Overexpression of GDNF in Spinal Cord Attenuates Morphine Analgesic Tolerance in Rats with Bone Cancer Pain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Intrathecal Catheterization
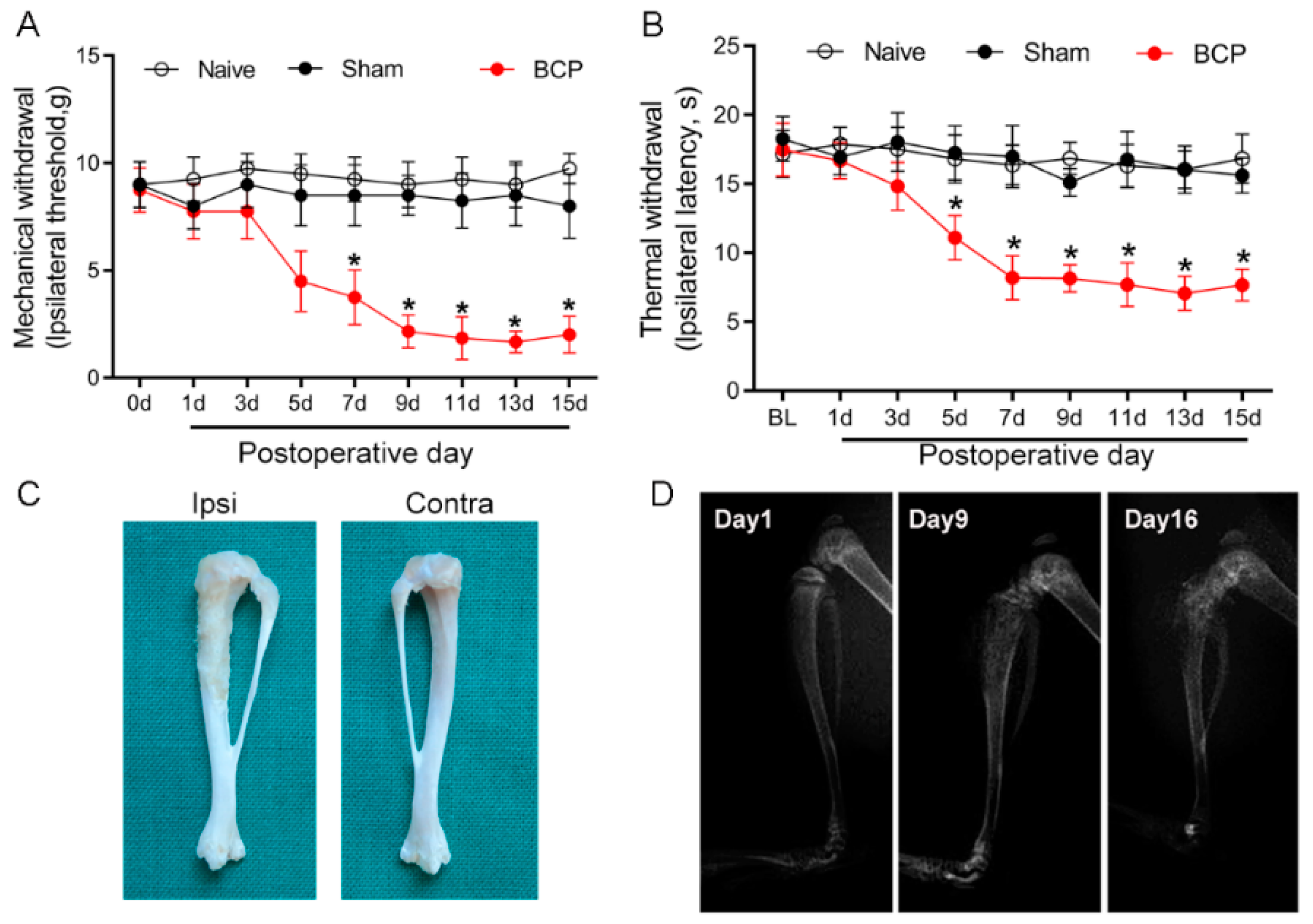
2.3. Induction of Bone Cancer Pain and Morphine Tolerance Model
2.4. Behavioral Test
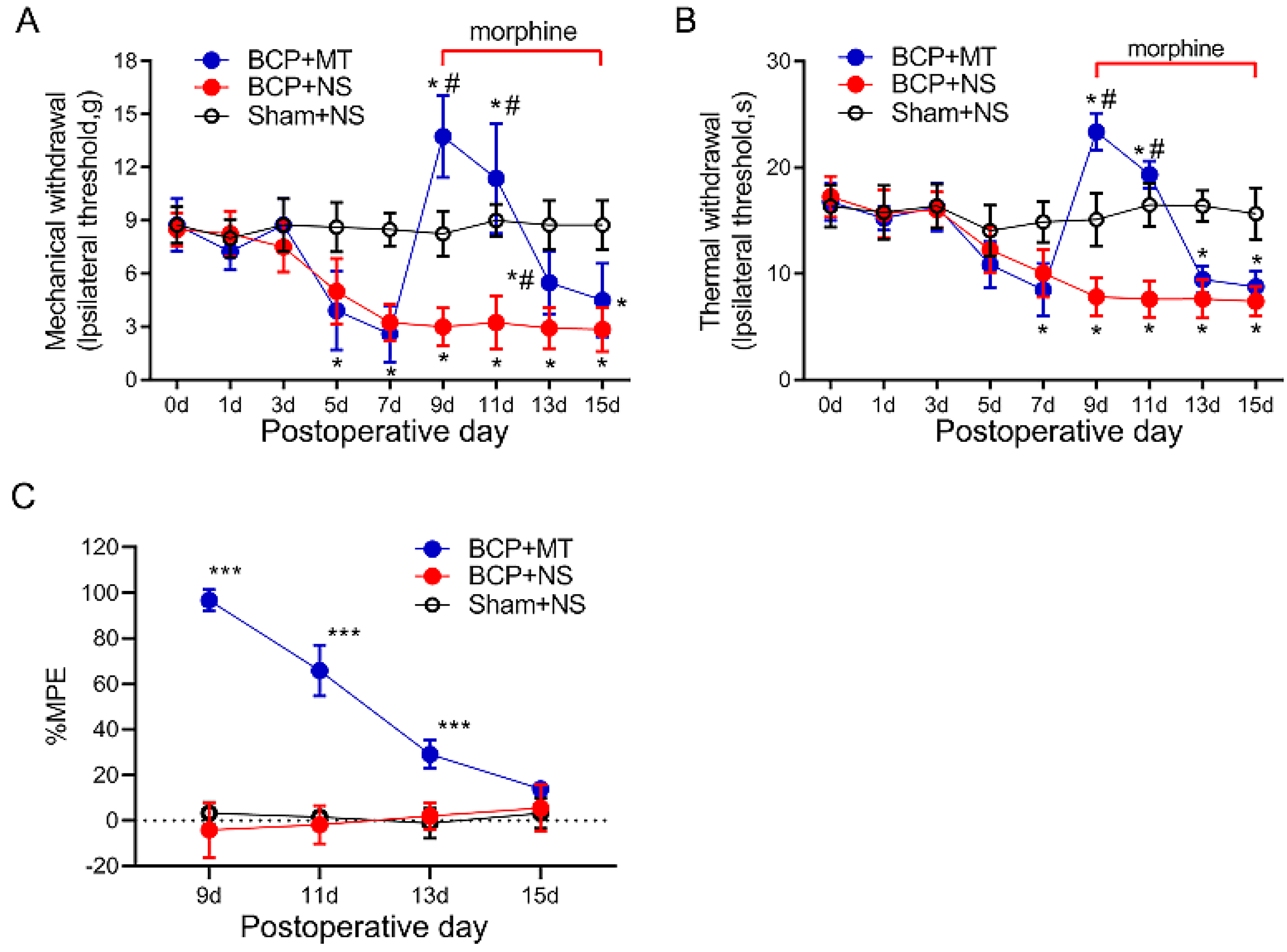
2.4.1. Mechanical Paw Withdrawal Threshold (PWT) Test
2.4.2. Paw Withdrawal Latency (PWL) Test
2.4.3. Tail-Flick Latency (TF) Test
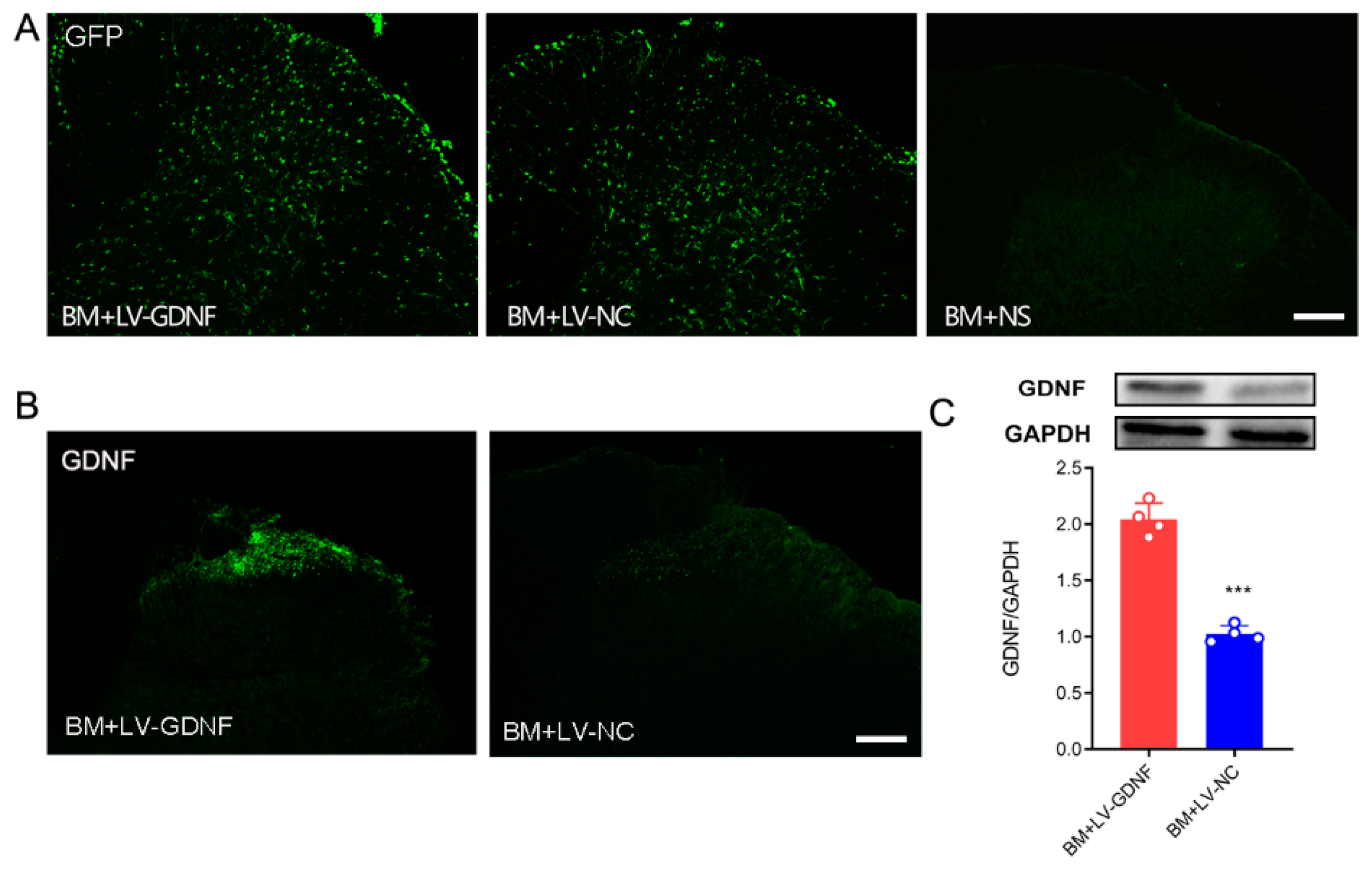
2.5. Lentivirus Delivery
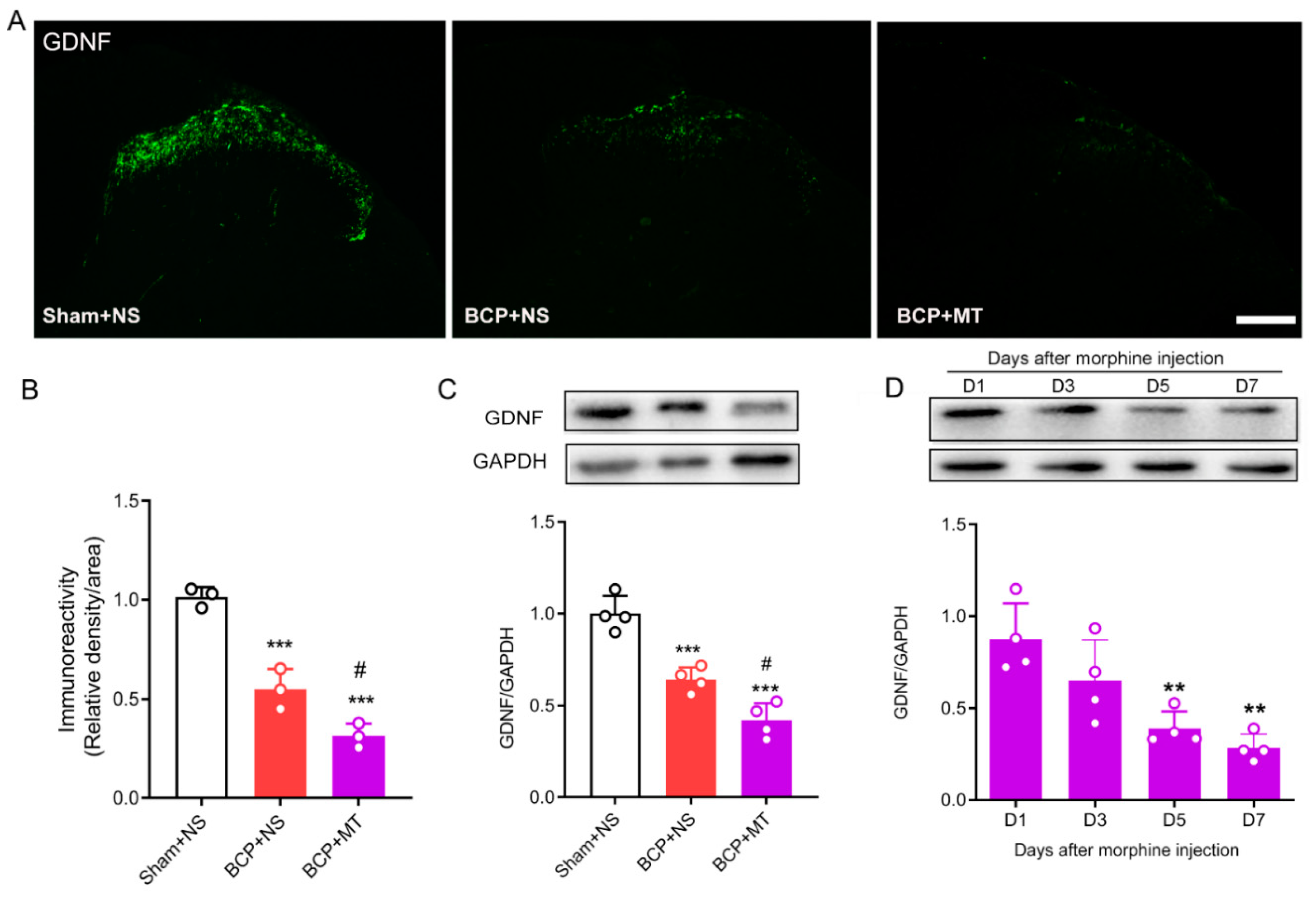
2.6. Immunohistochemistry
2.7. Western Blotting
2.8. Statistical Analyses
3. Results
3.1. Establishment of Morphine Tolerance in Rats with Bone Cancer Pain
3.2. Chronic Morphine Reduced the Level of GDNF in the Spinal Cord of BCP Rats
3.3. Intrathecal Injections of Lentiviral Vector Mediated GDNF Induce Persistent and Local Gene Expressions in Spinal Cord
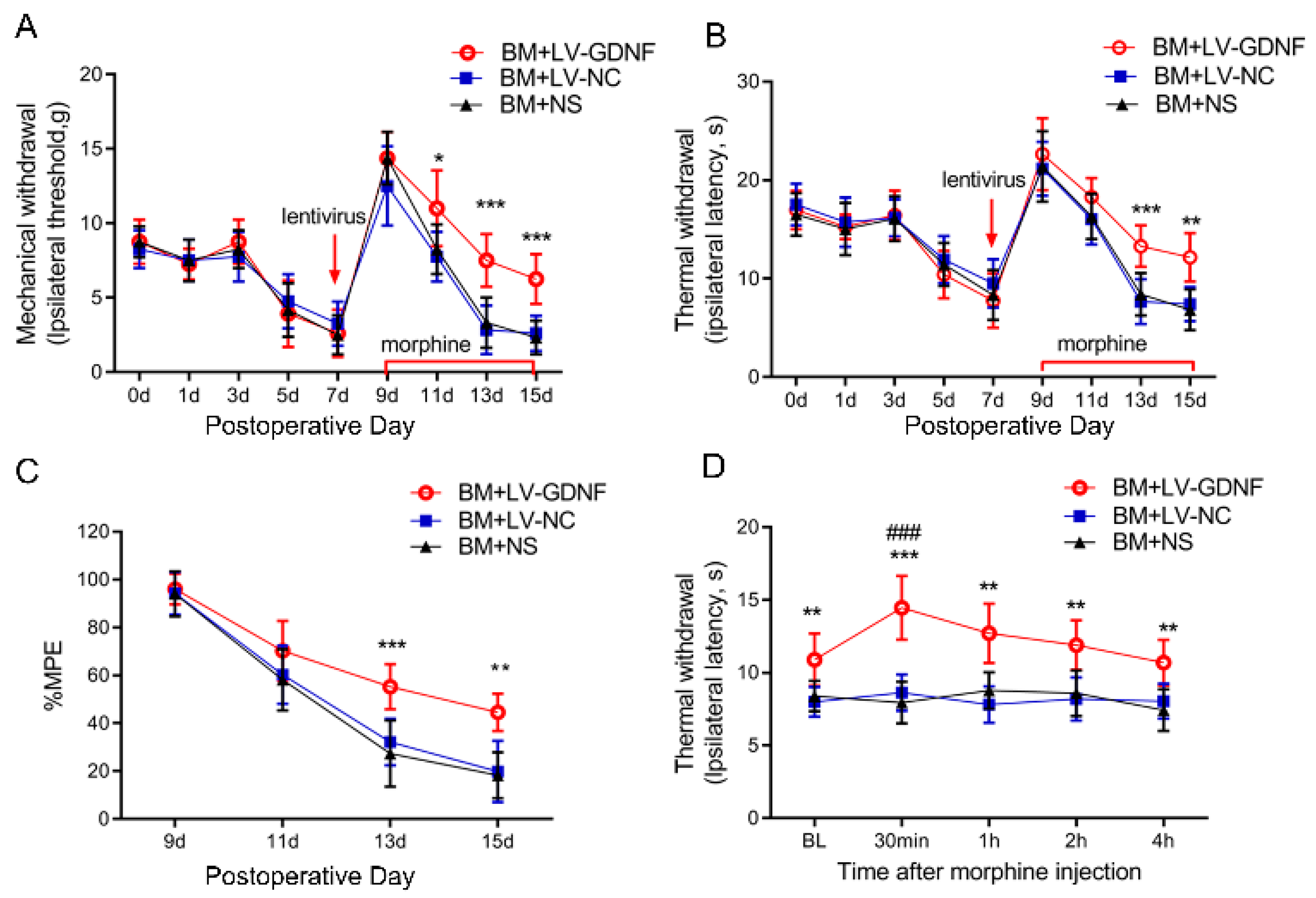
3.4. Upregulation of GDNF Alleviated the Morphine Tolerance in Bone Cancer Pain Rats
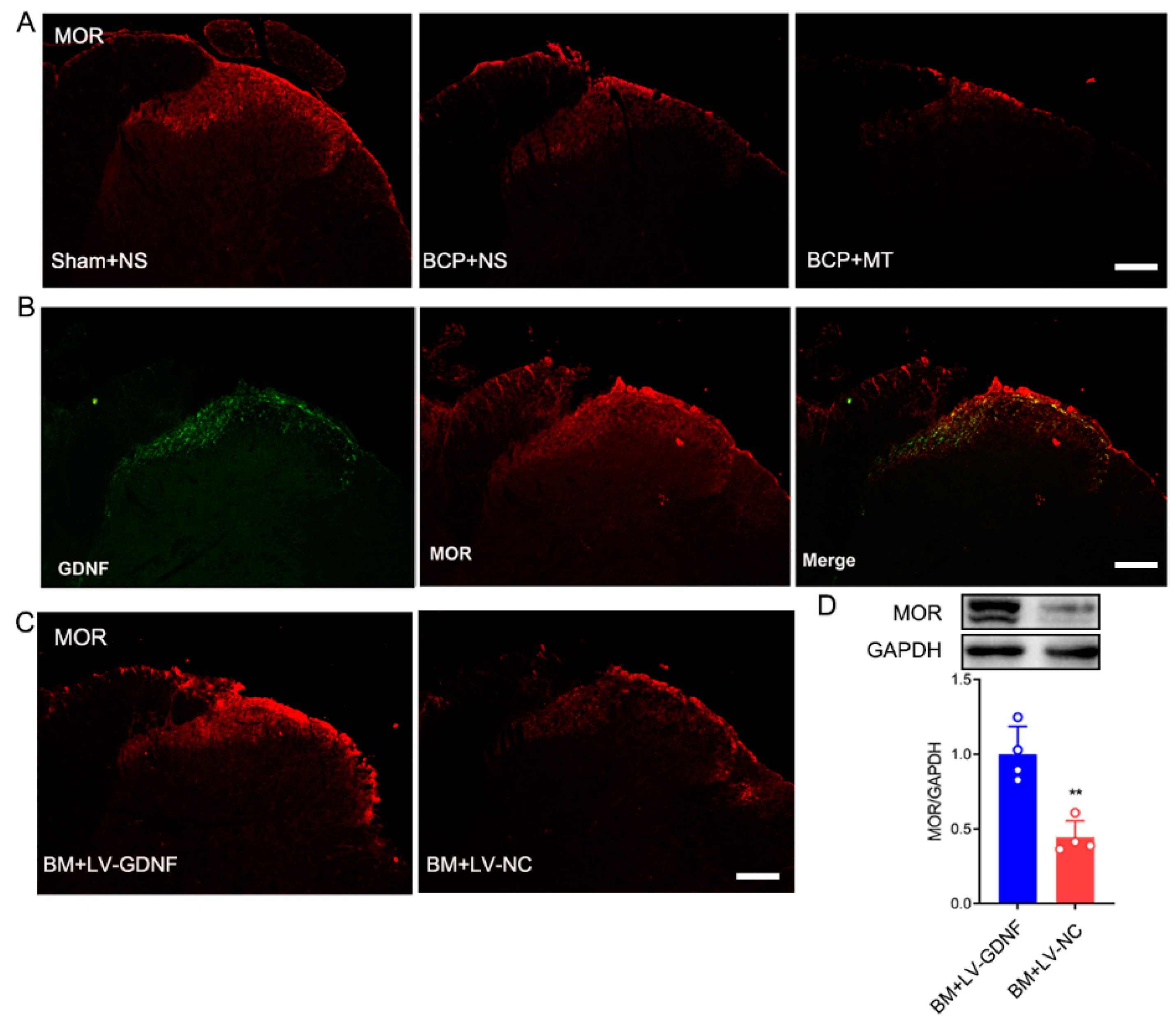
3.5. Changes of μ-Opioid Receptor (MOR) in the Spinal Cord after LV-GDNF Injection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mantyh, P. Bone cancer pain: Causes, consequences, and therapeutic opportunities. Pain 2013, 154 (Suppl. S1), S54–S62. [Google Scholar] [CrossRef]
