Long-Term Seizure Outcomes and Predictors in Patients with Dysembryoplastic Neuroepithelial Tumors Associated with Epilepsy
Abstract
1. Introduction
2. Materials and Methods
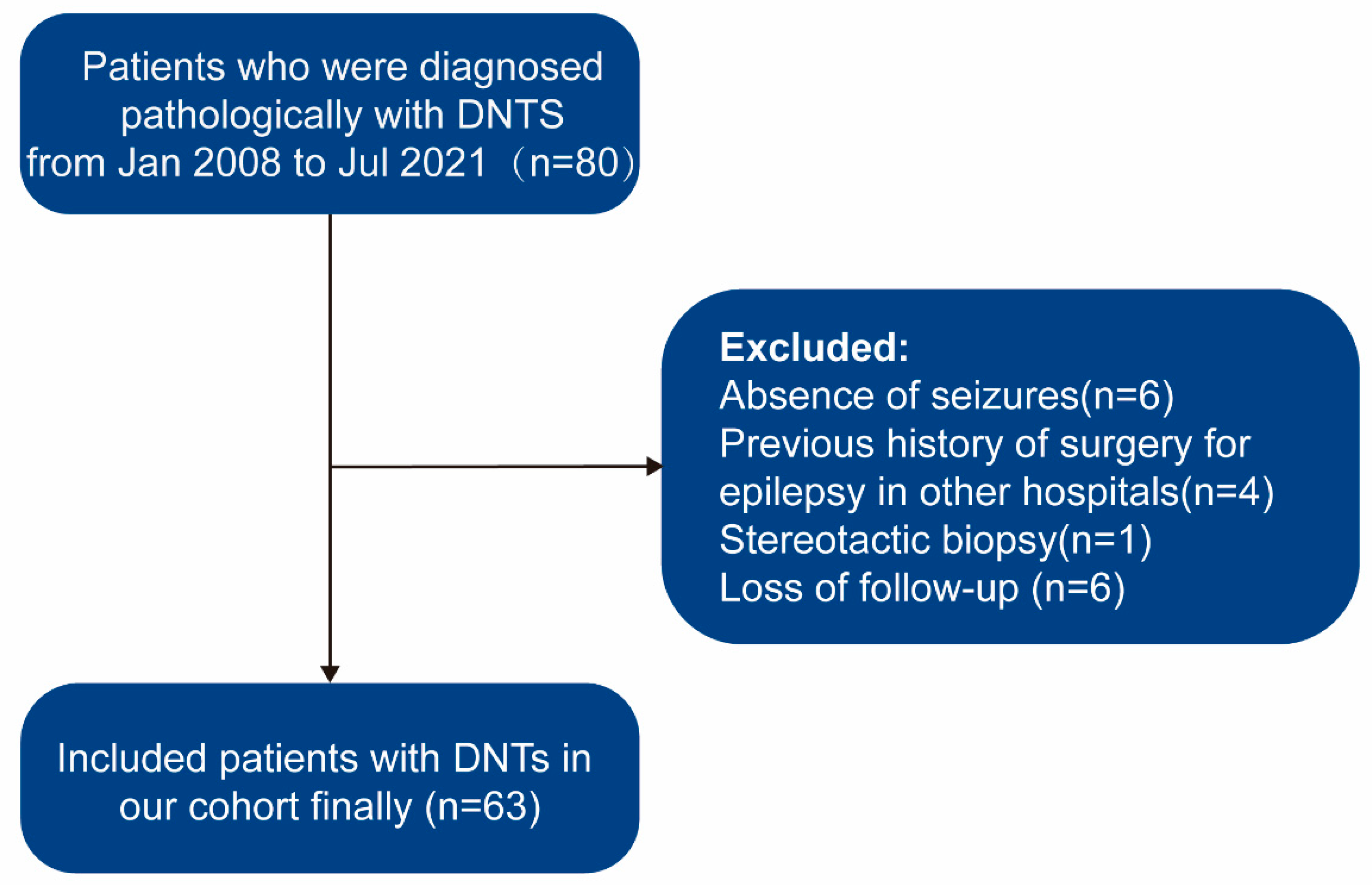
2.1. Patient Selection
2.2. Presurgical Assessments
2.3. Surgical Procedure
2.4. Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Demographic Characteristics
3.2. Clinical Characteristics
3.3. Surgical Complications
3.4. Follow-Up and Outcomes
3.5. Univariable Survival Analysis
3.6. Multivariable Cox Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Time, Years | Cumulative Rate of Seizure Recurrence-Free (%) | Standard Error | 95% CI (%) |
|---|---|---|---|
| 1 | 84.1 | 0.0460 | 75.6–93.7 |
| 2 | 82.5 | 0.0478 | 73.7–92.5 |
| 3 | 79.0 | 0.0518 | 69.5–89.9 |
| 4 | 79.0 | 0.0518 | 69.5–89.9 |
| 5 | 79.0 | 0.0518 | 69.5–89.9 |
| 6 | 76.5 | 0.0561 | 66.2–88.3 |
| 7 | 76.5 | 0.0561 | 66.2–88.3 |
| 8 | 76.5 | 0.0561 | 66.2–88.3 |
| 9 | 76.5 | 0.0561 | 66.2–88.3 |
| 10 | 76.5 | 0.0561 | 66.2–88.3 |
| 11 | 76.5 | 0.0561 | 66.2–88.3 |
| 12 | 76.5 | 0.0561 | 66.2–88.3 |
| 13 | 76.5 | 0.0561 | 66.2–88.3 |
| Variable | Chi-Square | p-Value |
|---|---|---|
| Sex | 1.984 | 0.159 |
| Age at onset | 7.400 | 0.007 * |
| Duration | 10.076 | 0.002 * |
| Age at surgery | 0.443 | 0.506 |
| Auras | 0.002 | 0.964 |
| Seizure frequency | 1.597 | 0.660 |
| Seizure type | 0.343 | 0.842 |
| Seizure kind | 0.640 | 0.726 |
| Site of lesion | 0.846 | 0.358 |
| MRI subtype 1 | 8.920 | 0.003 * |
| IEDs 2 | 14.009 | 0.003 * |
| Ictal onset rhythms | 4.964 | 0.174 |
| Surgical type | 18.607 | <0.001 * |
| Histopathological types | 7.480 | 0.058 |
| Complications | 0.315 | 0.854 |
| Variable | HR | p-Value | 95% CI |
|---|---|---|---|
| Sex | 2.19 | 0.160 | 0.73–6.54 |
| Age at onset | 0.25 | 0.012 * | 0.08–0.74 |
| Duration | 4.87 | 0.005 * | 1.63–14.56 |
| Age at surgery | 1.42 | 0.510 | 0.50–4.06 |
| Auras | 1.07 | 0.915 | 0.33–3.4 |
| Seizure frequency | 1.33 | 0.325 | 0.75–2.35 |
| Seizure type | 0.97 | 0.936 | 0.52–1.83 |
| Seizure kind | 0.86 | 0.753 | 0.34–2.17 |
| Lateral | 0.82 | 0.713 | 0.28–2.37 |
| Site of lesion | 1.62 | 0.373 | 0.56–4.67 |
| MRI subtype | 1.82 | 0.027 * | 1.07–3.10 |
| IEDs | 1.64 | 0.017 * | 1.09–2.48 |
| Ictal onset rhythms | 1.23 | 0.382 | 0.78–1.94 |
| Surgical type | 7.90 | <0.001 * | 2.71–23.06 |
| Histopathological types | 1.37 | 0.482 | 0.57–3.32 |
| Complications | 1.51 | 0.528 | 0.42–5.42 |
References
