Abstract
Background: Mental disorders linked with dysfunction in the temporal cortex, such as anxiety and depression, can increase the morbidity and mortality of people living with HIV (PLWHA). Expressions of both nucleobindin 1 (NUCB1) and cannabinoid receptor 1 (CNR1) in the neurons have been found to alter in patients with depressive disorder, but whether it is involved in the development of depression in the context of HIV infection is unknown. Objectives To investigate the effects of NUCB1 on depressive disorder among PLWHA and preliminarily explore the underlying molecular mechanisms. Methods: Individuals who were newly HIV diagnosed were assessed on the Hospital Anxiety and Depression scale (HADS). Then SHIV-infected rhesus monkeys were used to investigate the possible involvement of the NUCB1 and the CNR1 protein in depression-like behavior. Results: The prevalence rate of depression among PLWHA was 27.33% (41/150). The mechanism results showing elevated NUCB1 levels in cerebrospinal fluid from HIV-infected patients suffering from depression were confirmed compared to those of HIV-infected patients. Moreover, the immunohistochemical analysis indicated the expression of NUCB1 in the temporal cortex neurons of SHIV-infected monkeys was higher than that of the healthy control. Conversely, CNR1 expression was down-regulated at protein levels. Conclusions: Depression symptoms are common among PLWHA and associate with NUCB1 expression increases, and NUCB1 may be a potential target for depression.
1. Introduction
Human immunodefciency virus (HIV) is one of the major public health problems that had affected over 37 million people worldwide by the end of 2020 [1]. People living with HIV (PLWH) present a series of mental disorders, such as depression and anxiety [2,3]. Studies have shown that the prevalence and complexity of depression is higher among PLWH than in the general population, as depression can be a risk factor for HIV acquisition as well as a consequence of HIV infection [4,5]. In recent years, research has explored possible mechanisms underlying depression among PLWHA, thereby discovering new diagnostic markers and novel therapeutic targets, becoming hotspots in the field of both AIDS and neurobiology research.
Nucleobindin 1 (NUCB1), also known as CALNUC or NUC, is a highly conserved, multifunctional protein widely expressed in nerve cells [6]. Importantly, it is believed to be a novel pan-neuronal calcium handling marker [7], associated with cancers [8], and stimulating insulin secretion [9]. According to the literature, NUCB1 protein is implicated in Alzheimer’s disease [10,11], other neurodegenerative diseases, or cellular processes known to be dysregulated early in tauopathy pathogenesis [12] such as synaptic plasticity, proteostasis, glucose metabolism, and mitochondrial function. Recently, a paper indicated that an adult-viable mutant that completely disrupts the G protein α-subunit binding and activates NUCB1 plays a neuroprotective role in the Drosophila model [13] of neurodegenerative disease. Furthermore, in 2020, an LC-MS/MS analysis predicted a significantly negative correlation between plasma protein levels of NUCB1 and degree of depression [14]. Therefore, we put forward the hypotheses that alterations in NUCB1 expression might be linked to depression among PLWHA.
It has been reported that depressive disorder is related to temporal lobe dysfunction [15,16], and bilateral thinning within the temporal cortex in HIV patients has been detected [17,18]. The present study aimed to survey the prevalence of depression among PLWHA and preliminarily explore the underlying mechanisms. However, brain tissues from PLWH are not readily available, thereby limiting research access to mechanisms underlying depression in the context of HIV infection. Considering simian immunodeficiency virus (SIV) or simian/human immunodeficiency virus-infected (SHIV) rhesus macaques (RMs) have been widely utilized in pathogenic mechanisms of neuroAIDS [19,20] to unravel the possible involvement of NUCB1 in the pathophysiology of depression among PLWHA, we studied SHIV-infected RMs because the viral replication and pathological changes in the brain of infected RMs resembles those in PLWHAs. Understanding the mechanism underlying the interaction between NUCB1 expression and depression in the context of HIV-infection may provide insights that may facilitate the development of a biomarker for diagnosis, new drug target, and treatment response.
2. Material and Methods
2.1. Research Subjects
In order to eliminate the potential effects of other factors, from January 2015 to October 2020, a total of 150 individuals were selected at the First Affiliated Hospital of the University of Science and Technology of China (USTC). Eligibility criteria inclusion for participation in the present study were newly diagnosed as HIV positive, no current prescription for antiretroviral medication, and having given informed consent/assent to participate in the study. The exclusion criteria were a diagnosis of schizophrenia or other psychotic disorder, bipolar disorder, substance dependence, dementia, or other neurodegenerative disease that could significantly impact cognitive functioning, or a mood disorder due to a general medical condition or substance use. All participants were asked to participate in the HADS questionnaires and the medical history systems. This study has been reviewed and approved by the Bioethics and Biological Safety Review Committee of USTC (2021-N(H)-236).
2.2. Investigation Procedures
HADS was used to assess severity of anxiety and depression symptoms, where A represents anxiety, while D represents depression [21,22]. The score of each subscale ranges between 0 and 21 points. Overall, the total scores of HAD-A and HAD-D were classified as normal (0–7) and anxiety or depressive (8–21), with higher scores indicating higher levels of symptoms [23].
