Global and Regional Structural Differences and Prediction of Autistic Traits during Adolescence
Abstract
:1. Introduction
Main Aim of the Study
2. Materials and Methods
2.1. Participants
2.2. Screening for Intelligence
2.3. Autistic Traits: Social Responsiveness Scale
2.4. Magnetic Resonance Imaging
2.5. Statistical Analyses
3. Results
3.1. Sample Characteristics
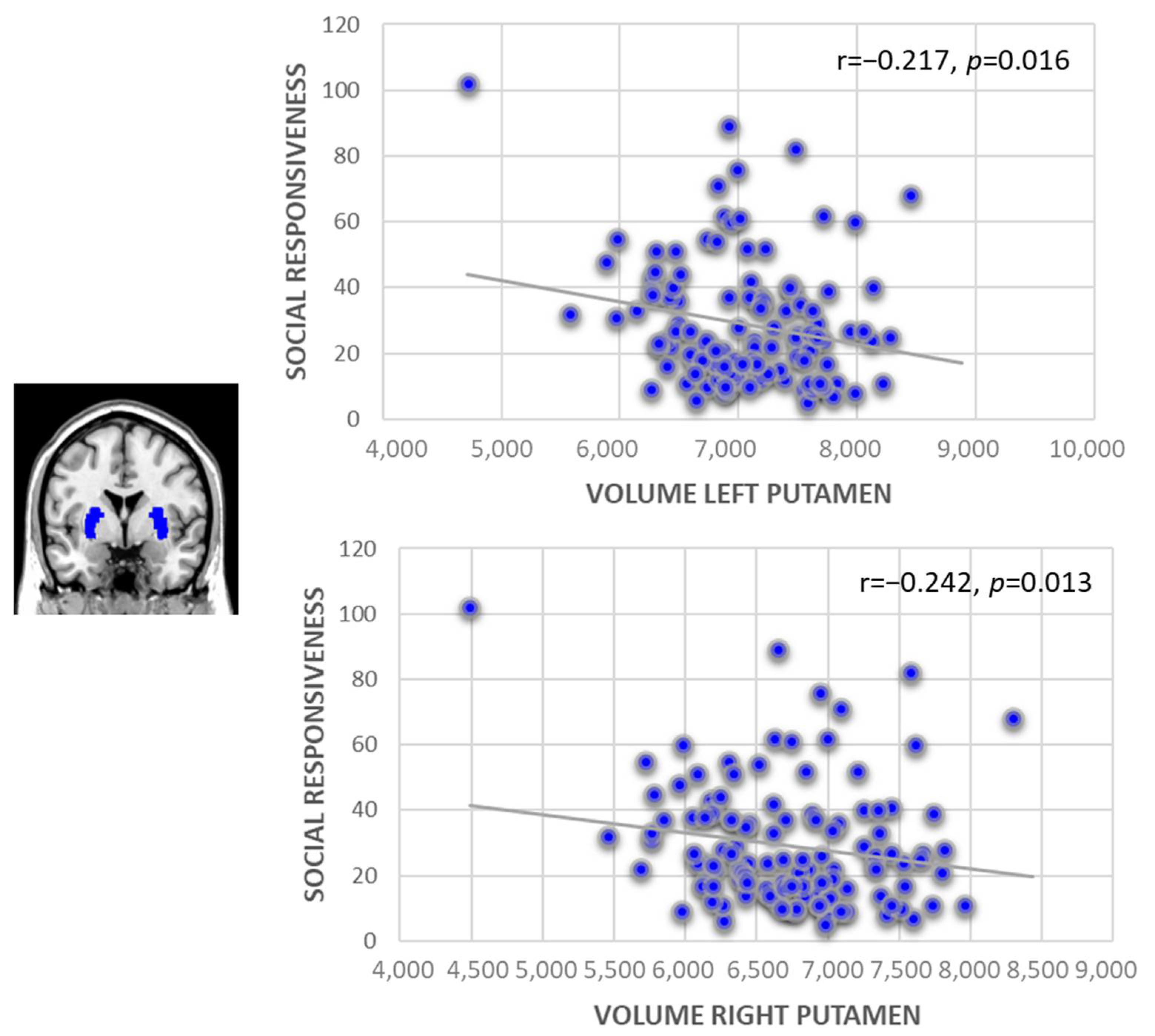
3.2. Subcortical Brain Volume and the Prediction of Social Responsiveness
3.3. Cortical Thickness and the Prediction of Social Responsiveness
4. Discussion
4.1. Volumetric Findings
4.2. Cortical Thickness and SRS
4.3. Autistic Traits and Brain Development
4.4. Strengths and Limitations
4.5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Constantino, J.N.; Todd, R.D. Autistic traits in the general population: A twin study. Arch. Gen. Psychiatry 2003, 60, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Bralten, J.; van Hulzen, K.J.; Martens, M.B.; Galesloot, T.E.; Arias Vasquez, A.; Kiemeney, L.A.; Buitelaar, J.K.; Muntjewerff, J.W.; Franke, B.; Poelmans, G. Autism spectrum disorders and autistic traits share genetics and biology. Mol. Psychiatry 2018, 23, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- van Rooij, D.; Anagnostou, E.; Arango, C.; Auzias, G.; Behrmann, M.; Busatto, G.F.; Calderoni, S.; Daly, E.; Deruelle, C.; Di Martino, A.; et al. Cortical and Subcortical Brain Morphometry Differences between Patients with Autism Spectrum Disorder and Healthy Individuals across the Lifespan: Results From the ENIGMA ASD Working Group. Am. J. Psychiatry 2018, 175, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Sussman, D.; Leung, R.C.; Vogan, V.M.; Lee, W.; Trelle, S.; Lin, S.; Cassel, D.B.; Chakravarty, M.M.; Lerch, J.P.; Anagnostou, E.; et al. The autism puzzle: Diffuse but not pervasive neuroanatomical abnormalities in children with ASD. Neuroimage Clin. 2015, 8, 170–179. [Google Scholar] [CrossRef]
- Lange, N.; Travers, B.G.; Bigler, E.D.; Prigge, M.B.; Froehlich, A.L.; Nielsen, J.A.; Cariello, A.N.; Zielinski, B.A.; Anderson, J.; Fletcher, P.T.; et al. Longitudinal volumetric brain changes in autism spectrum disorder ages 6-35 years. Autism Res. 2015, 8, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Campbell, K.; Solso, S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Res. 2011, 1380, 138–145. [Google Scholar] [CrossRef]
- Barnea-Goraly, N.; Lotspeich, L.J.; Reiss, A.L. Similar white matter aberrations in children with autism and their unaffected siblings: A diffusion tensor imaging study using tractbased spatial statistics. Arch. Gen. Psychiatry 2010, 67, 1052–1060. [Google Scholar] [CrossRef]
- Geurts, H.M.; Ridderinkhof, K.R.; Scholte, H.S. The relationship between grey-matter and ASD and ADHD traits in typical adults. J. Autism Dev. Disord. 2013, 43, 1630–1641. [Google Scholar] [CrossRef]