- Daumas-Duport, C.; Scheithauer, B.W.; Chodkiewicz, J.-P.; Laws, E.R.; Vedrenne, C. Dysembryoplastic Neuroepithelial Tumor: A Surgically Curable Tumor of Young Patients with Intractable Partial Seizures. Neurosurgery 1988, 23, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A Summary. Neuro-Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.S.; Doan, N.; Gelsomino, M.; Shabani, S. Dysembryoplastic Neuroectodermal Tumor: An Analysis from the Surveillance, Epidemiology, and End Results Program, 2004–2013. World Neurosurg. 2017, 103, 380–385. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.; Borofsky, S.; Jones, R.V.; Levy, L.M. Dysembryoplastic Neuroepithelial Tumor with Atypical Presentation: MRI and Diffusion Tensor Characteristics. J. Radiol. Case Rep. 2013, 7, 7–14. [Google Scholar] [CrossRef]
- Sharma, M.C.; Jain, D.; Gupta, A.; Sarkar, C.; Suri, V.; Garg, A.; Gaikwad, S.B.; Chandra, P.S. Dysembryoplastic Neuroepithelial Tumor: A Clinicopathological Study of 32 Cases. Neurosurg. Rev. 2009, 32, 161–170. [Google Scholar] [CrossRef]
- Blumcke, I.; Aronica, E.; Urbach, H.; Alexopoulos, A.; Gonzalez-Martinez, J.A. A Neuropathology-Based Approach to Epilepsy Surgery in Brain Tumors and Proposal for a New Terminology Use for Long-Term Epilepsy-Associated Brain Tumors. Acta Neuropathol. 2014, 128, 39–54. [Google Scholar] [CrossRef]
- Piao, Y.-S.; Lu, D.-H.; Chen, L.; Liu, J.; Wang, W.; Liu, L.; Yu, T.; Wang, Y.-P.; Li, Y.-J. Neuropathological Findings in Intractable Epilepsy: 435 Chinese Cases. Brain Pathol. 2010, 20, 902–908. [Google Scholar] [CrossRef]
- Sperling, M.R.; Ko, J. Seizures and Brain Tumors. Semin. Oncol. 2006, 33, 333–341. [Google Scholar] [CrossRef]
- Thom, M.; Toma, A.; An, S.; Martinian, L.; Hadjivassiliou, G.; Ratilal, B.; Dean, A.; McEvoy, A.; Sisodiya, S.M.; Brandner, S. One Hundred and One Dysembryoplastic Neuroepithelial Tumors: An Adult Epilepsy Series With Immunohistochemical, Molecular Genetic, and Clinical Correlations and a Review of the Literature. J. Neuropathol. Exp. Neurol. 2011, 70, 859–878. [Google Scholar] [CrossRef]
- Cosson, R.S.; Varlet, P.; Beuvon, F.; Duport, C.D.; Devaux, B.; Chassoux, F.; Frédy, D.; Meder, J.F. Dysembryoplastic Neuroepithelial Tumors: CT, MR Findings and Imaging Follow-up: A Study of 53 Cases. J. Neuroradiol. J. Neuroradiol. 2001, 28, 230–240. [Google Scholar]
- Chassoux, F.; Rodrigo, S.; Mellerio, C.; Landre, E.; Miquel, C.; Turak, B.; Laschet, J.; Meder, J.-F.; Roux, F.-X.; Daumas-Duport, C.; et al. Dysembryoplastic Neuroepithelial Tumors: An MRI-Based Scheme for Epilepsy Surgery. Neurology 2012, 79, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Sontowska, I.; Matyja, E.; Malejczyk, J.; Grajkowska, W. Dysembryoplastic Neuroepithelial Tumour: Insight into the Pathology and Pathogenesis. Folia Neuropathol. 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Pagès, M.; Debily, M.; Fina, F.; Jones, D.T.W.; Saffroy, R.; Castel, D.; Blauwblomme, T.; Métais, A.; Bourgeois, M.; Lechapt-Zalcman, E.; et al. The Genomic Landscape of Dysembryoplastic Neuroepithelial Tumours and a Comprehensive Analysis of Recurrent Cases. Neuropathol. Appl. Neurobiol. 2022, 48, e12834. [Google Scholar] [CrossRef] [PubMed]