2.3. Western Blot Assay
Night cerebrospinal fluid (CSF) samples (HAD-D score ≥ 16) were obtained by lumbar puncture in the morning; 3 mL CSF was taken from each subject. Western blot was performed as previously described [24]. Briefly, equal amounts of proteins from the CSF samples (40 microg/each lane) were separated by SDS-PAGE gel and electrophoresed, then transferred to a nitrocellulose membrane followed by blocking with 5% non-fat milk for 1 h and incubated with primary antibody (anti-NUCB1 Rabbit pAb, ABclonal, A3994) at 4 °C overnight. Appropriate secondary antibodies (1:2000, Santa Cruz, CA, USA) were used for two-hour incubation at room temperature. Membranes were visualized by ECL plus kit (GE Healthcare and Life Science, Piscataway, NJ, USA).
2.4. Animals and Ethics Statement
The RMs used in this study were from the Tianqin Breeding and Research Center (SCXK (e) 2021–0010), Hubei Province, China. The monkeys were housed in an air-conditioned room with an ambient temperature of 16–26 °C, a relative humidity of 40–70%, and a 12 h light-dark cycle at the Animal Bio-Safety Level-III laboratory of the Wuhan University (SCXK (e) 2019–0013). The RMs were individually housed in stainless steel wire-bottomed cages with sufficient space (800 mm wide, 800 mm depth, and 1600 mm height) and provided with a commercial monkey diet. In addition, animal health was monitored daily by the animal care staff and veterinary personnel. All study protocols were approved by the Institutional Animal Care and Use Committee of USTC (2021-N(A) -349) in accordance with the regulations of the National Institute of Health “Guide for the Care and Use of Laboratory Animals”, and all details of animal welfare and steps taken to ameliorate suffering were in accordance with the recommendations of the Weatherall report, “The use of nonhuman primates in research”.
2.5. Virus Inoculation and Sample Collection
Seven Chinese-origin RMs from three different projects were enrolled in this study. Of these, five RMs were inoculated with 103–108 TCID50 of SIV/SHIV by either intravenous or intravaginal route under anesthesia with intramuscular injection of ketamine hydrochloride (10 mg/kg) plus intramuscular injection of atropine (0.04 mg/kg), while two healthy RMs were inoculated with the same volume of medium for mock infection and were used as negative controls.
2.6. Histology and Immunohistochemistry
At day of sacrifice, a depressive monkey and a healthy control were anesthetized with ketamine-HCl and euthanized by intravenous pentobarbital overdose. Formalin-fixed, paraffin-embedded brain sections from the cerebrum were obtained; 4 microns sections were processed and stained with hematoxylin and eosin (H&E) staining and immunohistochemistry (IHC). For IHC, sections were secured using an automated system, the Dako Autostainer Link. Formalin-fixed paraffin sections were rehydrated with water. Heat-induced epitope retrieval was performed with the FLEX TRS High-pH Retrieval Buffer for 20 min. After peroxidase blocking, the specific monoclonal antibody (IHC-plus CNR1/CB1 pAb, Lifespan, LS-B8253; anti-NUCB1 Rabbit pAb, ABclonal, A3994) was applied at room temperature for 20 min. The FLEX + Rabbit EnVision System was used for detection. DAB chromogen was then applied for 10 min. Slides were counterstained with Mayers hematoxylin for 5 s and then dehydrated and coverslipped. Images were then processed and analyzed using CaseViewer software (2.1 v, 3D Histech Ltd., Budapest, Hungary). Negative controls were included in the run.
2.7. Western Blotting
Western blotting was performed in cerebral cortex tissue lysates as previously described [25]. Briefly, tissue lysates of the cerebral cortex from SHIV-infected RMs were lysed with radioimmune precipitation assay buffer. Subsequently, proteins were transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA, USA), and appropriate primary antibodies and HRP-conjugated secondary antibodies were used. Proteins were detected with the enhanced chemiluminescent (ECL) reagent (Thermo Scientific, Waltham, MA, USA), followed by quantification using ImageJ software.
2.8. Statistical Analysis
Statistical analysis of the data was performed using the chi-square test (level of significance was 0.05) with SPSS software version 23.0 (Chicago, IL, USA) and GraphPad prism 5.0 (GraphPad Software, San Diego, CA, USA). In each aspect, univariate analysis was used to determine the variables significantly related to the dependent variable. The confidence interval was 95%.
3. Results
3.1. Prevalence of Depression among HIV-Infected Patients
Amongst all study participants, 6.00% (9/150) of patients newly testing positive for HIV met the criteria for depression (HAD-D score ≥ 8), and the prevalence of baseline anxiety was 14.00% (21/150) according to the HAD-A score ≥ 8 criterion. Combined anxiety and depression accounted for 21.33% (32/150) of the variance in reported body dissatisfaction. Furthermore, the number of HIV-infected individuals with HAD-A score ≥ 8 was reduced significantly from 53 at baseline to 27 at week 8 (p = 0.006), but no statistically significant differences in the number of patients with HAD-D score ≥ 8 was observed comparing baseline to week 8 (p = 0.189, two sample t-test). Prevalence of anxiety and depression among PLWHAs are listed in Table 1, respectively.

Table 1.
Prevalence of anxiety and depression among HIV/AIDS patients.
