Impact of Rural vs. Urban Residence on Survival Rates of Patients with Glioblastoma: A Tertiary Care Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. Data Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef]
- Iacob, G.; Dinca, E.B. Current data and strategy in glioblastoma multiforme. J. Med. Life 2009, 2, 386–393. [Google Scholar]
- Khurana, V.; Jain, S.; Smee, R.; Cook, R.; Dobes, M.; Shadbolt, B.; Smith, S.; Dexter, M. Increasing incidence of glioblastoma multiforme and meningioma, and decreasing incidence of Schwannoma (2000–2008): Findings of a multicenter Australian study. Surg. Neurol. Int. 2011, 2, 176. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef]
- Kleihues, P.; Ohgaki, H. Primary and secondary glioblastomas: From concept to clinical diagnosis. Neuro Oncol. 1999, 1, 44–51. [Google Scholar] [CrossRef]
- Ohgaki, H.; Kleihues, P. The definition of primary and secondary glioblastoma. Clin. Cancer Res. 2013, 19, 764–772. [Google Scholar] [CrossRef]
- Allahdini, F.; Amirjamshidi, A.; Reza-Zarei, M.; Abdollahi, M. Evaluating the prognostic factors effective on the outcome of patients with glioblastoma multiformis: Does maximal resection of the tumor lengthen the median survival? World Neurosurg. 2010, 73, 128–134, discussion:e16. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef]
- Sun, M.Z.; Oh, T.; Ivan, M.E.; Clark, A.J.; Safaee, M.; Sayegh, E.T.; Kaur, G.; Parsa, A.T.; Bloch, O. Survival impact of time to initiation of chemoradiotherapy after resection of newly diagnosed glioblastoma. J. Neurosurg. 2015, 122, 1144–1150. [Google Scholar] [CrossRef]
- Alnaami, I.; VanderPluym, J.; Murtha, A.; Walling, S.; Mehta, V.; Gourishankar, S.; Senthilselvan, A. The potential impact of delayed radiation therapy on patients with glioblastoma. Can. J. Neurol. Sci. 2013, 40, 790–794. [Google Scholar] [CrossRef]
- Afshar, N.; English, D.R.; Milne, R.L. Rural-urban residence and cancer survival in high-income countries: A systematic review. Cancer 2019, 125, 2172–2184. [Google Scholar] [CrossRef]
- Xu, H.; Chen, J.; Xu, H.; Qin, Z. Geographic variations in the incidence of glioblastoma and prognostic factors predictive of overall survival in US adults from 2004–2013. Front. Aging Neurosci. 2017, 9, 352. [Google Scholar] [CrossRef]
- Ostrom, Q.; Gittleman, H.; Farah, P.; Ondracek, A.; Chen, Y.; Wolinsky, Y.; Stroup, N.E.; Kruchko, C.; Barnholtz-Sloan, J. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013, 15 (Suppl. 2), ii1–ii56. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Efird, J.T. Epidemiology of Glioma. In Glioma—Exploring Its Biology and Practical Relevance; InTechOpen: London, UK, 2011. [Google Scholar] [CrossRef]
- Malay, S.; Somasundaram, E.; Patil, N.; Buerki, R.; Sloan, A.; Barnholtz-Sloan, J.S. Treatment and surgical factors associated with longer-term glioblastoma survival: A National Cancer Database study. Neuro-Oncol. Adv. 2020, 2, 1–10. [Google Scholar] [CrossRef]