- Rivera, B.; Gayden, T.; Carrot-Zhang, J.; Nadaf, J.; Boshari, T.; Faury, D.; Zeinieh, M.; Blanc, R.; Burk, D.L.; Fahiminiya, S.; et al. Germline and Somatic FGFR1 Abnormalities in Dysembryoplastic Neuroepithelial Tumors. Acta Neuropathol. 2016, 131, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Bonney, P.A.; Boettcher, L.B.; Conner, A.K.; Glenn, C.A.; Briggs, R.G.; Santucci, J.A.; Bellew, M.R.; Battiste, J.D.; Sughrue, M.E. Review of Seizure Outcomes after Surgical Resection of Dysembryoplastic Neuroepithelial Tumors. J. Neurooncol. 2016, 126, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Takita, H.; Shimono, T.; Uda, T.; Ikota, H.; Kawashima, T.; Horiuchi, D.; Terayama, E.; Tsukamoto, T.; Miki, Y. Malignant Transformation of a Dysembryoplastic Neuroepithelial Tumor Presenting with Intraventricular Hemorrhage. Radiol. Case Rep. 2022, 17, 939–943. [Google Scholar] [CrossRef]
- Ataseven, E.; Özcan, M.; Ölçülü, C.B.; Bolat, E.; Ertan, Y.; Kitiş, Ö.; Tekgül, H.; Kantar, M. Dysembryoplastic Neuroepithelial Tumors of Childhood: Ege University Experience. Childs Nerv. Syst. 2022, 38, 1699–1706. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, D.; Yang, Z.; Chen, X.; Liu, J.; Zhang, J.; Li, S.; Li, J.; Yang, Z. Factors Associated with Prognosis of Dysembryoplastic Neuroepithelial Tumors Patients after Surgical Resection: A Retrospective Observational Study. Br. J. Neurosurg. 2021, 1–6. [Google Scholar] [CrossRef]
- Luzzi, S.; Elia, A.; Maestro, M.D.; Elbabaa, S.K.; Carnevale, S.; Guerrini, F.; Caulo, M.; Morbini, P.; Galzio, R. Dysembryoplastic Neuroepithelial Tumors: What You Need to Know. World Neurosurg. 2019, 127, 255–265. [Google Scholar] [CrossRef]
- Yang, J.; Kim, S.-K.; Kim, K.J.; Chae, J.H.; Lim, B.C.; Wang, K.-C.; Park, S.-H.; Phi, J.H. Satellite Lesions of DNET: Implications for Seizure and Tumor Control after Resection. J. Neurooncol. 2019, 143, 437–445. [Google Scholar] [CrossRef]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational Classification of Seizure Types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.L.; et al. ILAE Classification of the Epilepsies: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Sanai, N.; Mirzadeh, Z.; Berger, M.S. Functional Outcome after Language Mapping for Glioma Resection. N. Engl. J. Med. 2008, 358, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Chassoux, F.; Daumas-Duport, C. Dysembryoplastic Neuroepithelial Tumors: Where Are We Now? Epilepsia 2013, 54, 129–134. [Google Scholar] [CrossRef]