- Wang, K.; Gu, Y.; Liao, Y.; Bang, S.; Donnelly, C.R.; Chen, O.; Tao, X.; Mirando, A.J.; Hilton, M.J.; Ji, R.R. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J. Clin. Investig. 2020, 130, 3603–3620. [Google Scholar] [CrossRef]
- Ruiz-Garcia, V.; Lopez-Briz, E. Morphine remains gold standard in breakthrough cancer pain. BMJ 2008, 337, a3104. [Google Scholar] [CrossRef]
- Jani, M.; Girard, N.; Bates, D.W.; Buckeridge, D.L.; Sheppard, T.; Li, J.; Iqbal, U.; Vik, S.; Weaver, C.; Seidel, J.; et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: A population-based cohort study. PLoS Med. 2021, 18, e1003829. [Google Scholar] [CrossRef]
- Nosek, K.; Leppert, W.; Nosek, H.; Wordliczek, J.; Onichimowski, D. A comparison of oral controlled-release morphine and oxycodone with transdermal formulations of buprenorphine and fentanyl in the treatment of severe pain in cancer patients. Drug Des. Devel. Ther. 2017, 11, 2409–2419. [Google Scholar] [CrossRef]
- Zollner, C.; Shaqura, M.A.; Bopaiah, C.P.; Mousa, S.; Stein, C.; Schafer, M. Painful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol. Pharmacol. 2003, 64, 202–210. [Google Scholar] [CrossRef]
- Hestehave, S.; Abelson, K.; Bronnum, P.T.; Munro, G. The analgesic efficacy of morphine varies with rat strain and experimental pain model: Implications for target validation efforts in pain drug discovery. Eur. J. Pain 2019, 23, 539–554. [Google Scholar] [CrossRef]
