A Comprehensive Neuropsychological Study of Familial Hypercholesterolemia and Its Relationship with Psychosocial Functioning: A Biopsychosocial Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Setting
2.2. Diagnosis of Familial Hypercholesterolemia
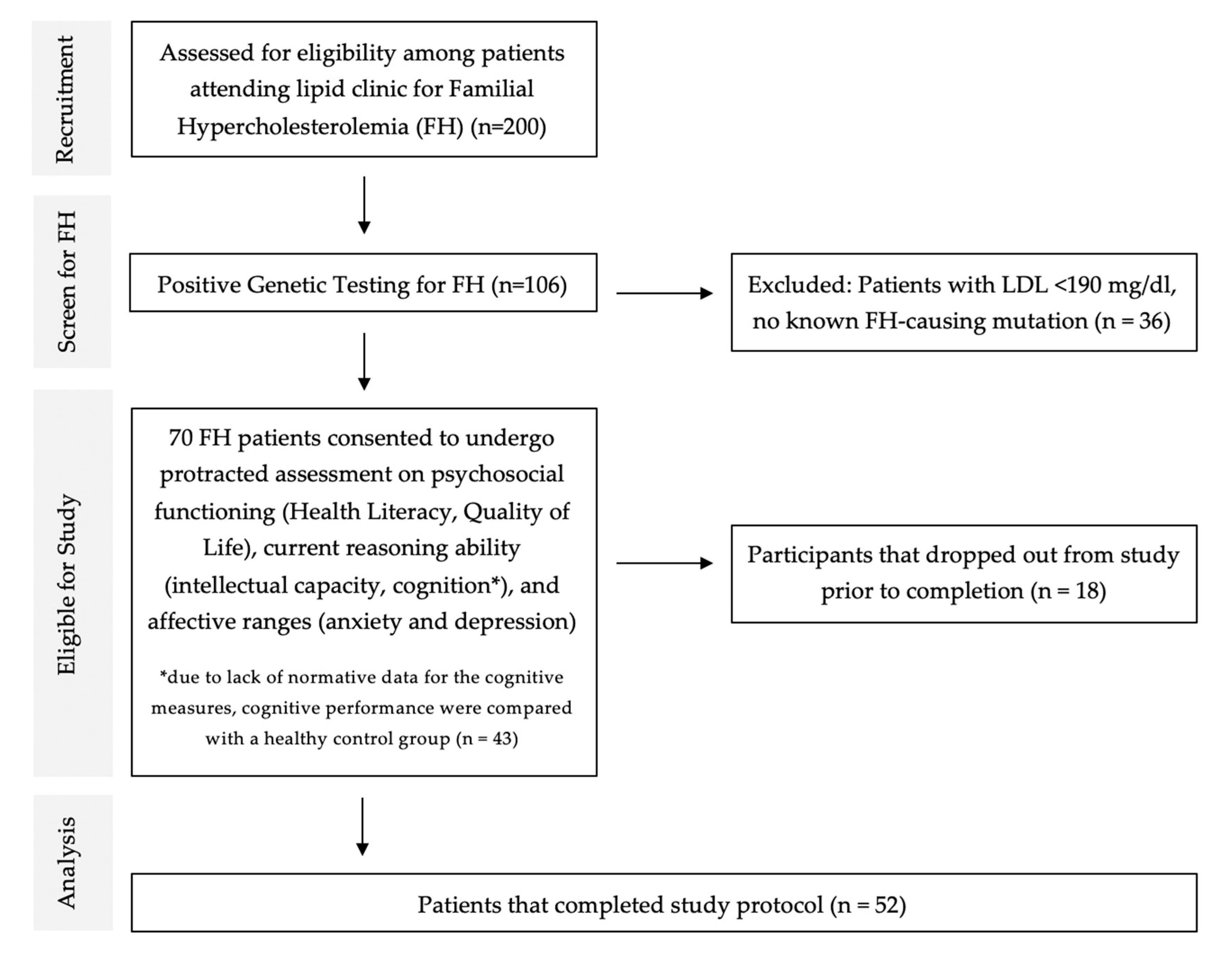
2.3. Inclusion/Exclusion Criteria
2.4. Control Group
2.5. Ethical Consideration
2.6. Outcome Measures
2.6.1. Psychosocial Functioning
- Health Literacy:
- Quality of Life:
2.6.2. Intellectual Ability and Cognition
- Raven’s Progressive Matrices:
- Attention and Concentration:
- Learning and Remembering—Memory:
- Executive functioning:
- Verbal Fluency Test:
- Trail Making Test:
2.6.3. Affective Range
2.7. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Aim 1: To Examine the Psychosocial Status of Patients with FH
3.3. Aim 2: To Compare the Intellectual Capacity and Cognitive Outcomes with the Reference Group
3.4. Aim 2: To Compare the Intellectual Capacity and Cognitive Outcomes with the Reference Group
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, P.; Dharmayat, K.I.; Stevens, C.A.; Sharabiani, M.T.; Jones, R.S.; Watts, G.F.; Genest, J.; Ray, K.; Vallejo-Vaz, A.J. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients with Atherosclerotic Cardiovascular Disease: A systematic review and meta-analysis. Circulation 2020, 141, 1742–1759. [Google Scholar] [CrossRef] [PubMed]
- EAS Familial Hypercholesterolaemia Studies Collaboration; Vallejo-Vaz, A.J.; De Marco, M.; Stevens, C.A.; Akram, A.; Freiberger, T.; Hovingh, G.K.; Kastelein, J.J.; Mata, P.; Raal, F.J.; et al. Overview of the current status of familial hypercholesterolaemia care in over 60 countries—The EAS Familial Hypercholesterolaemia Studies Collaboration (FHSC). Atherosclerosis 2018, 277, 234–255. [Google Scholar] [CrossRef]
- Ariza, M.; Cuenca, N.; Mauri, M.; Jurado, M.A.; Garolera, M. Neuropsychological performance of young familial hypercholesterolemia patients. Eur. J. Intern. Med. 2016, 34, e29–e31. [Google Scholar] [CrossRef]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; Melo, H.M.; Lopes, S.C.; Ribeiro, C.T.; Delanogare, E.; Moreira, J.C.F.; Gelain, D.P.; Prediger, R.D.; et al. LDL Receptor Deficiency Does Not Alter Brain Amyloid-β Levels but Causes an Exacerbation of Apoptosis. J. Alzheimer Dis. 2020, 73, 585–596. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.; Engel, D.F.; de Paula, G.C.; dos Santos, D.B.; Lopes, J.B.; Farina, M.; Moreira, E.L.; de Bem, A.F. High Cholesterol Diet Exacerbates Blood-Brain Barrier Disruption in LDLr–/– Mice: Impact on Cognitive Function. J. Alzheimer Dis. 2020, 78, 97–115. [Google Scholar] [CrossRef]