3.2. Univariate Analyses of Variables Related to Symptoms of Depression among Individuals with HIV Infection
We included personal information and clinical information in univariate analyses to observe whether the variables were related to anxiety and depressive symptoms. A total of 150 participants were included in the study. Nearly all participants were male (146/150; 97.33%). The mean age and weight of the study participants was 34.87 (± standard deviation 14.15) years and 67.02 ± 11.91 kg, respectively. Participants were mostly living without a spouse (118/150; 78.67%), were private employees (96/150; 64.0%), and had some education (104/150; 69.33%). Most of the study participants were homosexual. The time elapsed from the first positive HIV test to ART initiation of over half (108/150; 72.0%) was less than 4 weeks. Moreover, 28 (18.7%) had comorbid smoking, and drinking alcohol (16.0%). Demographic characteristics are shown in Table 2. Among the several items, “age” and “marital status” were significantly related to depression in those patients using univariate analyses (both p < 0.05). In addition, “weight” was significantly related to the patient’s anxiety symptom (p = 0.043) among the variables.

Table 2.
Factors associated with anxiety and depression among patients with HIV/AIDS.
3.3. NUCB1 Levels Are Elevated in CSF from HIV Cases Currently Suffering from Depression
CSF is a proximal fluid which communicates closely with brain tissue and contains numerous brain-derived proteins. Thus, NUCB1 expressions of CSF in HIV-infected individuals were examined via Western blot analysis. Comparing with only HIV cases, the protein expression of NUCB1 in the CSF was significantly increased in those HIV cases currently suffering from depression. Notably, we did not enroll a control group of healthy subjects because of the invasiveness of the CSF procedure for which the Ethics Committee did not allow. Protein levels of NUCB1 in CSF are reported in Figure 1.

Figure 1.
Representative image of protein levels of NUCB1. Western blotting results showed that in HIV-infected individuals, having comorbid depression significantly increased the expression of NUCB1 in the cerebrospinal fluid.
3.4. SHIVKU-1 Infection Triggers a Reduction in the Number of Neurons in Cerebral Cortex of Rhesus Monkeys
To explore the pathogenesis of depression among PLWHA, in this study, SIV/SHIV of five RMs established persistent infections. Of note, during the late stage of infection, Macaque WSH01 infected with SHIVKU-1 presented depression-like syndromes [26,27] that mimic those observed in human neuroAIDS, including difficulties in standing, head tilting, weakening of muscle strength, decreased appetite and movement, loss of body mass, ataxia, fear, psychomotor changes, sleep disturbance, and total loss of motoric function on the left side of the body. The animal had a slow progressing course lasting for about 18 months after the triggering infection and was euthanized at week 72. This study demonstrated that SHIVKU-1-infected RMs can resemble human neuroAIDS and will become an important tool for studying pathogenesis and evaluating treatment and preventive drugs of neuroAIDS.
H&E staining analysis (Figure 2A,B) showed marked histological damages with increased infiltration and vasculitis in the brain of Macaque WSH01. Importantly, the cerebral cortex exhibited a decrease in the number of neurons. Accompanied with purulent meningitis, the intactness of the cerebral cortex could be not revealed more clearly, and many cavities appeared after liquefaction of cerebral cortex tissue. Specifically, the neuronal loss in the cerebral cortex of the SHIVKU-1-infected monkey was observed, and there were also substantial changes in the spatial arrangement of neurons. In addition, residual nerve cells bodies in the cerebral cortex changed from swelling to shrinkage, Nissl body staining was weak, the dendritic length due to cell death or an inflammatory process was irregular, and neural axone was thin (Figure 2a,b).
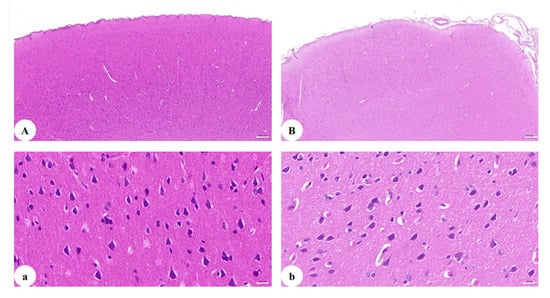
Figure 2.
H&E staining of the cerebral cortex: (A) section of the cerebral cortex of healthy monkey (50×); (B) section of the cerebral cortex of the SHIVKU-1-infected monkey brain (50×); (a) section of the cerebral cortex of healthy monkey (400×); (b) section of the cerebral cortex of the SHIVKU-1-infected monkey brain (400×).
3.5. Up-Regulation of NUCB1 Protein in Cerebral Cortex of SHIVKU-1-Infected Monkey
We next verified whether alterations in the protein expression of NUCB1 occurred in an SHIVKU-1-infected monkey via IHC staining analysis (Figure 3A,B). The results revealed that the NUCB1 protein was expressed in the cerebral cortex of the healthy monkey but, in the infected one, the expression of the NUCB1 protein was higher. Furthermore, these findings were supported by data from quantitative Western blotting of NUCB1 protein levels in the whole cerebrum in vivo (p = 0.0056) (Figure 3C). The results suggest that NUCB1 plays an important role in the pathological processes leading to depression.
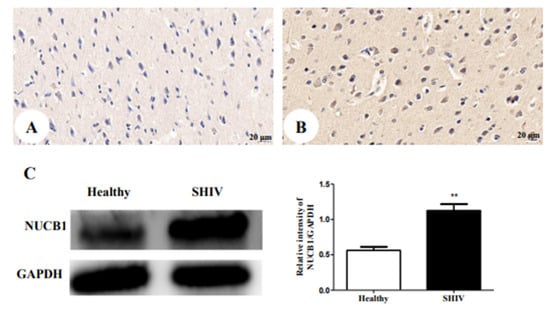
Figure 3.