- Saito, Y.; Suga, M.; Tochigi, M.; Abe, O.; Yahata, N.; Kawakubo, Y.; Liu, X.; Kawamura, Y.; Sasaki, T.; Kasai, K.; et al. Neural correlate of autistic-like traits and a common allele in the oxytocin receptor gene. SCAN 2014, 9, 1443–1450. [Google Scholar] [CrossRef] [PubMed]
- Wallace, G.L.; Shaw, P.; Lee, N.R.; Clasen, L.S.; Raznahan, A.; Lenroot, R.K.; Martin, A.; Giedd, J.N. Distinct cortical correlates of autistic versus antisocial traits in a longitudinal sample of typically developing youth. J. Neurosci. 2012, 32, 4856–4860. [Google Scholar] [CrossRef]
- Tu, P.C.; Hsu, J.W.; Lan, C.C.; Liu, C.C.; Su, T.P.; Chen, Y.S. Structural and functional correlates of a quantitative autistic trait measured using the social responsive scale in neurotypical male adolescents. Autism Res. 2012, 9, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Blanken, L.M.E.; Mous, S.E.; Ghassabian, A.; Muetzel, R.L.; Schoemaker, N.K.; Marroun, H.E.; Van Der Lugt, A.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; et al. Cortical morphology in 6- to 10-year old children with autistic traits: A population-based neuroimaging study. Am. J. Psychiatry 2015, 172, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Koolschijn, P.C.; Geurts, H.M.; van der Leij, A.R.; Scholte, H.S. Are Autistic Traits in the General Population Related to Global and Regional Brain Differences? J. Autism Dev. Disord. 2015, 45, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Duerden, E.G.; Mak-Fan, K.M.; Taylor, M.J.; Roberts, S.W. Regional differences in grey and white matter in children and adults with autism spectrum disorders: An activation likelihood estimate (ALE) meta-analysis. Autism Res. 2012, 5, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Constantino, J.N.; Gruber, C.P. Social Responsiveness Scale (SRS); Western Psychological Services: Los Angeles, CA, USA, 2005. [Google Scholar]
- Baron-Cohen, S.; Wheelwright, S.; Skinner, R.; Martin, J.; Clubley, E. The autism-spectrum quotient (AQ): Evidence from Asperger syndrome/high-functioning autism, males and females, scientists and mathematicians. J. Autism Dev. Disord. 2001, 31, 5–17. [Google Scholar] [CrossRef]
- Ecker, C.; Shahidiani, A.; Feng, Y.; Daly, E.; Murphy, C.; D’Almeida, V.; Deoni, S.; Williams, S.C.; Gillan, N.; Gudbrandsen, M.; et al. The effect of age, diagnosis, and their interaction on vertex-based measures of cortical thickness and surface area in autism spectrum disorder. J. Neural Transmiss. 2014, 121, 1157–1170. [Google Scholar] [CrossRef]
- Goodman, R.; Ford, T.; Richards, H.; Gatward, R.; Meltzer, H. The Development and well-being assessment: Description and initial validation of an integrated assessment of child and adolescent psychopathology. J. Child Psychol. Psychiatry 2000, 41, 645–655. [Google Scholar] [CrossRef]