- de Bem, A.F.; Krolow, R.; Farias, H.R.; de Rezende, V.L.; Gelain, D.P.; Moreira, J.C.F.; Duarte, J.M.d.N.; de Oliveira, J. Animal Models of Metabolic Disorders in the Study of Neurodegenerative Diseases: An Overview. Front. Neurosci. 2021, 14, 604150. [Google Scholar] [CrossRef]
- Zambón, D.; Quintana, M.; Mata, P.; Alonso, R.; Benavent, J.; Cruz-Sánchez, F.; Gich, J.; Pocoví, M.; Civeira, F.; Capurro, S.; et al. Higher Incidence of Mild Cognitive Impairment in Familial Hypercholesterolemia. Am. J. Med. 2010, 123, 267–274. [Google Scholar] [CrossRef]
- Bagnasco, M.S. Psychological issues and cognitive impairment in adults with familial hypercholesterolemia. Fam. Pract. 2017, 34, 520–524. [Google Scholar] [CrossRef]
- Lopes, J.B.; De Oliveira, J.; Engel, D.; De Paula, G.C.; Moreira, E.; de Bem, A.F. Efficacy of Donepezil for Cognitive Impairments in Familial Hypercholesterolemia: Preclinical Proof of Concept. CNS Neurosci. Ther. 2015, 21, 964–966. [Google Scholar] [CrossRef]
- Hyttinen, L.; Tuulio-Henriksson, A.; Vuorio, A.F.; Kuosmanen, N.; Härkänen, T.; Koskinen, S.; Strandberg, T.E. Long-Term Statin Therapy is Associated with Better Episodic Memory in Aged Familial Hypercholesterolemia Patients in Comparison with Population Controls. J. Alzheimer Dis. 2010, 21, 611–617. [Google Scholar] [CrossRef]
- Shepardson, N.E.; Shankar, G.M.; Selkoe, D.J. Cholesterol Level and Statin Use in Alzheimer Disease: I. Review of epidemiological and preclinical studies. Arch. Neurol. 2011, 68, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.; Igland, J.; Mundal, L.J.; Urke, E.B.; Krogh, H.W.; Holven, K.B.; Bogsrud, M.P.; Leren, T.P.; Retterstøl, K. No increased risk of cognitive impairment in familial hypercholesterolemia compared with controls. Atherosclerosis 2020, 315, e40. [Google Scholar] [CrossRef]
- Todate, Y.; Uwano, I.; Yashiro, S.; Chida, A.; Hasegawa, Y.; Oda, T.; Nagasawa, K.; Honma, H.; Sasaki, M.; Ishigaki, Y. High Prevalence of Cerebral Small Vessel Disease on 7T Magnetic Resonance Imaging in Familial Hypercholesterolemia. J. Atheroscler. Thromb. 2019, 26, 1045–1053. [Google Scholar] [CrossRef]
- Mata, N.; Alonso, R.; Banegas, J.R.; Zambón, D.; Brea, A.; Mata, P. Quality of life in a cohort of familial hypercholesterolemia patients from the south of Europe. Eur. J. Public Health 2014, 24, 221–225. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hollman, G.; Gullberg, M.; Ek, A.-C.; Eriksson, M.; Olsson, A.G. Quality of life in patients with familial hypercholesterolaemia. J. Intern. Med. 2002, 251, 331–337. [Google Scholar] [CrossRef]