NUCB1 protein expression in cerebral cortex: (A) the healthy control (400×); (B) SHIVKU-1-infected monkey (400×) (C) comparison of NUCB1 expression of cerebral cortex in healthy control and SHIVKU-1-infected monkey using Western blotting. In the column, from the t test, statistically significant differences of the two groups can be determined (p = 0.0056). ** p < 0.01.
3.6. Down-Regulation of CNR1 Protein in Cerebral Cortex of SHIVKU-1-Infected Monkey
Finally, 16 potential targets relative to depression were acquired from the TCMSP database (https://old.tcmsp-e.com/disease.php?qd=228, accessed on 19 March 2022) and further analyzed by the online STRING database (http://string.embl.de/, accessed on 15 March 2022) to explore the functional mechanism of the NUCB1 protein.
The network showed CNR1, neuroprotective and highly expressed in the neurons [28,29], is in close contact with NUCB1 (Figure 4A). Thereby, we investigated the impact of SHIVKU-1 infection on CNR1 expression in neurons. Accordingly, we observed, using an immunohistochemical technique, the down-regulation of CNR1 protein expression in the infected monkey compared to the healthy control (Figure 4B,C). This was supported by protein levels of CNR1 via Western blot analysis (p = 0.0366) (Figure 4D). We speculate that decreased CNR1 expression by SHIVKU-1 infection results in depressive disorder because there is support for existing cannabinoid signaling pathways that can decrease neuronal injury [30].
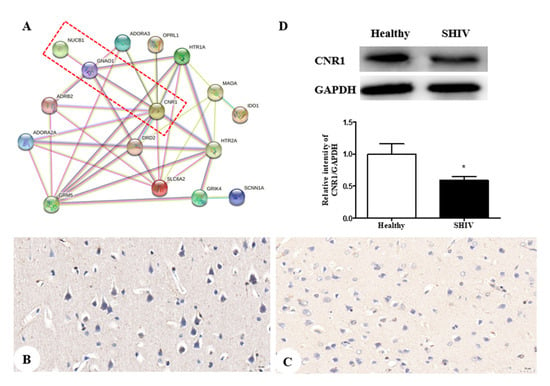
Figure 4.
CNR1 protein expression in both an SHIVKU-1-infected monkey and healthy control: (A) the protein-protein interactome networks; blue rectangle nodes represent down-regulated proteins; red circular nodes stand for the up-regulated proteins; the lines represent the regulation of relationship between two nodes; (B) IHC staining against CNR1 of the healthy control (400×); (C) IHC staining against CNR1 of the SHIVKU-1-infected monkey (400×); (D) comparison of CNR1 expression of cerebral cortex in healthy control and the SHIVKU-1-infected monkey using Western blotting. In the column, from the t test, statistically significant differences of two groups can be determined (p = 0.0366). * p < 0.05.
4. Discussion
Many studies have reported significantly higher prevalence of depression in HIV-infected patients when compared to the general population [31,32]. In the present study, the prevalence of depression among HIV-infected patients was 27.33% (41/150). Moreover, univariable logistic regression analysis indicated that age and marital status were associated with depression (p < 0.05). Unfortunately, the current diagnoses of depression are still based on clinical manifestations and self-rating scales as the main diagnostic criteria, as a lack of relevant objective laboratory indicators exists. Thus, there is an urgent need to search for and identify new clinical biomarkers of depression.
Increased functional connectivity between the temporal lobe dysfunction and the DMN (default mode network) has been shown in depressive disorder [33,34]. Evidence from recent studies provides a deeper understanding of human learning neural networks, particularly on human PFC crucial role, proposing a theoretical model to conceptualize these psychophysiological processes. The neurovisceral integration model of fear (NVI-f) that can be impaired in the context of a psychiatric disorder [35,36] might also contribute to the advancement of alternative, more precise and individualized treatments for psychiatric disorders. Moreover, recent studies demonstrated regulatory and functional aspects of the kynurenine pathway, which has been shown to possess neuroprotective and antidepressant-like properties [37,38]. In addition, there is evidence [39,40] that diet may also protect from depressive disorders and improve mental health through mechanisms that are related to inflammation, endogenous metabolic factors in cognitive and emotional functions, and pathological neural substrates of depressive disorder, especially on frontal lobe dysfunction [41].
Fortunately, our understanding of the (endocannabinoid system, ECS) comprised neuromodulatory lipids and their receptors associated with depression has increased [42,43]. CNR1, also known as CB1, is the most abundant G protein-coupled receptor (GPCR) in the mammalian brain. Rocha et al. [44] found that CNR1 knockout mice lost weight and appetite, reduced rearing and exploratory behaviors, and increased anxiety, compared to wild-type littermates. These symptoms were similar to human depression patients. In addition, a clinical phase I/II trial with SR14716A (rimonabant), a CNR1 antagonist agonist showed that it produced serious adverse neuropsychiatric events such as anxiety, depression, and even suicidal ideation [45,46].