- Campos, A.R.; Clusmann, H.; von Lehe, M.; Niehusmann, P.; Becker, A.J.; Schramm, J.; Urbach, H. Simple and Complex Dysembryoplastic Neuroepithelial Tumors (DNT) Variants: Clinical Profile, MRI, and Histopathology. Neuroradiology 2009, 51, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Arzimanoglou, A.; Berg, A.T.; Brodie, M.J.; Hauser, W.A.; Mathern, G.; Moshé, S.L.; Perucca, E.; Wiebe, S.; French, J. Definition of Drug Resistant Epilepsy: Consensus Proposal by the Ad Hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 2010, 51, 1069–1077. [Google Scholar] [CrossRef]
- Blümcke, I.; Thom, M.; Aronica, E.; Armstrong, D.D.; Vinters, H.V.; Palmini, A.; Jacques, T.S.; Avanzini, G.; Barkovich, A.J.; Battaglia, G.; et al. The Clinicopathologic Spectrum of Focal Cortical Dysplasias: A Consensus Classification Proposed by an Ad Hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia 2011, 52, 158–174. [Google Scholar] [CrossRef]
- Nolan, M.A.; Sakuta, R.; Chuang, N.; Otsubo, H.; Rutka, J.T.; Snead, O.C.; Hawkins, C.E.; Weiss, S.K. Dysembryoplastic Neuroepithelial Tumors in Childhood: Long-Term Outcome and Prognostic Features. Neurology 2004, 62, 2270–2276. [Google Scholar] [CrossRef]
- Chang, E.F.; Christie, C.; Sullivan, J.E.; Garcia, P.A.; Tihan, T.; Gupta, N.; Berger, M.S.; Barbaro, N.M. Seizure Control Outcomes after Resection of Dysembryoplastic Neuroepithelial Tumor in 50 Patients. J. Neurosurg. Pediatr. 2010, 5, 123–130. [Google Scholar] [CrossRef]
- Sakuta, R.; Otsubo, H.; Nolan, M.A.; Weiss, S.K.; Hawkins, C.; Rutka, J.T.; Chuang, N.A.; Chuang, S.H.; Snead, O.C. Recurrent Intractable Seizures in Children With Cortical Dysplasia Adjacent to Dysembryoplastic Neuroepithelial Tumor. J. Child Neurol. 2005, 20, 377–384. [Google Scholar] [CrossRef]
- Ko, A.; Kim, S.H.; Kim, S.H.; Park, E.K.; Shim, K.-W.; Kang, H.-C.; Kim, D.-S.; Kim, H.D.; Lee, J.S. Epilepsy Surgery for Children With Low-Grade Epilepsy-Associated Tumors: Factors Associated With Seizure Recurrence and Cognitive Function. Pediatr. Neurol. 2019, 91, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Minkin, K.; Klein, O.; Mancini, J.; Lena, G. Surgical Strategies and Seizure Control in Pediatric Patients with Dysembryoplastic Neuroepithelial Tumors: A Single-Institution Experience. J. Neurosurg. Pediatr. 2008, 1, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Morris, H.H.; Matkovic, Z.; Estes, M.L.; Prayson, Y.A.; Comair, Y.G.; Turnbull, J.; Najm, I.; Kotagal, P.; Wyllie, E. Ganglioglioma and Intractable Epilepsy: Clinical and Neurophysiologic Features and Predictors of Outcome After Surgery. Epilepsia 2005, 39, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Berger, M.S.; Barbaro, N.M.; Chang, E.F. Factors Associated with Seizure Freedom in the Surgical Resection of Glioneuronal Tumors. Epilepsia 2012, 53, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Aronica, E.; Leenstra, S.; van Veelen, C.W.M.; van Rijen, P.C.; Hulsebos, T.J.; Tersmette, A.C.; Yankaya, B.; Troost, D. Glioneuronal Tumors and Medically Intractable Epilepsy: A Clinical Study with Long-Term Follow-up of Seizure Outcome after Surgery. Epilepsy Res. 2001, 43, 179–191. [Google Scholar] [CrossRef]