- Mortensen, G.L.; Madsen, I.B.; Kruse, C.; Bundgaard, H. Familial hypercholesterolaemia reduces the quality of life of patients not reaching treatment targets. Dan. Med. J. 2016, 63, A5224. [Google Scholar]
- Akioyamen, L.E.; Genest, J.; Shan, S.D.; Inibhunu, H.; Chu, A.; Tu, J.V. Anxiety, depression, and health-related quality of life in heterozygous familial hypercholesterolemia: A systematic review and meta-analysis. J. Psychosom. Res. 2018, 109, 32–43. [Google Scholar] [CrossRef]
- Millan, M.J.; Agid, Y.; Brüne, M.; Bullmore, E.; Carter, C.S.; Clayton, N.; Connor, R.; Davis, S.; Deakin, J.; DeRubeis, R.; et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012, 11, 141–168. [Google Scholar] [CrossRef]
- Chew, L.D.; Bradley, K.A.; Boyko, E.J. Brief questions to identify patients with inadequate health literacy. Fam. Med. 2004, 36, 588–594. [Google Scholar]
- Baker, D.W. The meaning and the measure of health literacy. J. Gen. Intern. Med. 2006, 21, 878–883. [Google Scholar] [CrossRef]
- Nam, H.; Yoon, J. Linking Health Literacy to Self-Care in Hypertensive Patients with Physical Disabilities: A Path Analysis Using a Multi-Mediation Model. Int. J. Environ. Res. Public Health 2021, 18, 3363. [Google Scholar] [CrossRef] [PubMed]
- Chew, L.D.; Griffin, J.M.; Partin, M.R.; Noorbaloochi, S.; Grill, J.P.; Snyder, A.; Bradley, K.A.; Nugent, S.M.; Baines, A.D.; VanRyn, M. Validation of Screening Questions for Limited Health Literacy in a Large VA Outpatient Population. J. Gen. Intern. Med. 2008, 23, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Megwalu, U.C.; Lee, J.Y. Health Literacy Assessment in an Otolaryngology Clinic Population. Otolaryngol. Head Neck Surg. 2016, 155, 969–973. [Google Scholar] [CrossRef]
- Baker, D.W.; Wolf, M.S.; Feinglass, J.; Thompson, J.A. Health Literacy, Cognitive Abilities, and Mortality among Elderly Persons. J. Gen. Intern. Med. 2008, 23, 723–726. [Google Scholar] [CrossRef]
- Gupta, V.; Shivaprakash, G.; Bhattacherjee, D.; Udupa, K.; Poojar, B.; Sori, R.; Mishra, S. Association of health literacy and cognition levels with severity of adverse drug reactions in cancer patients: A South Asian experience. Int. J. Clin. Pharm. 2020, 42, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Levinthal, B.R.; Morrow, D.G.; Tu, W.; Wu, J.; Murray, M.D. Cognition and Health Literacy in Patients with Hypertension. J. Gen. Intern. Med. 2008, 23, 1172–1176. [Google Scholar] [CrossRef]
- Segall, A. Cultural Factors in Sick-Role Expectations. In Health Behavior; Springer: Boston, MA, USA, 1988; pp. 249–260. [Google Scholar]