Several studies have showed that HIV gp120 stimulates increased cortical fatty acid amide hydrolase (FAAH) [47,48], subsequently allowing for rapid 2-AG and AEA production [49], selective ligands for the NUCB1 linked to degree of depressive behavior [50], in the postsynaptic neuron, whereas decreased CNR1 expression promotes the release of 2-AG and AEA [51,52]. Herein, down-regulation of CNR1 expression and up-regulation of NUCB1 expression in neurons were found in the co-occurrence of depression disorder and SHIV infection, as detected by IHC and Western blot. By combining our experimental results, we propose a schematic presentation of a possible mechanism of NUCB1 involvement in depression in PLWH (Figure 5). Therefore, NUCB1 inhibition may be considered as a therapeutic agent to relieve depressive disorder. Based on the published articles, we speculate that both a ZiBuPiYin recipe [53], which was recorded in the book of Bujuji written by Cheng Wu in the Qing dynasty, and Luks-PV [54] which is a pore-forming leukocidin secreted by Staphylococcus aureus, have potential roles in the prevention and treatment of depression because of their reductions in the levels of the NUCB1 protein.
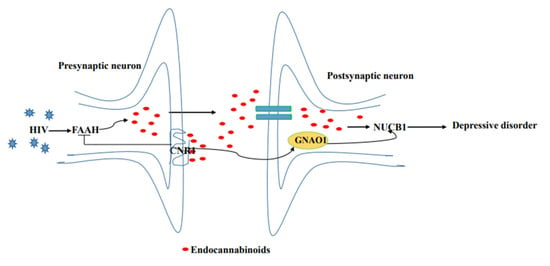
Figure 5.
Proposed model of the molecular events in NUCB1-mediated HIV comorbid depression.
There are several limitations in our study. Firstly, with an animal model we were unable to conduct questionnaires, interviews, or oral reports. Therefore, the severity of depression cannot be quantified. Moreover, there is no way for a human investigator to really know whether a monkey is feeling depressed. What we can do is observe the behavior that a monkey makes in response to viral infection. Second, there are too little data on NUCB1 levels in CSF from HIV-infected individuals currently suffering from depression to draw a convincing conclusion. The sample size should be enlarged, etc. Third, a total of 150 participants were randomly selected rather than by performing a statistical power analysis to estimate the appropriate number of participants required to generate results. Finally, further studies are needed to demonstrate the expression of NUCB1 in specific neuronal subpopulations. Despite all this, this study suggests that the NUCB1 protein may be a novel clinical biomarker for depression, and inhibiting its activity might have a potential function that predicts and monitors responsiveness of treatment. In future studies, we will investigate the molecular mechanisms underlying the regulation of NUCB1 expression and the role of NUCB1 in other mental disorders.
Overall, this study indicates a high prevalence of depressive symptoms in newly diagnosed HIV-infected patients and identifies NUCB1-CNR1 signaling as a novel mediator of impaired neuronal function in HIV-infected people. Together, these findings illustrate that NUCB1 inhibition can provide an additional neuroprotective benefit, extending the significance of our findings beyond HIV.
Author Contributions
Conceptualization, K.Z. and C.Z.; methodology, Y.Y.; software, Q.Z. and J.Y.; validation, J.Y., Y.W. and C.Z.; formal analysis, Y.Y. and C.Z.; investigation, Q.Z.; resources, J.Y.; data curation, Q.Z.; writing—original draft preparation, Y.Y. and J.Y.; writing—review and editing, K.Z. and C.Z.; visualization, Y.W.; supervision, Y.W.; project administration, Y.Y. and J.Y.; funding acquisition, C.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Chinese Federation of Public Health foundation (GWLM202007 for C.Z.) and Open project of the Third People’s Hospital of Shenzhen (#13 for C.Z.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Bioethics and Biological Safety Review Committee of USTC (2021-N(H)-236). All the animal procedures were approved by the Ethics Review Committee for Animal Experimentation of USTC (2021-N(A) -349).
Informed Consent Statement
Informed consent was obtained from all subjects in the study.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors appreciate all the participants for participating in our study. And we thank Ying Dong, Fei Li and Ting Liu for their constant support and useful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, X.; Wang, H.; Zhu, Z.; Zhang, L.; Cao, J.; Zhang, L.; Yang, H.; Wen, H.; Hu, Y.; Chen, C.; et al. Exploring bridge symptoms in HIV-positive people with comorbid depressive and anxiety disorders. BMC Psychiatry 2022, 22, 448. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.C.; Harman, J.S.; Cook, R.L.; Marlow, N.M.; Harle, C.A.; Duncan, R.P.; Bengtson, A.M.; Pence, B.W. Comparative effectiveness of dual-action versus single-action antidepressants for the treatment of depression in people living with HIV/AIDS. J. Affect. Disord. 2017, 215, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Algoodkar, S.; Kidangazhiathmana, A.; Rejani, P.P.; Shaji, K.S. Prevalence and Factors associated with Depression among Clinically Stable People Living with HIV/AIDS on Antiretroviral Therapy. Indian J. Psychol. Med. 2017, 39, 789–793. [Google Scholar] [CrossRef]
