh, T.; Kawahara, K.; Nakayama, T.; Kohno, N. Mano-videoendoscopic assessment in the evaluation of the pharyngeal contraction and upper esophageal sphincter function in dysphagic patients. Auris Nasus Larynx 2017, 44, 79–85. [Google Scholar] [CrossRef]
- Jiao, H.; Mei, L.; Sharma, T.; Kern, M.; Sanvanson, P.; Shaker, R. A human model of restricted upper esophageal sphincter opening and its pharyngeal and UES deglutitive pressure phenomena. Am. J. Physiol. Gastrointest Liver Physiol. 2016, 311, G84–G90. [Google Scholar] [CrossRef]
- Lee, T.; Park, J.H.; Sohn, C.; Yoon, K.J.; Lee, Y.T.; Park, J.H.; Jung, I.S. Failed deglutitive upper esophageal sphincter relaxation is a risk factor for aspiration in stroke patients with oropharyngeal dysphagia. J. Neurogastroenterol. Motil. 2017, 23, 34–40. [Google Scholar] [CrossRef][Green Version]
- Barker, J.; Martino, R.; Yau, T.M. Changes in Cardiac Function During a Swallow Exercise Program in Patients with Coronary Artery Disease. Dysphagia 2022. ahead of print. [Google Scholar] [CrossRef]
- Han, X.; Ye, Q.; Meng, Z.; Pan, D.; Wei, X.; Wen, H.; Dou, Z. Biomechanical mechanism of reduced aspiration by the Passy-Muir valve in tracheostomized patients following acquired brain injury: Evidences from subglottic pressure. Front. Neurosci. 2022, 16, 1004013. [Google Scholar] [CrossRef]
- Rogus-Pulia, N.; Rusche, N.; Hind, J.A.; Zielinski, J.; Gangnon, R.; Safdar, N.; Robbins, J. Effects of device-facilitated isometric progressive resistance oropharyngeal therapy on swallowing and health-related outcomes in older adults with dysphagia. J. Am. Geriatr. Soc. 2016, 64, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, A.; Hind, J.; Kays, S.; Nicosia, M.; Doyle, J.; Tompkins, W.; Gangnon, R.; Robbins, J. Standardized instrument for lingual pressure measurement. Dysphagia 2008, 23, 16–25. [Google Scholar] [CrossRef] [PubMed]



| Patient Group (n = 15) | Control Group (n = 15) | p Value | |
|---|---|---|---|
| Age (years), mean ± SD | 59.21 ± 10.53 | 57.32 ± 9.65 | 0.595 |
| Gender (male), n (%) | 7 (46.7) | 10 (66.7) | 0.462 |
| BMI (kg/m2), mean ± SD | 18.97 ± 1.51 | 20.28 ± 1.65 | 0.026 |
| BI score, mean ± SD | 29.00 ± 15.72 | 98.00 ± 3.68 | <0.001 |
| FOIS score, n (%) | <0.001 | ||
| 1–3 | 15 (100) | 0 | |
| 4–5 | 0 | 0 | |
| 6–7 | 0 | 15 (100) | |
| Brain injury etiology, n (%) | |||
| Stroke | 11 (73.3) | - | - |
| Brain tumor | 2 (13.3) | - | - |
| Traumatic brain injury | 2 (13.3) | - | - |
| Lesion location, n (%) | |||
| Supratentorial | 4 (26.7) | - | - |
| Infratentorial | 11 (73.3) | - | - |
| Lesion side, n% | |||
| Left | 3 (20) | - | - |
| Right | 6 (40) | - | - |
| Both | 6 (40) | - | - |
| NIHSS (for patients with stroke), mean ± SD | 6.75 ± 3.42 | - | - |
| Time from disease onset (months), mean ± SD | 3.41 ± 1.36 | - | - |
| Duration of tracheal intubation (months), mean ± SD | 3.27 ± 1.62 | - | - |
| Aspiration pneumonia, n (%) | 13 (86.7) | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.-X.; Qiao, J.; Meng, Z.-A.; Pan, D.-M.; Zhang, K.; Wei, X.-M.; Dou, Z.-L. The Biomechanical Characteristics of Swallowing in Tracheostomized Patients with Aspiration following Acquired Brain Injury: A Cross-Sectional Study. Brain Sci. 2023, 13, 91. https://doi.org/10.3390/brainsci13010091
Han X-X, Qiao J, Meng Z-A, Pan D-M, Zhang K, Wei X-M, Dou Z-L. The Biomechanical Characteristics of Swallowing in Tracheostomized Patients with Aspiration following Acquired Brain Injury: A Cross-Sectional Study. Brain Sciences. 2023; 13(1):91. https://doi.org/10.3390/brainsci13010091
Chicago/Turabian StyleHan, Xiao-Xiao, Jia Qiao, Zhan-Ao Meng, Dong-Mei Pan, Ke Zhang, Xiao-Mei Wei, and Zu-Lin Dou. 2023. "The Biomechanical Characteristics of Swallowing in Tracheostomized Patients with Aspiration following Acquired Brain Injury: A Cross-Sectional Study" Brain Sciences 13, no. 1: 91. https://doi.org/10.3390/brainsci13010091
APA StyleHan, X.-X., Qiao, J., Meng, Z.-A., Pan, D.-M., Zhang, K., Wei, X.-M., & Dou, Z.-L. (2023). The Biomechanical Characteristics of Swallowing in Tracheostomized Patients with Aspiration following Acquired Brain Injury: A Cross-Sectional Study. Brain Sciences, 13(1), 91. https://doi.org/10.3390/brainsci13010091