- Ranger, A.; Diosy, D. Seizures in Children with Dysembryoplastic Neuroepithelial Tumors of the Brain—A Review of Surgical Outcomes across Several Studies. Childs Nerv. Syst. 2015, 31, 847–855. [Google Scholar] [CrossRef]
- Honavar, M.; Janota, I.; Polkey, C.E. Histological Heterogeneity of Dysembryoplastic Neuroepithelial Tumour: Identification and Differential Diagnosis in a Series of 74 Cases. Histopathology 1999, 34, 342–356. [Google Scholar] [CrossRef]
- Lee, D.Y.; Chung, C.K.; Hwang, Y.S.; Choe, G.; Chi, J.G.; Kim, H.J.; Cho, B.K. Dysembryoplastic Neuroepithelial Tumor: Radiological Findings (Including PET, SPECT, and MRS) and Surgical Strategy. J. Neurooncol. 2000, 47, 167–174. [Google Scholar] [CrossRef]
- van Klink, N.E.; Zweiphenning, W.J.; Ferrier, C.H.; Gosselaar, P.H.; Miller, K.J.; Aronica, E.; Braun, K.P.; Zijlmans, M. Can We Use Intraoperative High-Frequency Oscillations to Guide Tumor-Related Epilepsy Surgery? Epilepsia 2021, 62, 997–1004. [Google Scholar] [CrossRef]
- Burneo, J.G.; Tellez-Zenteno, J.; Steven, D.A.; Niaz, N.; Hader, W.; Pillay, N.; Wiebe, S. Adult-Onset Epilepsy Associated with Dysembryoplastic Neuroepithelial Tumors. Seizure 2008, 17, 498–504. [Google Scholar] [CrossRef]
- Isler, C.; Cetin, O.E.; Ugurlar, D.; Ozkara, C.; Comunoglu, N.; Kizilkilic, O.; Oz, B.; Kayadibi, Y.; Tanriverdi, T.; Uzan, M. Dysembryoplastic Neuroepithelial Tumours: Clinical, Radiological, Pathological Features and Outcome. Br. J. Neurosurg. 2018, 32, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Ramantani, G.; Kadish, N.E.; Anastasopoulos, C.; Brandt, A.; Wagner, K.; Strobl, K.; Mayer, H.; Schubert-Bast, S.; Stathi, A.; Korinthenberg, R.; et al. Epilepsy Surgery for Glioneuronal Tumors in Childhood. Neurosurgery 2014, 74, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Jeong, W.; Chung, C.K. Clinical Relevance of Interictal Spikes in Tumor-Related Epilepsy: An Electrocorticographic Study. J. Epilepsy Res. 2020, 9, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Mittal, S.; Barkmeier, D.; Hua, J.; Pai, D.S.; Fuerst, D.; Basha, M.; Loeb, J.A.; Shah, A.K. Intracranial EEG Analysis in Tumor-Related Epilepsy: Evidence of Distant Epileptic Abnormalities. Clin. Neurophysiol. 2016, 127, 238–244. [Google Scholar] [CrossRef]
- Chassoux, F.; Landré, E.; Mellerio, C.; Laschet, J.; Devaux, B.; Daumas-Duport, C. Dysembryoplastic Neuroepithelial Tumors: Epileptogenicity Related to Histologic Subtypes. Clin. Neurophysiol. 2013, 124, 1068–1078. [Google Scholar] [CrossRef]