- Titze, I.; Tewes, U. Messung der Intelligenz bei Kindern mit dem HAWIK-R; Seattle: Bern, Switzerland; Göttingen, Germany; Toronto, Canada, 2000; pp. 13–21. [Google Scholar]
- Fischl, B.; Salat, D.H.; Busa, E.; Albert, M.; Dieterich, M.; Haselgrove, C.; van der Kouwe, A.; Killiany, R.; Kennedy, D.; Klaveness, S.; et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 2002, 33, 341–355. [Google Scholar] [CrossRef]
- Buckner, R.L.; Head, D.; Parker, J.; Fotenos, A.F.; Marcus, D.; Morris, J.C.; Snyder, A.Z. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage 2004, 23, 724–738. [Google Scholar] [CrossRef]
- Fischl, B.; Dale, A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. USA 2000, 97, 11050–11055. [Google Scholar] [CrossRef] [Green Version]
- Lyall, K.; Constantino, J.N.; Weisskopf, M.G.; Roberts, A.L.; Ascherio, A.; Santangelo, S.L. Parental social responsiveness and risk of autism spectrum disorder in offspring. JAMA Psychiatry 2014, 71, 936–942. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.B.; Grossrubatscher, I.; Frank, L. Dynamic Hippocampal Circuits Support Learning- and Memory-Guided Behaviors. Cold Spring Harbour. Symp. Quant. Biol. 2014, 79, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Anckarsäter, H.; Stahlberg, O.; Larson, T.; Hakansson, C.; Jutblad, S.B.; Niklasson, L. The impact of ADHD and autism spectrum disorders on temperament, character, and personality development. Am. J. Psychiatry 2000, 163, 1239–1244. [Google Scholar] [CrossRef]
- Bozdagi, O.; Sakurai, T.; Papapetrou, D.; Wang, X.; Dickstein, D.L.; Takahashi, N.; Kajiwara, Y.; Yang, M.; Katz, A.M.; Scattoni, M.L.; et al. Haploinsufficiency of the autism-associated Shank3 gene leads to deficits in synaptic function, social interaction, and social communication. Mol. Autism 2010, 1, 15. [Google Scholar] [CrossRef] [PubMed]
- Groen, W.; Teluij, M.; Buitelaar, J.; Tendolkar, I. Amygdala and hippocampus enlargement during adolescence in autism. J. A Acad. Child Adol. Psychiatry 2010, 49, 552–560. [Google Scholar]
- Haznedar, M.M.; Buchsbaum, M.S.; Wie, T.C.; Hof, P.R.; Cartwright, C.; Bienstock, C.A.; Hollander, E. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am. J. Psychiatry 2000, 157, 1994–2001. [Google Scholar] [CrossRef]
- Stoodley, C.J. The Cerebellum and Neurodevelopmental Disorders. Cerebellum 2016, 15, 34–37. [Google Scholar] [CrossRef]
- Pote, I.; Wang, S.; Sethna, V.; Blasi, A.; Daly, E.; Kuklisova-Murgasova, M.; Lloyd-Fox, S.; Mercure, E.; Busuulwa, P.; Stoencheva, V.; et al. BASIS Team (2019): Familial risk of autism alters subcortical and cerebellar brain anatomy in infants and predicts the emergence of repetitive behaviors in early childhood. Autism Res. 2013, 12, 614–627. [Google Scholar] [CrossRef]