- Cholera, R.; Pence, B.W.; Gaynes, B.N.; Bassett, J.; Qangule, N.; Pettifor, A.; Macphail, C.; Miller, W.C. Depression and Engagement in Care among Newly Diagnosed HIV-Infected Adults in Johannesburg, South Africa. AIDS Behav. 2017, 21, 1632–1640. [Google Scholar] [CrossRef] [PubMed]
- Malava, J.K.; Lancaster, K.E.; Hosseinipour, M.C.; Hosseinipour, M.C.; Rosenberg, N.E.; O’Donnell, J.K.; Kauye, F.; Mbirimtengerenji, N.; Chaweza, T.; Tweya, H.; et al. Prevalence and correlates of probable depression diagnosis and suicidal ideation among patients receiving HIV care in Lilongwe, Malawi. Malawi Med. J. 2018, 30, 236–242. [Google Scholar] [CrossRef]
- Kamthan, A.; Kamthan, M.; Kumar, A.; Sharma, P.; Ansari, S.; Thakur, S.S.; Chaudhuri, A.; Datta, A. A calmodulin like EF hand protein positively regulates oxalate decarboxylase expression by interacting with E-box elements of the promoter. Sci. Rep. 2015, 5, 14578. [Google Scholar] [CrossRef] [PubMed]
- Kanuru, M.; Aradhyam, G.K. Chaperone-like Activity of Calnuc Prevents Amyloid Aggregation. Biochemistry 2017, 56, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Barbazan, J.; Dunkel, Y.; Li, H.; Nitsche, U.; Janssen, K.-P.; Messer, K.; Ghosh, P. Prognostic Impact of Modulators of G proteins in Circulating Tumor Cells from Patients with Metastatic Colorectal Cancer. Sci. Rep. 2016, 6, 22112. [Google Scholar] [CrossRef]
- Ramesh, N.; Mohan, H.; Unniappan, S. Nucleobindin-1 encodes a nesfatin-1-like peptide that stimulates insulin secretion. Gen. Comp. Endocrinol. 2015, 216, 182–189. [Google Scholar] [CrossRef]
- Horvatić, A.; Gelemanović, A.; Pirkić, B.; Smolec, O.; Ljubić, B.B.; Rubić, I.; Eckersall, P.D.; Mrljak, V.; McLaughlin, M.; Samardžija, M.; et al. Multi-Omics Approach to Elucidate Cerebrospinal Fluid Changes in Dogs with Intervertebral Disc Herniation. Int. J. Mol. Sci. 2021, 22, 11678. [Google Scholar] [CrossRef]
- Sargeant, J.; Hay, J.C. Ca2+ regulation of constitutive vesicle trafficking. Fac. Rev. 2022, 11, 6. [Google Scholar] [CrossRef] [PubMed]
- Koren, S.A.; Hamm, M.J.; Cloyd, R.; Fontaine, S.N.; Chishti, E.; Lanzillotta, C.; Rodriguez-Rivera, J.; Ingram, A.; Bell, M.; Galvis-Escobar, S.M.; et al. Broad Kinase Inhibition Mitigates Early Neuronal Dysfunction in Tauopathy. Int. J. Mol. Sci. 2021, 22, 1186. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Srinivasan, B. Genetic analyses uncover pleiotropic compensatory roles for Drosophila Nucleobindin-1 in inositol trisphosphate-mediated intracellular calcium homeostasis. Genome 2020, 63, 61–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Ahn, H.-S.; Lee, M.Y.; Yu, J.; Yeom, J.; Jeong, H.; Min, H.; Lee, H.J.; Kim, K.; Ahn, Y.M. An Exploratory Pilot Study with Plasma Protein Signatures Associated with Response of Patients with Depression to Antidepressant Treatment for 10 Weeks. Biomedicines 2020, 8, 455. [Google Scholar] [CrossRef]
- Cacciaguerra, L.; Mistri, D.; Valsasina, P.; Martinelli, V.; Filippi, M.; Rocca, M.A. Time-varying connectivity of the precuneus and its association with cognition and depressive symptoms in neuromyelitis optica: A pilot MRI study. Mult. Scler. 2022, 7, 13524585221107125. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, K.; Wei, Y.; Guo, X.; Wen, J.; Luo, Y. Minimal EEG channel selection for depression detection with connectivity features during sleep. Comput. Biol. Med. 2022, 147, 105690. [Google Scholar] [CrossRef] [PubMed]
- Küper, M.; Rabe, K.; Esser, S.; Gizewski, E.R.; Husstedt, I.W.; Maschke, M.; Obermann, M. Structural gray and white matter changes in patients with HIV. J. Neurol. 2011, 258, 1066–1075. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Kimura, S.; Kiryu, Y.; Watanabe, A.; Kinai, E.; Oka, S.; Kikuchi, Y.; Kimura, S.; Ogata, M.; Takano, M.; et al. Neurocognitive dysfunction and brain FDG-PET/CT findings in HIV-infected hemophilia patients and HIV-infected non-hemophilia patients. PLoS ONE 2020, 15, e0230292. [Google Scholar] [CrossRef]