- Moazzam, A.A.; Wagle, N.; Shiroishi, M.S.; Tomita, T.; Volk, J.M.; Shen, W.; Pundy, T. Glioneuronal Tumors of Cerebral Hemisphere in Children: Correlation of Surgical Resection with Seizure Outcomes and Tumor Recurrences. Childs Nerv. Syst. 2016, 32, 1839–1848. [Google Scholar] [CrossRef]
- Hudetz, J.A.; Gandhi, S.D.; Iqbal, Z.; Patterson, K.M.; Pagel, P.S. Elevated Postoperative Inflammatory Biomarkers Are Associated with Short- and Medium-Term Cognitive Dysfunction after Coronary Artery Surgery. J. Anesth. 2011, 25, 1–9. [Google Scholar] [CrossRef]
- Skvarc, D.R.; Berk, M.; Byrne, L.K.; Dean, O.M.; Dodd, S.; Lewis, M.; Marriott, A.; Moore, E.M.; Morris, G.; Page, R.S.; et al. Post-Operative Cognitive Dysfunction: An Exploration of the Inflammatory Hypothesis and Novel Therapies. Neurosci. Biobehav. Rev. 2018, 84, 116–133. [Google Scholar] [CrossRef]
- Peng, L.; Xu, L.; Ouyang, W. Role of Peripheral Inflammatory Markers in Postoperative Cognitive Dysfunction (POCD): A Meta-Analysis. PLoS ONE 2013, 8, e79624. [Google Scholar] [CrossRef]
- Donato, R.; Heizmann, C.W. S100B Protein in the Nervous System and Cardiovascular Apparatus in Normal and Pathological Conditions. Cardiovasc. Psychiatry Neurol. 2010, 2010, 929712. [Google Scholar] [CrossRef]
- Harmon, D.; Eustace, N.; Ghori, K.; Butler, M.; O’Callaghan, S.; O’Donnell, A.; Moore-Groarke, G.M.; Shorten, G. Plasma Concentrations of Nitric Oxide Products and Cognitive Dysfunction Following Coronary Artery Bypass Surgery. Eur. J. Anaesthesiol. 2005, 22, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Vacas, S.; Degos, V.; Tracey, K.J.; Maze, M. High-Mobility Group Box 1 Protein Initiates Postoperative Cognitive Decline by Engaging Bone Marrow–Derived Macrophages. Anesthesiology 2014, 120, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Satoer, D.; Visch-Brink, E.; Dirven, C.; Vincent, A. Glioma Surgery in Eloquent Areas: Can We Preserve Cognition? Acta Neurochir. 2016, 158, 35–50. [Google Scholar] [CrossRef] [PubMed]
- Ko, A.; Lee, J.S. Factors Associated with Seizure and Cognitive Outcomes after Epilepsy Surgery for Low-Grade Epilepsy-Associated Neuroepithelial Tumors in Children. Clin. Exp. Pediatr. 2019, 63, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Baxendale, S.; Donnachie, E.; Thompson, P.; Sander, J.W. Dysembryoplastic Neuroepithelial Tumors: A Model for Examining the Effects of Pathology versus Seizures on Cognitive Dysfunction in Epilepsy. Epilepsia 2013, 54, 2214–2218. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, E.J.; Habets, E.J.J.; Taphoorn, M.J.B.; Douw, L.; Zwinderman, A.H.; Vandertop, W.P.; Barkhof, F.; Klein, M.; De Witt Hamer, P.C. Linking Late Cognitive Outcome with Glioma Surgery Location Using Resection Cavity Maps. Hum. Brain Mapp. 2018, 39, 2064–2074. [Google Scholar] [CrossRef] [PubMed]