- FitzGerald, T.H.; Friston, K.J.; Dolan, R.J. Action-specific value signals in reward-related regions of the human brain. J. Neurosci. 2012, 32, 16417–16423. [Google Scholar] [CrossRef]
- Monchi, O.; Petrides, M.; Strafella, A.P.; Worsley, K.J.; Doyon, J. Functional role of the basal ganglia in the planning and execution of actions. Ann. Neurol. 2006, 59, 257–264. [Google Scholar] [CrossRef]
- Langen, M.; Leemans, A.; Johnston, P.; Ecker, C.; Daly, E.; Murphy, C.M.; Dell’Acqua, F.; Durston, S.; Murphy, D.G. Fronto-striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex 2012, 48, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ide, J.S.; Li, C.S. Resting-state functional connectivity of the medial superior frontal cortex. Cereb. Cortex 2012, 22, 99–111. [Google Scholar]
- Just, M.A.; Keller, T.A.; Malave, V.L.; Kana, R.K.; Varma, S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neurosci. Biobehav. Rev. 2012, 36, 1292–1313. [Google Scholar] [CrossRef] [PubMed]
- Hardan, A.Y.; Minshew, N.J.; Mallikarjuhn, M.; Keshavan, M.S. Brain volume in autism. J. Child Neurol. 2001, 16, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.K.; O’Brien, L.M.; Lange, N.; Bakardjiev, A.; Hodgson, J.; Adrien, K.T.; Steele, S.; Makris, N.; et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 2003, 126, 1182–1192. [Google Scholar] [CrossRef]
- Wegiel, J.; Flory, M.; Kuchna, I.; Nowicki, K.; Ma, S.Y.; Imaki, H.; Wegiel, J.; Cohen, I.L.; London, E.; Wisniewski, T.; et al. Stereological study of the neuronal number and volume of 38 brain subdivisions of subjects diagnosed with autism reveals significant alterations restricted to the striatum, amygdala and cerebellum. Acta Neuropathol. Comm. 2014, 2, 141. [Google Scholar] [CrossRef]
- Riva, D.; Annunziata, S.; Contarino, V.; Erbetta, A.; Aquino, D.; Bulgheroni, S. Gray matter reduction in the vermis and CRUS-II is associated with social and interaction deficits in low-functioning children with autistic spectrum disorders: A VBM-DARTEL Study. Cerebellum 2013, 12, 676–685. [Google Scholar] [CrossRef]
- Ecker, C.; Ginestet, C.; Feng, Y.; Johnston, P.; Lombardo, M.V.; Lai, M.C.; Suckling, J.; Palaniyappan, L.; Daly, E.; Murphy, C.M.; et al. Brain surface anatomy in adults with autism: The relationship between surface area, cortical thickness, and autistic symptoms. JAMA Psychiatry 2013, 70, 59–70. [Google Scholar] [CrossRef]
- Ayoub, M.; Keegan, L.; Tager-Flusberg, H.; Gill, S.V. Neuroimaging Techniques as Descriptive and Diagnostic Tools for Infants at Risk for Autism Spectrum Disorder: A Systematic Review. Brain Sci. 2022, 12, 602. [Google Scholar] [CrossRef]
- Yang, S.; Jin, D.; Liu, J.; He, Y. Identification of Young High-Functioning Autism Individuals Based on Functional Connectome Using Graph Isomorphism Network: A Pilot Study. Brain Sci. 2022, 12, 883. [Google Scholar] [CrossRef]