- Westeel, V.; Pitard, A.; Martin, M.; Thaon, I.; Depierre, A.; Dalphin, J.-C.; Arveux, P. Negative impact of rurality on lung cancer survival in a population-based study. J. Thorac. Oncol. 2007, 2, 613–618. [Google Scholar] [CrossRef]
- Wrensch, M.; Minn, Y.; Chew, T.; Bondy, M.; Berger, M.S. Epidemiology of primary brain tumors: Current concepts and review of the literature. Neuro-oncology 2002, 4, 278–299. [Google Scholar] [CrossRef]
- Reschovsky, J.D.; Staiti, A.B. Access and quality: Does rural America lag behind? Health Aff. 2005, 24, 1128–1139. [Google Scholar] [CrossRef]
- Miranda-Filho, A.; Piñeros, M.; Soerjomataram, I.; Deltour, I.; Bray, F. Cancers of the brain and CNS: Global patterns and trends in incidence. Neuro-Oncol. 2017, 19, 270–280. [Google Scholar] [CrossRef]
- Jaimes, R. Reaching remote areas in Latin America. Plan. Parent. Chall. 1994, 43–46. [Google Scholar] [PubMed]
- Alfaqeeh, G.; Cook, E.J.; Randhawa, G.; Ali, N. Access and utilisation of primary health care services comparing urban and rural areas of Riyadh Providence, Kingdom of Saudi Arabia. BMC Health Serv. Res. 2017, 17, 106. [Google Scholar] [CrossRef]
- Altwairgi, A.K.; Algareeb, W.; Yahya, G.; Maklad, A.M.; Aly, M.M.; AL Shakweer, W.; Balbaid, A.; Alsaeed, E.; Alhussain, H.; Orz, Y.; et al. Outcome of patients with glioblastoma in Saudi Arabia: Single center experience. Mol. Clin. Oncol. 2016, 4, 756–762. [Google Scholar] [CrossRef][Green Version]
- Flanigan, P.M.; Jahangiri, A.; Kuang, R.; Truong, A.; Choi, S.; Chou, A.; Rick, J.W.; Chang, S.M.; Molinaro, A.M.; McDermott, M.W.; et al. Improved survival with decreased wait time to surgery in glioblastoma patients presenting with seizure. Neurosurgery 2017, 81, 824–833. [Google Scholar] [CrossRef]
- Katsigiannis, S.; Krischek, B.; Barleanu, S.; Grau, S.; Galldiks, N.; Timmer, M.; Kabbasch, C.; Goldbrunner, R.; Stavrinou, P. Impact of time to initiation of radiotherapy on survival after resection of newly diagnosed glioblastoma. Radiat. Oncol. 2019, 14, 73. [Google Scholar] [CrossRef]
- Noel, G.; Huchet, A.; Feuvret, L.; Maire, J.P.; Verrelle, P.; Le Rhun, E.; Aumont, M.; Thillays, F.; Sunyach, M.P.; Henzen, C.; et al. Waiting times before initiation of radiotherapy might not affect outcomes for patients with glioblastoma: A French retrospective analysis of patients treated in the era of concomitant temozolomide and radiotherapy. J. Neurooncol. 2012, 109, 167–175. [Google Scholar] [CrossRef]
- Adeberg, S.; Bostel, T.; Harrabi, S.; Bernhardt, D.; Welzel, T.; Wick, W.; Debus, J.; Combs, S. Impact of delays in initiating postoperative chemoradiation while determining the MGMT promoter-methylation statuses of patients with primary glioblastoma. BMC Cancer 2015, 15, 558. [Google Scholar] [CrossRef][Green Version]