- Faramand, A.; Barnes, N.; Harrison, S.; Gunny, R.; Jacques, T.; Tahir, M.; Varadkar, S.; Cross, H.; Harkness, W.; Tisdall, M. Seizure and Cognitive Outcomes after Resection of Glioneuronal Tumors in Children. Epilepsia 2018, 59, 170–178. [Google Scholar] [CrossRef]
- Hansson, L.; Lithell, H.; Skoog, I.; Baro, F.; Bánki, C.M.; Breteler, M.; Castaigne, A.; Correia, M.; Degaute, J.P.; Elmfeldt, D.; et al. Study on COgnition and Prognosis in the Elderly (SCOPE): Baseline Characteristics. Blood Press. 2000, 9, 146–151. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Hoffermann, M.; Bruckmann, L.; Mahdy Ali, K.; Zaar, K.; Avian, A.; von Campe, G. Pre- and Postoperative Neurocognitive Deficits in Brain Tumor Patients Assessed by a Computer Based Screening Test. J. Clin. Neurosci. Off. J. Neurosurg. Soc. Australas. 2017, 36, 31–36. [Google Scholar] [CrossRef]
- Gehring, K.; Aaronson, N.K.; Taphoorn, M.J.; Sitskoorn, M.M. Interventions for Cognitive Deficits in Patients with a Brain Tumor: An Update. Expert Rev. Anticancer Ther. 2010, 10, 1779–1795. [Google Scholar] [CrossRef] [PubMed]



| Characteristics | SF (n = 49) | NSF (n = 14) | p-Value |
|---|---|---|---|
| Sex | |||
| Male | 28 (57.1%) | 5 (35.7%) | 0.266 |
| Female | 21 (42.9%) | 9 (64.3%) | |
| Age at seizure onset | |||
| ≤4 years | 4 (8.2%) | 5 (35.7%) | 0.030 * |
| >4 years | 45 (91.8%) | 9 (64.3%) | |
| Duration of seizures | |||
| ≤4 years | 39 (79.6%) | 5 (35.7%) | 0.005 * |
| >4 years | 10 (20.4%) | 9 (64.3%) | |
| Age at surgery | |||
| ≤16 years | 27 (55.1%) | 6 (42.9%) | 0.613 |
| >16 years | 22 (44.9%) | 8 (57.1%) | |
| Aura | |||
| Yes | 35 (71.4%) | 10 (71.4%) | 1.000 |
| No | 14 (28.6%) | 4 (28.6%) | |
| Seizure frequency | |||
| Daily | 10 (20.4%) | 1 (7.1%) | 0.654 |
| Weekly | 15 (30.6%) | 4 (28.6%) | |
| Monthly | 17 (34.7%) | 7 (50.0%) | |
| Yearly | 7 (14.3%) | 2 (14.3%) | |
| Seizure types | |||
| Focal only | 27 (55.1%) | 7 (50.0%) | 0.848 |
| Generalized only | 10 (20.4%) | 4 (28.6%) | |
| Both | 12 (24.5%) | 3 (21.4%) | |
| IEDs | |||
| Regional | 31 (63.3%) | 2 (14.3%) | 0.002 * |
| Unilateral | 3 (6.1%) | 2 (14.3%) | |
| Bilateral | 5 (10.2%) | 6 (42.9%) | |
| Nonspecific | 10 (20.4%) | 4 (28.6%) | |
| Ictal onset rhythms | |||
| Regional | 16 (32.7%) | 2 (14.3%) | 0.523 |
| Unilateral | 6 (12.2%) | 2 (14.3%) | |
| Bilateral | 13 (26.5%) | 6 (42.9%) | |
| Not captured | 14 (28.6%) | 4 (28.6%) | |
| Laterality of tumor in preoperative MRI | |||
| Left | 26 (53.1%) | 8 (57.1%) | 1.000 |
| Right | 23 (46.9%) | 6 (42.9%) | |
| Site of lesion | |||
| Temporal | 21 (42.9%) | 8 (57.1%) | 0.521 |
| Extratemporal | 28 (57.1%) | 6 (42.9%) | |
| MRI subtype | |||
| Type 1 | 42 (85.7%) | 8 (57.1%) | 0.020 * |
| Type 2 | 2 (4.1%) | 0 (0%) | |