- Hou, X.; Weng, Y.; Wang, T.; Ouyang, B.; Li, Y.; Song, Z.; Pan, Y.; Zhang, Z.; Zou, W.; Huang, C.; et al. Suppression of HDAC2 in Spinal Cord Alleviates Mechanical Hyperalgesia and Restores KCC2 Expression in a Rat Model of Bone Cancer Pain. Neuroscience 2018, 377, 138–149. [Google Scholar] [CrossRef]
- Hou, X.; Weng, Y.; Ouyang, B.; Ding, Z.; Song, Z.; Zou, W.; Huang, C.; Guo, Q. HDAC inhibitor TSA ameliorates mechanical hypersensitivity and potentiates analgesic effect of morphine in a rat model of bone cancer pain by restoring mu-opioid receptor in spinal cord. Brain Res. 2017, 1669, 97–105. [Google Scholar] [CrossRef]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar] [CrossRef]
- Messer, C.J.; Eisch, A.J.; Carlezon, W.J.; Whisler, K.; Shen, L.; Wolf, D.H.; Westphal, H.; Collins, F.; Russell, D.S.; Nestler, E.J. Role for GDNF in biochemical and behavioral adaptations to drugs of abuse. Neuron 2000, 26, 247–257. [Google Scholar] [CrossRef]
- Niwa, M.; Nitta, A.; Shen, L.; Noda, Y.; Nabeshima, T. Involvement of glial cell line-derived neurotrophic factor in inhibitory effects of a hydrophobic dipeptide Leu-Ile on morphine-induced sensitization and rewarding effects. Behav. Brain Res. 2007, 179, 167–171. [Google Scholar] [CrossRef]