- Han, S.J.; Rutledge, W.C.; Molinaro, A.M.; Chang, S.M.; Clarke, J.L.; Prados, M.D.; Taylor, J.W.; Berger, M.S.; Butowski, N.A. The effect of timing of concurrent Chemoradiation in patients with newly diagnosed glioblastoma. Neurosurgery 2015, 77, 248–253. [Google Scholar] [CrossRef]
- Seidlitz, A.; Siepmann, T.; Löck, S.; Juratli, T.; Baumann, M.; Krause, M. Impact of waiting time after surgery and overall time of postoperative radiochemotherapy on treatment outcome in glioblastoma multiforme. Radiat. Oncol. 2015, 10, 172. [Google Scholar] [CrossRef][Green Version]
- Schaff, L.R.; Yan, D.; Thyparambil, S.; Tian, Y.; Cecchi, F.; Rosenblum, M.; Reiner, A.S.; Panageas, K.S.; Hembrough, T.; Lin, A.L. Characterization of MGMT and EGFR protein expression in glioblastoma and association with survival. J. Neurooncol. 2020, 146, 163–170. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Bio-Demographic Data | Residence | p Value | |||
|---|---|---|---|---|---|
| Riyadh | Outside Riyadh | ||||
| No | % | No | % | ||
| Age in years | 0.814 | ||||
| < 30 | 10 | 16.4% | 8 | 12.5% | |
| 30–49 | 19 | 31.1% | 20 | 31.3% | |
| > 50 | 32 | 52.5% | 36 | 56.3% | |
| Gender | 0.508 | ||||
| Male | 47 | 77.0% | 46 | 71.9% | |
| Female | 14 | 23.0% | 18 | 28.1% | |
| Elderly (> 65 years) | 0.896 | ||||
| Yes | 12 | 19.7% | 12 | 18.8% | |
| No | 49 | 80.3% | 52 | 81.3% | |
| Eastern Cooperative Oncology Group Performance Status | 0.430 | ||||
| <2 | 35 | 64.8% | 31 | 57.4% | |
| >2 | 19 | 35.2% | 23 | 42.6% | |
| DM | 0.177 | ||||
| Yes | 16 | 26.2% | 24 | 37.5% | |
| No | 45 | 73.8% | 40 | 62.5% | |
| HTN | 0.571 | ||||
| Yes | 18 | 29.5% | 16 | 25.0% | |
| No | 43 | 70.5% | 48 | 75.0% | |
| Seizures | 0.858 | ||||
| Yes | 16 | 27.1% | 18 | 28.6% | |
| No | 43 | 72.9% | 45 | 71.4% | |
| Clinical Data | Residence | p Value | |||
|---|---|---|---|---|---|
| Riyadh | Outside Riyadh | ||||
| No | % | No | % | ||
| Brain Hemisphere | 0.970 | ||||
| Bilateral | 7 | 11.5% | 8 | 12.5% | |
| Left | 25 | 41.0% | 25 | 39.1% | |
| Right | 29 | 47.5% | 31 | 48.4% | |
| Brain Location | 0.384 | ||||
| Cerebellar | 1 | 1.6% | 0 | 0.0% | |
| Frontal | 20 | 32.8% | 13 | 20.3% | |
| Multifocal | 12 | 19.7% | 19 | 29.7% | |
| Occipital | 2 | 3.3% | 4 | 6.3% | |
| Others | 4 | 6.6% | 2 | 3.1% | |
| Parietal | 10 | 16.4% | 7 | 10.9% | |
| Temporal | 10 | 16.4% | 16 | 25.0% | |
| Thalamic | 2 | 3.3% | 3 | 4.7% | |
| Time to surgery in days | 0.021 * | ||||
| Same day | 0 | 0.0% | 9 | 14.1% | |
| 1–7 | 19 | 31.1% | 20 | 31.3% | |
| 8–15 | 21 | 34.4% | 16 | 25.0% | |
| > 15 | 21 | 34.4% | 19 | 29.7% | |
| Type of surgery | 0.137 | ||||
| Biopsy | 8 | 13.1% | 15 | 23.4% | |
| Resection | 53 | 86.9% | 49 | 76.6% | |
| Residual | 0.240 | ||||