- Shilling, C. Culture, the ‘sick role’ and the consumption of health. Br. J. Sociol. 2002, 53, 621–638. [Google Scholar] [CrossRef]
- Al-Lawati, J.; Al-Lawati, N.; Al-Siddiqui, M.; Antony, S.X.; Al-Naamani, A.; Martin, R.G.; Kolbe, R.; Theodorsson, T.; Osman, Y.; Al-Hussainim, A.A.; et al. Psychological morbidity in primary health care in Oman: A preliminary study. Sultan Qaboos Univ. Med. J. 2000, 2, 105–110. [Google Scholar]
- Representatives of the Global Familial Hypercholesterolemia Community; Wilemon, K.A.; Patel, J.; Aguilar-Salinas, C.; Ahmed, C.D.; Alkhnifsawi, M.; Almahmeed, W.; Alonso, R.; Al-Rasadi, K.; Badimon, L.; et al. Reducing the Clinical and Public Health Burden of Familial Hypercholesterolemia: A Global Call to Action. JAMA Cardiol. 2020, 5, 217–229. [Google Scholar] [CrossRef]
- Al-Waili, K.; Al-Rasadi, K.; Zadjali, F.; Al-Hashmi, K.; Al-Mukhaini, S.; Al-Kindi, M.; Al-Sabti, H.; Al-Hinai, A.T.; Farhan, H.; Al-Zakwani, I. Clinical and Genetic Characteristics of Familial Hypercholesterolemia at Sultan Qaboos University Hospital in Oman. Oman Med J. 2020, 35, e141. [Google Scholar] [CrossRef]
- Amerizadeh, A.; Javanmard, S.H.; Sarrafzadegan, N.; Vaseghi, G. Familial Hypercholesterolemia (FH) Registry Worldwide: A Systematic Review. Curr. Probl. Cardiol. 2021, 100999. [Google Scholar] [CrossRef] [PubMed]
- Alkhunizan, M.; Alkhenizan, A.; Basudan, L. Prevalence of Mild Cognitive Impairment and Dementia in Saudi Arabia: A Community-Based Study. Dement. Geriatr. Cogn. Disord. Extra 2018, 8, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Al-Rasadi, K.; Al-Zakwani, I.; Alsheikh-Ali, A.A.; Almahmeed, W.; Rashed, W.; Ridha, M.; Santos, R.D.; Zubaid, M. Prevalence, management, and outcomes of familial hypercholesterolemia in patients with acute coronary syndromes in the Arabian Gulf. J. Clin. Lipidol. 2018, 12, 685–692.e2. [Google Scholar] [CrossRef] [PubMed]
- Al-Adawi, S.; Al-Naamani, A.; Jaju, S.; Al-Farsi, Y.M.; Dorvlo, A.S.S.; Al-Maashani, A.; Al-Adawi, S.S.H.; Moustafa, A.A.; Al-Sibani, N.; Essa, M.M.; et al. Methylphenidate improves executive functions in patients with traumatic brain injuries: A feasibility trial via the idiographic approach. BMC Neurol. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Monastero, R.; Monastero, G.; Ciaccio, C.; Padovani, A.; Camarda, R. Cognitive deficits in beta-thalassemia major. Acta Neurol. Scand. 2000, 102, 162–168. [Google Scholar] [CrossRef]
- Duman, O.; Arayici, S.; Fettahoglu, C.; Eryilmaz, N.; Ozkaynak, S.; Yesilipek, A.; Hazar, V. Neurocognitive func-tion in patients with β-thalassemia major. Pediatrics Int. 2011, 53, 519–523. [Google Scholar] [CrossRef]