| Type 3 | 5 (10.2%) | 6 (42.9%) | |
| Surgical type 1 | |||
| GTR | 44 (89.8%) | 6 (42.9%) | <0.001 * |
| NTR | 5 (10.2%) | 6 (42.9%) | |
| STR | 0 (0%) | 2 (14.3%) | |
| Histopathological types 2 | |||
| Simple form | 36 (73.5%) | 10 (71.4%) | 0.146 |
| Complex form | 12 (24.5%) | 2 (14.3%) | |
| Nonspecific form | 1 (2.0%) | 2 (14.3%) | |
| Complication | |||
| No | 42 (85.7%) | 12 (85.7%) | 0.822 |
| Temporary | 5 (10.2%) | 1 (7.1%) | |
| Permanent | 2 (4.1%) | 1 (7.1%) | |
| Variables | Univariable Analysis | Multivariable Analysis | ||||
|---|---|---|---|---|---|---|
| HR | p-Value | 95%CI | HR | p-Value | 95%CI | |
| Age at seizure onset | ||||||
| ≤4 years | 1.00 | |||||
| >4 years | 0.25 | 0.012 * | 0.08–0.74 | 0.94 | 0.939 | 0.20–4.43 |
| Duration of seizures | ||||||
| ≤4 years | 1.00 | |||||
| >4 years | 4.87 | 0.005 * | 1.63–14.56 | 4.32 | 0.016 * | 1.31–14.2 |
| IEDs | ||||||
| Regional | 1.00 | |||||
| Unilateral | 10.09 | 0.021 * | 1.41–72.09 | 4.91 | 0.130 | 0.63–38.47 |
| Bilateral | 12.09 | 0.002 * | 2.43–60.09 | 6.38 | 0.047 * | 1.03–39.64 |
| Nonspecific | 5.33 | 0.054 | 0.97–29.13 | 1.26 | 0.817 | 0.18–8.91 |
| MRI subtype | ||||||
| Type 1 | 1.00 | |||||
| Type 2 | 0.00 | 0.999 | 0.00–Inf | 0.00 | 0.999 | 0.00–Inf |
| Type 3 | 4.26 | 0.007 * | 1.47–12.32 | 1.31 | 0.705 | 0.33–5.25 |
| Surgical type | ||||||
| GTR | 1.00 | |||||
| Non-GTR | 7.86 | <0.001 * | 2.70–22.95 | 6.38 | 0.031 * | 1.18–34.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Hu, Y.; Aihemaitiniyazi, A.; Li, T.; Zhou, J.; Guan, Y.; Qi, X.; Zhang, X.; Wang, M.; Liu, C.; et al. Long-Term Seizure Outcomes and Predictors in Patients with Dysembryoplastic Neuroepithelial Tumors Associated with Epilepsy. Brain Sci. 2023, 13, 24. https://doi.org/10.3390/brainsci13010024
Zhang H, Hu Y, Aihemaitiniyazi A, Li T, Zhou J, Guan Y, Qi X, Zhang X, Wang M, Liu C, et al. Long-Term Seizure Outcomes and Predictors in Patients with Dysembryoplastic Neuroepithelial Tumors Associated with Epilepsy. Brain Sciences. 2023; 13(1):24. https://doi.org/10.3390/brainsci13010024
Chicago/Turabian StyleZhang, Huawei, Yue Hu, Adilijiang Aihemaitiniyazi, Tiemin Li, Jian Zhou, Yuguang Guan, Xueling Qi, Xufei Zhang, Mengyang Wang, Changqing Liu, and et al. 2023. "Long-Term Seizure Outcomes and Predictors in Patients with Dysembryoplastic Neuroepithelial Tumors Associated with Epilepsy" Brain Sciences 13, no. 1: 24. https://doi.org/10.3390/brainsci13010024
APA StyleZhang, H., Hu, Y., Aihemaitiniyazi, A., Li, T., Zhou, J., Guan, Y., Qi, X., Zhang, X., Wang, M., Liu, C., & Luan, G. (2023). Long-Term Seizure Outcomes and Predictors in Patients with Dysembryoplastic Neuroepithelial Tumors Associated with Epilepsy. Brain Sciences, 13(1), 24. https://doi.org/10.3390/brainsci13010024