- Ding, Z.; Xu, W.; Zhang, J.; Zou, W.; Guo, Q.; Huang, C.; Liu, C.; Zhong, T.; Zhang, J.M.; Song, Z. Normalizing GDNF expression in the spinal cord alleviates cutaneous hyperalgesia but not ongoing pain in a rat model of bone cancer pain. Int. J. Cancer 2017, 140, 411–422. [Google Scholar] [CrossRef]
- Song, Z.; Zou, W.; Liu, C.; Guo, Q. Gene knockdown with lentiviral vector-mediated intrathecal RNA interference of protein kinase C gamma reverses chronic morphine tolerance in rats. J. Gene Med. 2010, 12, 873–880. [Google Scholar] [CrossRef]
- Wang, J.; Xu, W.; Zhong, T.; Song, Z.; Zou, Y.; Ding, Z.; Guo, Q.; Dong, X.; Zou, W. miR-365 targets beta-arrestin 2 to reverse morphine tolerance in rats. Sci. Rep. 2016, 6, 38285. [Google Scholar] [CrossRef]
- Liu, D.Q.; Zhou, Y.Q.; Gao, F. Targeting Cytokines for Morphine Tolerance: A Narrative Review. Curr. Neuropharmacol. 2019, 17, 366–376. [Google Scholar] [CrossRef]
- Kordower, J.H.; Emborg, M.E.; Bloch, J.; Ma, S.Y.; Chu, Y.; Leventhal, L.; McBride, J.; Chen, E.Y.; Palfi, S.; Roitberg, B.Z.; et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 2000, 290, 767–773. [Google Scholar] [CrossRef]
- Pezet, S.; Krzyzanowska, A.; Wong, L.F.; Grist, J.; Mazarakis, N.D.; Georgievska, B.; McMahon, S.B. Reversal of neurochemical changes and pain-related behavior in a model of neuropathic pain using modified lentiviral vectors expressing GDNF. Mol. Ther. 2006, 13, 1101–1109. [Google Scholar] [CrossRef]