- Hagger, M.S.; Hardcastle, S.J.; Hu, M.; Kwok, S.; Lin, J.; Nawawi, H.; Pang, J.; Santos, R.D.; Soran, H.; Su, T.-C.; et al. Health literacy in familial hypercholesterolemia: A cross-national study. Eur. J. Prev. Cardiol. 2018, 25, 936–943. [Google Scholar] [CrossRef]
- Dardas, L.A.; Ahmad, M.M. Validation of the World Health Organization’s Quality of Life Questionnaire with Parents of Children with Autistic Disorder. J. Autism Dev. Disord. 2014, 44, 2257–2263. [Google Scholar] [CrossRef]
- Al-Farsi, O.A.; Al-Farsi, Y.M.; Al-Sharbati, M.M.; Al-Adawi, S.; Cucchi, A.; Essa, M.M.; Qoronfleh, M.W. Quality of life of caregivers of autism spectrum disorder, intellectual disability and typically developing children: A comparison study. Appl. Res. Qual. Life 2020, 17, 129–145. [Google Scholar] [CrossRef]
- Silva, P.A.B.; Soares, S.M.; Santos, J.F.G.; Silva, L.B. Cut-off point for WHOQOL-bref as a measure of quality of life of older adults. Rev. Saude. Publica. 2014, 48, 390–397. [Google Scholar] [CrossRef]
- Raven, J. The Raven’s Progressive Matrices: Change and Stability over Culture and Time. Cogn. Psychol. 2000, 41, 1–48. [Google Scholar] [CrossRef] [PubMed]
- Grizzle, R. Wechsler Intelligence Scale for Children, Fourth Edition. In Encyclopedia of Child Behavior and Development; Goldstein, S., Naglieri, J.A., Eds.; Springer: Boston, MA, USA, 2011. [Google Scholar] [CrossRef]
- Monaco, M.; Costa, A.; Caltagirone, C.; Carlesimo, G.A. Forward and backward span for verbal and visuo-spatial data: Standardization and normative data from an Italian adult population. Neurol. Sci. 2013, 34, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Yi, A. California Verbal Learning Test (California Verbal Learning Test-II). In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011. [Google Scholar] [CrossRef]
- Ruff, R.M.; Light, R.H.; Parker, S.B.; Levin, H.S. Benton controlled oral word association test: Reliability and updated norms. Arch. Clin. Neuropsychol. 1996, 11, 329–338. [Google Scholar] [CrossRef]
- Arnett, J.A.; Labovitz, S.S. Effect of physical layout in performance of the Trail Making Test. Psychol. Assess. 1995, 7, 220–221. [Google Scholar] [CrossRef]
- Al-Ghatani, A.M.; Obonsawin, M.; Al Moutaery, K.R. Normative data for the two equivalent forms of the Arabic verbal fluency test. Pan Arab. J. Neurosurg. 2009, 13, 57–65. [Google Scholar]