- Giannotti, M.; Bonatti, S.M.; Tanaka, S.; Kojima, H.; de Falco, S. Parenting Stress and Social Style in Mothers and Fathers of Children with Autism Spectrum Disorder: A Cross-Cultural Investigation in Italy and Japan. Brain Sci. 2022, 11, 1419. [Google Scholar] [CrossRef] [PubMed]
- Correale, C.; Borgi, M.; Cirulli, F.; Laghi, F.; Trimarco, B.; Ferraro, M.; Venerosi, A. The Impact of Health and Social Services on the Quality of Life in Families of Adults with Autism Spectrum Disorder (ASD): A Focus Group Study. Brain Sci. 2022, 12, 177. [Google Scholar] [CrossRef] [PubMed]

| Total (N = 285) M (SD), Range | Male (N = 123) M (SD), Range | Female (N = 162) M (SD), Range | Significance Male vs. Female | |
|---|---|---|---|---|
| WISC Verbal Comprehension | 110.21 (13.56) | 110.18 (14.93) | 110.22 (14.98) | n.s. |
| WISC Reasoning | 107.33 (14.19) | 108.64 (14.22) | 106.83 (15.13) | n.s. |
| SRS social communication | 17.25 (4.15), 5–39 | 16.97 (4.31), 10–36 | 17.46 (4.03), 5–39 | n.s. |
| SRS social awareness | 9.33 (2.40), 1–15 | 9.01 (2.04), 4–15 | 9.57 (2.38), 1–15 | 0.049 |
| SRS social cognition | 7.67 (2.54), 0–17 | 7.46 (2.36), 3–17 | 7.83 (2.67), 0–17 | |
| SRS social motivation | 7.15 (2.62), 0–19 | 7.37 (2.73), 2–19 | 6.99 (2.53), 0–17 | n.s. |
| SRS subscale mannerisms | 2.87 (3.5), 0–20 | 2.96 (2.74), 0–19 | 2.83 (3.36), 0–20 | n.s. |
| SRS total score | 44.21 (11.10), 7–103 | 43.76 (11.04), 24–98 | 44.55 (11.16), 7–103 | n.s. |
| Total (N = 285) M (SD) | Male (N = 123) M (SD) | Female (N = 162) M (SD) | Significance Male vs. Female | |
|---|---|---|---|---|
| Brain volume | ||||
| Amygdala | Left: 1698.96 (220.34), Right: 1775.37 (252.99) | Left: 1781.61 (208.57), Right: 1874.36 (237.27) | Left: 1636.08 (208.50), Right: 1700.06 (238.83) | Left: <0.001, Right: <0.001 |
| Caudate nucleus | Left: 4100.49 (492.75), Right: 4185.45 (513.76) | Left: 4246.48 (466.01), Right: 4328.68 (505.21) | Left: 3989.44 (484.81), Right: 4076.48 (494.51) | Left: <0.001, Right: <0.001 |
| Hippocampus | Left: 4272.93 (446.6), Right: 4336.75 (422.48) | Left: 4508.88 (392.11), Right: 4543.96 (386.77) | Left: 4093.44 (400.44), Right: 4179.12 (379.04) | Left: <0.001, Right: <0.001 |
| Global pallidum | Left: 1775.37 (252.99), Right: 1811.54 (200.94) | Left: 2043.52 (256.73), Right: 1903.57 (178.84) | Left: 1904.02 (251.77), Right: 1741.53 (188.64) | Left: <0.001, Right: <0.001 |
| Nucleus accumbens | Left: 676.10 (118.08), Right: 758.87 (113.16) | Left: 716.55 (112.38), Right: 802.04 (104.68) | Left: 645.33 (113.24), Right: 726.04 (108.51) | Left: <0.001, Right: <0.001 |
| Putamen | Left: 6621.11 (687.93), Right: 6341.24 (663.80) | Left: 7044.90 (607.55), Right: 6732.77 (610.04) | Left: 6298.72 (559.63), Right: 6043.39 (537.35) | Left: <0.001, Right: <0.001 |