- Wang, R.; Guo, W.; Ossipov, M.H.; Vanderah, T.W.; Porreca, F.; Lai, J. Glial cell line-derived neurotrophic factor normalizes neurochemical changes in injured dorsal root ganglion neurons and prevents the expression of experimental neuropathic pain. Neuroscience 2003, 121, 815–824. [Google Scholar] [CrossRef]
- Airavaara, M.; Mijatovic, J.; Vihavainen, T.; Piepponen, T.P.; Saarma, M.; Ahtee, L. In heterozygous GDNF knockout mice the response of striatal dopaminergic system to acute morphine is altered. Synapse 2006, 59, 321–329. [Google Scholar] [CrossRef]
- Park, J.; Decker, J.T.; Smith, D.R.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Reducing inflammation through delivery of lentivirus encoding for anti-inflammatory cytokines attenuates neuropathic pain after spinal cord injury. J. Control Release 2018, 290, 88–101. [Google Scholar] [CrossRef]
- Hendriks, W.T.; Eggers, R.; Verhaagen, J.; Boer, G.J. Gene transfer to the spinal cord neural scar with lentiviral vectors: Predominant transgene expression in astrocytes but not in meningeal cells. J. Neurosci. Res. 2007, 85, 3041–3052. [Google Scholar] [CrossRef]
- Haeseleer, F.; Imanishi, Y.; Saperstein, D.A.; Palczewski, K. Gene transfer mediated by recombinant baculovirus into mouse eye. Investig. Ophthalmol. Vis. Sci. 2001, 42, 3294–3300. [Google Scholar]