- Razzak, R.A. A Preliminary Study on the Trail-Making Test in Arabic–English Bilingual Young Adults. Appl. Neuropsychol. Adult 2013, 20, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Al-Asmi, A.; Al-Rawahi, S.; Al-Moqbali, Z.S.; Alfarsi, Y.M.; Essa, M.M.; El-Bouri, M.; Koshy, R.P.; Gujjar, A.R.; Jacob, P.C.; Al-Hodar, A.; et al. Magnitude and concurrence of anxiety and depression among attendees with multiple sclerosis at a tertiary care Hospital in Oman. BMC Neurol. 2015, 15, 131. [Google Scholar] [CrossRef]
- Santos, R.D.; Maranhao, R.C. What is new in familial hypercholesterolemia? Curr. Opin. Lipidol. 2014, 25, 183–188. [Google Scholar] [CrossRef]
- Beheshti, S.O.; Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Worldwide Prevalence of Familial Hypercholesterolemia: Meta-Analyses of 11 Million Subjects. J. Am. Coll. Cardiol. 2020, 75, 2553–2566. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Familial Hypercholesterolemia: A Genetic Defect in the Low-Density Lipoprotein Receptor. N. Engl. J. Med. 1976, 294, 1386–1390. [Google Scholar] [CrossRef]
- Soutar, A.K.; Naoumova, R.P. Mechanisms of Disease: Genetic causes of familial hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 2007, 4, 214–225. [Google Scholar] [CrossRef] [PubMed]
- Frich, J.C.; Ose, L.; Malterud, K.; Fugelli, P. Perceived Vulnerability to Heart Disease in Patients with Familial Hypercholesterolemia: A Qualitative Interview Study. Ann. Fam. Med. 2006, 4, 198–204. [Google Scholar] [CrossRef]
- Tonstad, S.; Nøvik, T.S.; Vandvik, I.H. Psychosocial function during treatment for familial hypercholesterolemia. Pediatrics 1996, 98, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hollman, G.; Olsson, A.G.; Ek, A.-C. Familial hypercholesterolaemia and quality of life in family members. Prev. Med. 2003, 36, 569–574. [Google Scholar] [CrossRef]
- Kayikcioglu, M.; Kuman-Tunçel, O.; Pirildar, S.; Yílmaz, M.; Kaynar, L.; Aktan, M.; Durmus, R.B.; Gökçe, C.; Temizhan, A.; Özcebe, O.I.; et al. Clinical management, psychosocial characteristics, and quality of life in patients with homozygous familial hypercholesterolemia undergoing LDL-apheresis in Turkey: Results of a nationwide survey (A-HIT1 registry). J. Clin. Lipidol. 2019, 13, 455–467. [Google Scholar] [CrossRef]
- Violán, C.; Foguet-Boreu, Q.; Flores-Mateo, G.; Salisbury, C.; Blom, J.; Freitag, M.; Glynn, L.; Muth, C.; Valderas, J.M. Prevalence, Determinants and Patterns of Multimorbidity in Primary Care: A Systematic Review of Observational Studies. PLoS ONE 2014, 9, e102149. [Google Scholar] [CrossRef]
- Hajat, C.; Stein, E. The global burden of multiple chronic conditions: A narrative review. Prev. Med. Rep. 2018, 12, 284–293. [Google Scholar] [CrossRef]
- Tsoukas, H. The Validity of Idiographic Research Explanations. Acad. Manag. Rev. 1989, 14, 551–561. [Google Scholar] [CrossRef]