| Yes | 51 | 83.6% | 58 | 90.6% | |
| No | 10 | 16.4% | 6 | 9.4% | |
| Pathology | 0.201 | ||||
| GBM | 56 | 91.8% | 54 | 84.4% | |
| GBM variant | 5 | 8.2% | 10 | 15.6% | |
| Residence | Means and Medians for Survival Time | 5-Years Survival Rate | p Value | |||
|---|---|---|---|---|---|---|
| Mean | SE | Median | SE | |||
| Riyadh | 23.7 | 2.8 | 14.4 | 2.0 | 9.0% | 0.187 |
| Outside Riyadh | 21.3 | 2.6 | 12.2 | 2.1 | 0.0% | |
| Overall | 22.4 | 1.9 | 14.1 | 1.9 | 4.0% | |
| Adjuvant Therapy | Residence | p Value | |||
|---|---|---|---|---|---|
| Riyadh | Outside Riyadh | ||||
| No | % | No | % | ||
| Radiotherapy received | 0.437 | ||||
| Yes | 59 | 96.7% | 60 | 93.8% | |
| No | 2 | 3.3% | 4 | 6.3% | |
| Radiotherapy type | 0.110 | ||||
| Standard | 47 | 79.7% | 40 | 66.7% | |
| Hypofractionated | 12 | 20.3% | 20 | 33.3% | |
| Adjuvant chemotherapy | 0.078 | ||||
| Yes | 51 | 83.6% | 45 | 70.3% | |
| No | 10 | 16.4% | 19 | 29.7% | |
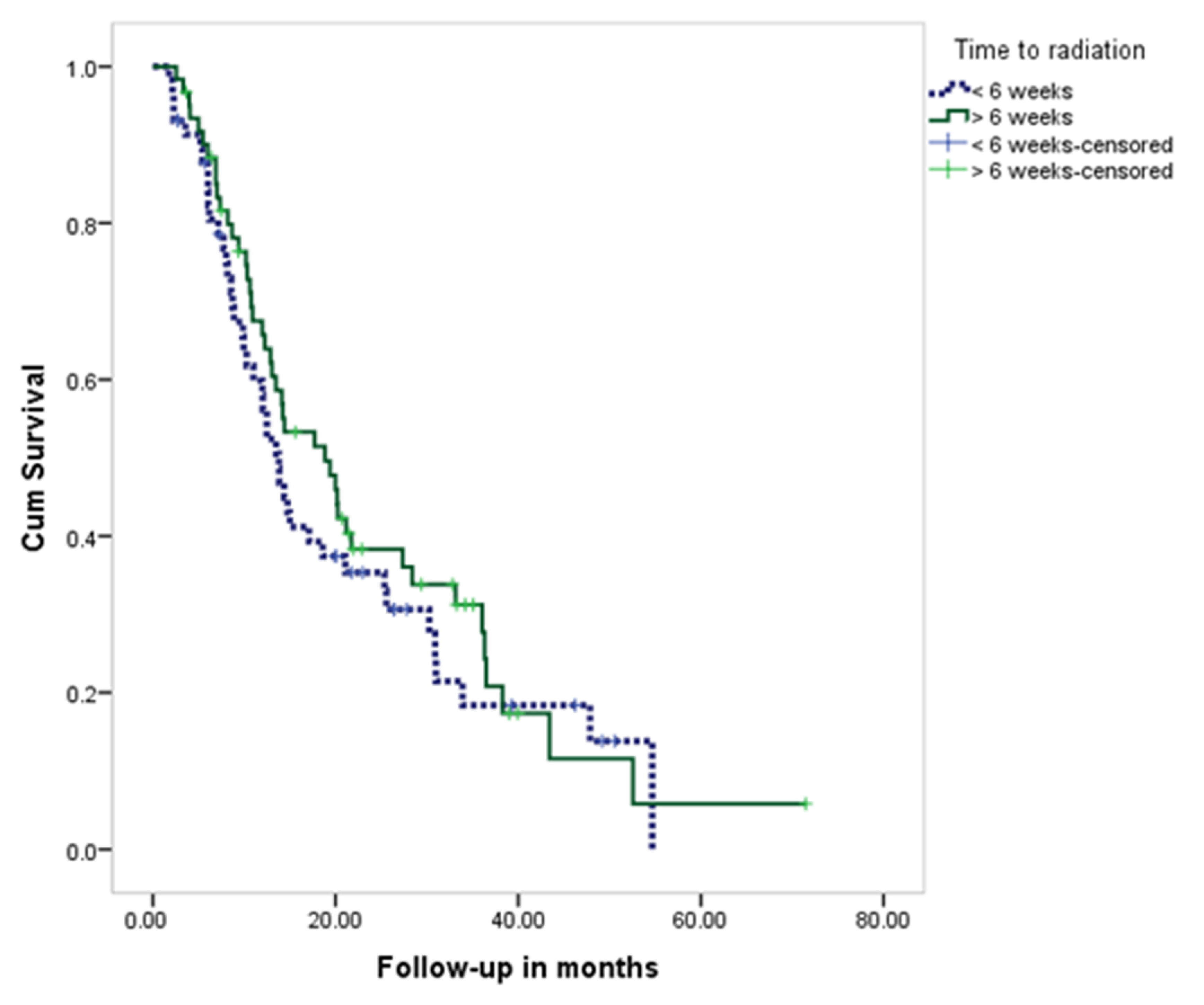
| Time to Radiation | Total | Residence | p Value | ||||
|---|---|---|---|---|---|---|---|
| From Riyadh | Outside Riyadh | ||||||
| No | % | No | % | No | % | ||
| ≤6 weeks | 58 | 48.7% | 28 | 47.5% | 30 | 50.0% | 0.781 |
| >6 weeks | 61 | 51.3% | 31 | 52.5% | 30 | 50.0% | |
| Mean ± SD (days) | 52.9 ± 32.8 | 51.1± 20.6 | 54.8 ± 41.5 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwadei, A.; Alnaami, I.; Alenazy, K.; Marei, A.; BaHammam, L.O.; Nasser, S.; Alswilem, A.M.; Maklad, A.; Shehata, S.F.; Alqahtani, M.S.; et al. Impact of Rural vs. Urban Residence on Survival Rates of Patients with Glioblastoma: A Tertiary Care Center Experience. Brain Sci. 2022, 12, 1186. https://doi.org/10.3390/brainsci12091186
Alwadei A, Alnaami I, Alenazy K, Marei A, BaHammam LO, Nasser S, Alswilem AM, Maklad A, Shehata SF, Alqahtani MS, et al. Impact of Rural vs. Urban Residence on Survival Rates of Patients with Glioblastoma: A Tertiary Care Center Experience. Brain Sciences. 2022; 12(9):1186. https://doi.org/10.3390/brainsci12091186
Chicago/Turabian StyleAlwadei, Ali, Ibrahim Alnaami, Kawthar Alenazy, Amal Marei, Leenh O. BaHammam, Sameh Nasser, Abdullah Mansour Alswilem, Ahmed Maklad, Shehata F. Shehata, Mohammad Salem Alqahtani, and et al. 2022. "Impact of Rural vs. Urban Residence on Survival Rates of Patients with Glioblastoma: A Tertiary Care Center Experience" Brain Sciences 12, no. 9: 1186. https://doi.org/10.3390/brainsci12091186
APA StyleAlwadei, A., Alnaami, I., Alenazy, K., Marei, A., BaHammam, L. O., Nasser, S., Alswilem, A. M., Maklad, A., Shehata, S. F., Alqahtani, M. S., Al-Shahrani, A., & Balbaid, A. (2022). Impact of Rural vs. Urban Residence on Survival Rates of Patients with Glioblastoma: A Tertiary Care Center Experience. Brain Sciences, 12(9), 1186. https://doi.org/10.3390/brainsci12091186