- Zollner, C.; Mousa, S.A.; Fischer, O.; Rittner, H.L.; Shaqura, M.; Brack, A.; Shakibaei, M.; Binder, W.; Urban, F.; Stein, C.; et al. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J. Clin. Investig. 2008, 118, 1065–1073. [Google Scholar] [CrossRef]
- Beck, K.D.; Valverde, J.; Alexi, T.; Poulsen, K.; Moffat, B.; Vandlen, R.A.; Rosenthal, A.; Hefti, F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 1995, 373, 339–341. [Google Scholar] [CrossRef]
- Airavaara, M.; Planken, A.; Gaddnas, H.; Piepponen, T.P.; Saarma, M.; Ahtee, L. Increased extracellular dopamine concentrations and FosB/DeltaFosB expression in striatal brain areas of heterozygous GDNF knockout mice. Eur. J. Neurosci. 2004, 20, 2336–2344. [Google Scholar] [CrossRef]
- Green-Sadan, T.; Kinor, N.; Roth-Deri, I.; Geffen-Aricha, R.; Schindler, C.J.; Yadid, G. Transplantation of glial cell line-derived neurotrophic factor-expressing cells into the striatum and nucleus accumbens attenuates acquisition of cocaine self-administration in rats. Eur. J. Neurosci. 2003, 18, 2093–2098. [Google Scholar] [CrossRef]
- Green-Sadan, T.; Kuttner, Y.; Lublin-Tennenbaum, T.; Kinor, N.; Boguslavsky, Y.; Margel, S.; Yadid, G. Glial cell line-derived neurotrophic factor-conjugated nanoparticles suppress acquisition of cocaine self-administration in rats. Exp. Neurol. 2005, 194, 97–105. [Google Scholar] [CrossRef]