- De Jongh, S.; Kerckhoffs, M.C.; Grootenhuis, M.A.; Bakker, H.D.; Heymans, H.S.; Last, B.F. Quality of life, anxiety and concerns among statin-treated children with familial hypercholesterolaemia and their parents. Acta Paediatr. 2003, 92, 1096–1101. [Google Scholar] [CrossRef]
- Tunçel, Ö.K.; Kayıkçıoğlu, M.; Pırıldar, Ş.; Yılmaz, M.; Kaynar, L.; Aktan, M.; Durmuş, R.B.; Gökçe, C.; Temizhan, A.; Özcebe, O.İ.; et al. Mental status and physical activity in patients with homozygous familial hypercholesterolemia: A subgroup analysis of a nationwide survey (A-HIT1 registry). J. Clin. Lipidol. 2020, 14, 361–370.e2. [Google Scholar] [CrossRef] [PubMed]
- Chang, N.-T.; Su, T.-C. Investigating the association between familial hypercholesterolemia and perceived depression. Atheroscler. Suppl. 2019, 36, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Patel, V.; Saxena, S.; Maj, M.; Maselko, J.; Phillips, M.R.; Rahman, A. No health without mental health. Lancet 2007, 370, 859–877. [Google Scholar] [CrossRef]
- Lichtman, J.H.; Froelicher, E.S.; Blumenthal, J.A.; Carney, R.M.; Doering, L.V.; Frasure-Smith, N.; Freedland, K.E.; Jaffe, A.S.; Leifheit-Limson, E.C.; Sheps, D.S.; et al. Depression as a Risk Factor for Poor Prognosis Among Patients with Acute Coronary Syndrome: Systematic Review and Recommendations: A scientific statement from the American Heart Association. Circulation 2014, 129, 1350–1369. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Khalek, A.M.; Lynn, R. Norms for Intelligence assessed by the Standard Progressive Matrices in Oman. Mank. Q. 2010, 51, 78–83. [Google Scholar] [CrossRef]
- Wicking, M.; Nees, F.; Steiger, F. Neuropsychological Measures of Hippocampal Function. Front. Neurol. Neurosci. 2014, 34, 60–70. [Google Scholar] [CrossRef]
- Mauri, M.; Cuenca, N.; Borrallo, R.; Romero, E.; Ottino, J.; García-García, I.; Jurado, M.; Garolera, M. Episodic memory performance in young adults with familial hypercholesterolemia. Atherosclerosis 2016, 252, e32. [Google Scholar] [CrossRef]
- Skorve, E.; Lundervold, A.J.; Torkildsen, Ø.; Myhr, K.-M. A two-year longitudinal follow-up of cognitive performance assessed by BICAMS in newly diagnosed patients with MS. Mult. Scler. Relat. Disord. 2020, 46, 102577. [Google Scholar] [CrossRef]
- Hammar, A.; Ardal, G. Cognitive functioning in major depression—A summary. Front. Hum. Neurosci. 2009, 3, 26. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services. Health Resources and Services Administration. Health Literacy. Available online: https://www.hrsa.gov/about/organization/bureaus/ohe/health-literacy/index.html (accessed on 11 July 2022).