- Omeragic, A.; Kayode, O.; Hoque, M.T.; Bendayan, R. Potential pharmacological approaches for the treatment of HIV-1 associated neurocognitive disorders. Fluids Barriers CNS 2020, 17, 42. [Google Scholar] [CrossRef]
- Williams, K.; Westmoreland, S.; Greco, J.; Ratai, E.; Lentz, M.; Kim, W.-K.; Fuller, R.A.; Kim, J.P.; Autissier, P.; Sehgal, P.K.; et al. Magnetic resonance spectroscopy reveals that activated monocytes contribute to neuronal injury in SIV neuroAIDS. J. Clin. Investig. 2005, 115, 2534–2545. [Google Scholar] [CrossRef] [Green Version]
- Serrat, M.; Almirall, M.; Musté, M.; Sanabria-Mazo, J.P.; Feliu-Soler, A.; Méndez-Ulrich, J.L.; Luciano, J.V.; Sanz, A. Effectiveness of a Multicomponent Treatment for Fibromyalgia Based on Pain Neuroscience Education, Exercise Therapy, Psychological Support, and Nature Exposure (NAT-FM): A Pragmatic Randomized Controlled Trial. J. Clin. Med. 2020, 9, 3348. [Google Scholar] [CrossRef] [PubMed]
- Feliu-Soler, A.; Borràs, X.; Peñarrubia-María, M.T.; Rozadilla-Sacanell, A.; D’Amico, F.; Moss-Morris, R.; Howard, M.A.; Fayed, N.; Soriano-Mas, C.; Puebla-Guedea, M.; et al. Cost-utility and biological underpinnings of Mindfulness-Based Stress Reduction (MBSR) versus a psychoeducational programme (FibroQoL) for fibromyalgia: A 12-month randomised controlled trial (EUDAIMON study). BMC Complement. Altern. Med. 2016, 16, 81. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatry Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, X.; Matei, N.; McBride, D.; Tang, J.; Yan, M.; Zhang, J.H. Ezetimibe, a NPC1L1 inhibitor, attenuates neuronal apoptosis through AMPK dependent autophagy activation after MCAO in rats. Exp. Neurol. 2018, 307, 12–23. [Google Scholar] [CrossRef]
- Kuwar, R.; Rolfe, A.; Di, L.; Blevins, H.; Xu, Y.; Sun, X.; Bloom, G.S.; Zhang, S.; Sun, D. A Novel Inhibitor Targeting NLRP3 Inflammasome Reduces Neuropathology and Improves Cognitive Function in Alzheimer’s Disease Transgenic Mice. J. Alzheimers Dis. 2021, 82, 1769–1783. [Google Scholar] [CrossRef]
- Yu, Q.; Xiao, Q.H.; Zhang, J.; Wang, Y.; Xian, Q.Y.; Cheng, J.X.; Zhuang, K. Pathological study of central nervous system injury in a Chinese rhesus macaque model of SHIVKU-1 infection. J. Pathog. Biol. 2019, 14, 896–900. [Google Scholar]
- Li, J.-L.; Zhuang, K.; Wu, G.-Y.; Ho, W.-Z. Magnetic resonance imaging study of a simian/human immunodeficiency virus-infected Chinese rhesus macaque with HIV-associated dementia. AIDS Res. Hum. Retrovir. 2015, 31, 272–273. [Google Scholar] [CrossRef]
- Ma, L.; Jia, J.; Niu, W.; Jiang, T.; Zhai, Q.; Yang, L.; Bai, F.; Wang, Q.; Xiong, L. Mitochondrial CB1 receptor is involved in ACEA-induced protective effects on neurons and mitochondrial functions. Sci. Rep. 2015, 5, 12440. [Google Scholar] [CrossRef]
- Schurman, L.D.; Lichtman, A.H. Endocannabinoids: A Promising Impact for Traumatic Brain Injury. Front. Pharmacol. 2017, 8, 69. [Google Scholar] [CrossRef]
- Liu, Q.; Bhat, M.; Bowen, W.D.; Cheng, J. Signaling pathways from cannabinoid receptor-1 activation to inhibition of N-methyl-d-aspartic acid mediated calcium influx and neurotoxicity in dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2009, 331, 1062–1070. [Google Scholar] [CrossRef]
- Fu, X.; Lawson, M.A.; Kelley, K.W.; Dantzer, R. HIV-1 Tat activates indoleamine 2,3 dioxygenase in murine organotypic hippocampal slice cultures in a p38 mitogen-activated protein kinase-dependent manner. J. Neuroinflam. 2011, 8, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kee, M.-K.; Lee, S.-Y.; Kim, N.-Y.; Lee, J.-S.; Kim, J.M.; Choi, J.Y.; Ku, N.S.; Kang, M.W.; Kim, M.J.; Woo, J.H.; et al. Anxiety and depressive symptoms among patients infected with human immunodeficiency virus in South Korea. AIDS Care 2015, 27, 1174–1182. [Google Scholar] [CrossRef] [PubMed]
- Tozzi, L.; Zhang, X.; Chesnut, M.; Holt-Gosselin, B.; Ramirez, C.A.; Williams, L.M. Reduced functional connectivity of default mode network subsystems in depression: Meta-analytic evidence and relationship with trait rumination. Neuroimage Clin. 2021, 30, 102570. [Google Scholar] [CrossRef] [PubMed]
- Selingardi, P.M.L.; de Lima Rodrigues, A.L.; Silva, V.; Fernandes, D.T.R.M.; Rosi, J.; Marcolin, M.A.; Yeng, L.T.; Brunoni, A.R.; Teixeira, M.J.; Galhardoni, R.; et al. Long-term deep-TMS does not negatively affect cognitive functions in stroke and spinal cord injury patients with central neuropathic pain. BMC Neurol. 2019, 19, 319. [Google Scholar] [CrossRef]