- Airavaara, M.; Tuomainen, H.; Piepponen, T.P.; Saarma, M.; Ahtee, L. Effects of repeated morphine on locomotion, place preference and dopamine in heterozygous glial cell line-derived neurotrophic factor knockout mice. Genes Brain Behav. 2007, 6, 287–298. [Google Scholar] [CrossRef]
- Levitt, E.S.; Williams, J.T. Desensitization and Tolerance of Mu Opioid Receptors on Pontine Kolliker-Fuse Neurons. Mol. Pharmacol. 2018, 93, 8–13. [Google Scholar] [CrossRef]
- Diaz, A.; Pazos, A.; Florez, J.; Hurle, M.A. Autoradiographic mapping of mu-opioid receptors during opiate tolerance and supersensitivity in the rat central nervous system. Naunyn Schmiedeberg’s Arch. Pharmacol. 2000, 362, 101–109. [Google Scholar] [CrossRef]
- Zhang, P.; Bu, J.; Wu, X.; Deng, L.; Chi, M.; Ma, C.; Shi, X.; Wang, G. Upregulation of mu-Opioid Receptor in the Rat Spinal Cord Contributes to the alpha2-Adrenoceptor Agonist Dexmedetomidine-Induced Attenuation of Chronic Morphine Tolerance in Cancer Pain. J. Pain Res. 2020, 13, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Hwang, C.K.; Zheng, H.; Wagley, Y.; Lin, H.Y.; Kim, D.K.; Law, P.Y.; Loh, H.H.; Wei, L.N. MicroRNA 339 down-regulates mu-opioid receptor at the post-transcriptional level in response to opioid treatment. FASEB J. 2013, 27, 522–535. [Google Scholar] [CrossRef] [PubMed]
- Ammon-Treiber, S.; Hollt, V. Morphine-induced changes of gene expression in the brain. Addict. Biol. 2005, 10, 81–89. [Google Scholar] [CrossRef]