| Cerebellum | Left: 58,435.23 (5751.47), Right: 59,223.91 (5691.18) | Left: 61,948.07 (4691.11), Right: 62,572.19 (4955.44) | Left: 55,762.88 (5005.98), Right: 56,676.75 (4840.20) | Left: <0.001, Right: <0.001 |
| Thalamus | Left: 7353.33 (727.58), Right: 7394.39 (723.82) | Left: 7731.56 (688.02), Right: 7773.15 (667.40) | Left: 7065.60 (618.35), Right: 7106.26 (627.22) | Left: <0.001, Right: <0.001 |
| Cortical thickness | ||||
| Caudal middle frontal | Left: 2.67 (0.20), Right: 2.66 (0.18) | Left: 2.67 (0.22), Right: 2.66 (0.19) | Left: 2.68 (0.19), Right: 2.66 (0.16) | Left: n.s., Right: n.s. |
| Lateral orbital frontal | Left: 2.83 (0.19), Right: 2.82 (0.20) | Left: 2.82 (0.19), Right: 2.81 (0.21) | Left: 2.83 (0.19), Right: 2.82 (0.20) | Left: n.s., Right: n.s. |
| Medial orbital frontal | Left: 2.66 (0.18), Right: 2.62 (0.21) | Left: 2.66 (0.18), Right: 2.63 (0.21) | Left: 2.66 (0.19), Right: 2.62 (0.22) | Left: n.s., Right: n.s. |
| Rostral middle frontal | Left: 2.46 (0.15), Right: 2.45 (0.17) | Left: 2.46 (0.16), Right: 2.45 (0.18) | Left: 2.46 (0.14), Right: 2.45 (0.16) | Left: n.s., Right: n.s. |
| Superior frontal | Left: 2.93 (0.19), Right: 2.90 (0.18) | Left: 2.91 (0.20), Right: 2.89 (0.19) | Left: 2.95 (0.18), Right: 2.91 (0.17) | Left: n.s., Right: n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nees, F.; Banaschewski, T.; Bokde, A.L.W.; Desrivières, S.; Grigis, A.; Garavan, H.; Gowland, P.; Grimmer, Y.; Heinz, A.; Brühl, R.; et al. Global and Regional Structural Differences and Prediction of Autistic Traits during Adolescence. Brain Sci. 2022, 12, 1187. https://doi.org/10.3390/brainsci12091187
Nees F, Banaschewski T, Bokde ALW, Desrivières S, Grigis A, Garavan H, Gowland P, Grimmer Y, Heinz A, Brühl R, et al. Global and Regional Structural Differences and Prediction of Autistic Traits during Adolescence. Brain Sciences. 2022; 12(9):1187. https://doi.org/10.3390/brainsci12091187
Chicago/Turabian StyleNees, Frauke, Tobias Banaschewski, Arun L. W. Bokde, Sylvane Desrivières, Antoine Grigis, Hugh Garavan, Penny Gowland, Yvonne Grimmer, Andreas Heinz, Rüdiger Brühl, and et al. 2022. "Global and Regional Structural Differences and Prediction of Autistic Traits during Adolescence" Brain Sciences 12, no. 9: 1187. https://doi.org/10.3390/brainsci12091187
APA StyleNees, F., Banaschewski, T., Bokde, A. L. W., Desrivières, S., Grigis, A., Garavan, H., Gowland, P., Grimmer, Y., Heinz, A., Brühl, R., Isensee, C., Becker, A., Martinot, J.-L., Paillère Martinot, M.-L., Artiges, E., Papadopoulos Orfanos, D., Lemaître, H., Stringaris, A., van Noort, B., ... on behalf of the IMAGEN Consortium. (2022). Global and Regional Structural Differences and Prediction of Autistic Traits during Adolescence. Brain Sciences, 12(9), 1187. https://doi.org/10.3390/brainsci12091187