- Battaglia, S.; Thayer, J.F. Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing cardiac autonomic dynamics of fear learning in humans. Psychophysiology 2022, e14122. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Crosstalk between Depression, Anxiety, and Dementia: Comorbidity in Behavioral Neurology and Neuropsychiatry. Biomedicines 2021, 9, 517. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Monitoring the kynurenine system: Concentrations, ratios or what else? Adv. Clin. Exp. Med. 2021, 30, 775–778. [Google Scholar] [CrossRef]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10, 849. [Google Scholar] [CrossRef]
- Ford, P.A.; Jaceldo-Siegl, K.; Lee, J.W.; Tonstad, S. Trans fatty acid intake is related to emotional affect in the Adventist Health Study-2. Nutr. Res. 2016, 36, 509–517. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Ren, M.-X.; Li, J.; Su, T.; Chen, S.-Y.; Shao, Y.; Lv, Y.-L. Resting-State Functional Magnetic Resonance Imaging and Functional Connectivity Density Mapping in Patients with Optic Neuritis. Front. Neurosci. 2021, 15, 718973. [Google Scholar] [CrossRef] [PubMed]
- Bassi, M.S.; Gilio, L.; Maffei, P.; Dolcetti, E.; Bruno, A.; Buttari, F.; Centonze, D.; Iezzi, E. Exploiting the Multifaceted Effects of Cannabinoids on Mood to Boost Their Therapeutic Use Against Anxiety and Depression. Front. Mol. Neurosci. 2018, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Rana, T.; Behl, T.; Sehgal, A.; Mehta, V.; Singh, S.; Kumar, R.; Bungau, S. Integrating Endocannabinoid Signalling in Depression. J. Mol. Neurosci. 2021, 71, 2022–2034. [Google Scholar] [CrossRef]
- Rocha, L.; Cinar, R.; Guevara-Guzmán, R.; Alonso-Vanegas, M.; San-Juan, D.; Martínez-Juárez, I.E.; Castañeda-Cabral, J.L.; Carmona-Cruz, F. Endocannabinoid System and Cannabinoid 1 Receptors in Patients with Pharmacoresistant Temporal Lobe Epilepsy and Comorbid Mood Disorders. Front. Behav. Neurosci. 2020, 14, 52. [Google Scholar] [CrossRef]
- Ettaro, R.; Laudermilk, L.; Clark, S.D.; Maitra, R. Behavioral assessment of rimonabant under acute and chronic conditions. Behav. Brain. Res. 2020, 390, 112697. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Thomas, B.F.; Zhang, Y. Overcoming the Psychiatric Side Effects of the Cannabinoid CB1 Receptor Antagonists: Current Approaches for Therapeutics Development. Curr. Top. Med. Chem. 2019, 19, 1418–1435. [Google Scholar] [CrossRef]
- Towe, S.L.; Meade, C.S.; Cloak, C.C.; Bell, R.P.; Baptiste, J.; Chang, L. Reciprocal Influences of HIV and Cannabinoids on the Brain and Cognitive Function. J. Neuroimmune Pharmacol. 2020, 15, 765–779. [Google Scholar] [CrossRef]
- Avraham, H.K.; Jiang, S.; Fu, Y.; Rockenstein, E.; Makriyannis, A.; Wood, J.; Wang, L.; Masliah, E.; Avraham, S. Impaired neurogenesis by HIV-1-Gp120 is rescued by genetic deletion of fatty acid amide hydrolase enzyme. Br. J. Pharmacol. 2015, 172, 4603–4614. [Google Scholar] [CrossRef]
- Starr, A.; Jordan-Sciutto, K.L.; Mironets, E. Confound, Cause, or Cure: The Effect of Cannabinoids on HIV-Associated Neurological Sequelae. Viruses 2021, 13, 1242. [Google Scholar] [CrossRef]
- Niphakis, M.J.; Lum, K.M.; Cognetta, A.B., III; Correia, B.E.; Ichu, T.-A.; Olucha, J.; Brown, S.J.; Kundu, S.; Piscitelli, F.; Rosen, H.; et al. A Global Map of Lipid-Binding Proteins and Their Ligandability in Cells. Cell 2015, 161, 1668–1680. [Google Scholar] [CrossRef]
- Hermes, D.J.; Yadav-Samudrala, B.J.; Xu, C.; Piscitelli, F.; Di Marzo, V.; Maradonna, F.; Calduch-Giner, J.; Pérez-Sánchez, J.; Carnevali, O. GPR18 drives FAAH inhibition-induced neuroprotection against HIV-1 Tat-induced neurodegeneration. Exp. Neurol. 2021, 341, 113699. [Google Scholar] [CrossRef] [PubMed]
- Forner-Piquer, I.; Fakriadis, I.; Mylonas, C.C.; Sui, H.; Zhang, L.; Zhao, C.; Lu, X. Effects of Dietary Bisphenol A on the Reproductive Function of Gilthead Sea Bream (Sparus aurata) Testes. Int. J. Mol. Sci. 2019, 20, 5003. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhou, W.; Zhan, L.; Sui, H.; Zhang, L.; Zhao, C.; Lu, X. The ZiBuPiYin recipe regulates proteomic alterations in brain mitochondria-associated ER membranes caused by chronic psychological stress exposure: Implications for cognitive decline in Zucker diabetic fatty rats. Aging 2020, 12, 23698–23726. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-C.; Yu, W.-W.; Qi, Y.-J.; Xu, L.-F.; Wang, Z.-R.; Qiang, Y.-W.; Ma, F.; Ma, X.-L. Quantitative proteomic analysis reveals that Luks-PV exerts antitumor activity by regulating the key proteins and metabolic pathways in HepG2 cells. Anticancer Drugs. 2020, 31, 223–230. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).