- Yamamoto, J.; Kawamata, T.; Niiyama, Y.; Omote, K.; Namiki, A. Down-regulation of mu opioid receptor expression within distinct subpopulations of dorsal root ganglion neurons in a murine model of bone cancer pain. Neuroscience 2008, 151, 843–853. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, W.; Ding, Z.; Song, Z.; Wang, J.; Zhang, J.; Zou, W. Overexpression of GDNF in Spinal Cord Attenuates Morphine Analgesic Tolerance in Rats with Bone Cancer Pain. Brain Sci. 2022, 12, 1188. https://doi.org/10.3390/brainsci12091188
Xu W, Ding Z, Song Z, Wang J, Zhang J, Zou W. Overexpression of GDNF in Spinal Cord Attenuates Morphine Analgesic Tolerance in Rats with Bone Cancer Pain. Brain Sciences. 2022; 12(9):1188. https://doi.org/10.3390/brainsci12091188
Chicago/Turabian StyleXu, Wei, Zhuofeng Ding, Zongbin Song, Jian Wang, Jie Zhang, and Wangyuan Zou. 2022. "Overexpression of GDNF in Spinal Cord Attenuates Morphine Analgesic Tolerance in Rats with Bone Cancer Pain" Brain Sciences 12, no. 9: 1188. https://doi.org/10.3390/brainsci12091188
APA StyleXu, W., Ding, Z., Song, Z., Wang, J., Zhang, J., & Zou, W. (2022). Overexpression of GDNF in Spinal Cord Attenuates Morphine Analgesic Tolerance in Rats with Bone Cancer Pain. Brain Sciences, 12(9), 1188. https://doi.org/10.3390/brainsci12091188