- Shaw, S.J.; Huebner, C.; Armin, J.; Orzech, K.; Vivian, J. The Role of Culture in Health Literacy and Chronic Disease Screening and Management. J. Immigr. Minor. Health 2009, 11, 460–467. [Google Scholar] [CrossRef]
- Al-Saadoon, M.; Al-Adawi, M.; Al-Adawi, S. Socio-Cultural Constraints in Protecting Child Rights in a Society in Transition: A Review and Synthesis from Oman. Child Indic. Res. 2021, 14, 239–267. [Google Scholar] [CrossRef] [PubMed]
- Marvanova, M.; Roumie, C.L.; Eden, S.K.; Cawthon, C.; Schnipper, J.L.; Kripalani, S. Health literacy and medication understanding among hospitalized adults. J. Hosp. Med. 2011, 6, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.V.; Baddeley, A. Raven’s Matrices and Working Memory: A Dual-Task Approach. Q. J. Exp. Psychol. 2013, 66, 1881–1887. [Google Scholar] [CrossRef] [PubMed]
- Little, D.R.; Lewandowsky, S.; Craig, S. Working memory capacity and fluid abilities: The more difficult the item, the more more is better. Front. Psychol. 2014, 5, 239. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Daseking, M.; Gawrilow, C.; Karbach, J.; Kerner Auch Koerner, J. Self-regulation in preschool: Are executive function and effortful control overlapping constructs? Dev. Sci. 2022, e13272. [Google Scholar] [CrossRef]
| Characteristics/Demographic | FH (N = 52) | Healthy Control (N = 43) | Statistics (p-Value) | |
|---|---|---|---|---|
| Sex | Male | 27 (51.9) | 17 (39.5) | 1.45 a (0.228) |
| Female | 25 (48.1) | 26 (60.5) | ||
| Marital status | Married | 43 (82.7) | 18 (41.9) | 17.08 a (<0.001 **) |
| Single | 9 (17.3) | 25 (58.1) | ||
| Age (years) | Mean ± SD | 37.2 ± 9.2 | 39.1 ± 5.8 | 1.25 b (0.216) |
| Median [Range] | 38.0 [21.0–52.0] | 40.0 [29.0–56.0] | ||
| Education level | High school/below | 26 (50.0) | 19 (44.2) | 0.32 a (0.572) |
| University/above | 26 (50.0) | 24 (55.8) | ||
| Socio-economic status (Family income) | High | 5 (9.6) | 7 (16.3) | 2.99 a (0.224) |
| Middle | 41 (78.8) | 27 (62.8) | ||
| Low | 6 (11.5) | 9 (20.9) | ||
| Cardiovascular disease | Yes | 8 (15.4) | NA | |
| No | 44 (84.6) | NA | ||
| Health Literacy (HL) Health Communication Questionnaire | Inadequate (≤9) | 18 (34.6) | NA | |
| Adequate | 34 (65.4) | NA | ||
| Quality of Life (QoL) WHO Quality of Life-BREF | Physical (Inadequate) | 12 (23.1) | NA | |
| Psychological (Inadequate) | 10 (19.2) | NA | ||
| Social Relationships (Inadequate) | 14 (26.9) | NA | ||
| Environmental (Inadequate) | 4 (7.7) | NA | ||
| General health (Inadequate) | 11 (21.2) | NA | ||
| General QoL (Inadequate) | 7 (13.5) | NA | ||
| Intellectual and Cognitive Functioning | FH (N = 52) | Healthy Control (N = 43) | Univariate | |
|---|---|---|---|---|
| n (%) | n (%) | Statistics (p-Value) | ||
| Current Reasoning Ability (Raven’s Progressive Matrices) | Mean ± SD | 31.8 ± 10.8 | 36.7 ± 8.9 | 2.37 a (0.020 *) |
| Median [Range] | 25.0 [24.0–75.0] | 36.0 [25.0–75.0] | ||
| Attention and Concentration Digit Span | Mean ± SD | 16.8 ± 2.6 | 18.8 ± 2.5 | 3.94 a (<0.001 **) |
| Median [Range] | 18.0 [12.0–23.0] | 18.0 [12.0–24.0] | ||
| Memory (Long Delay Free Recall) California Verbal Learning Test | Mean ± SD | 8.9 ± 2.5 | 11.4 ± 3.1 | 4.52 a (<0.001 **) |
| Median [Range] | 9.0 [5.0–15.0] | 11.0 [4.0–20.0] | ||
| Executive Functioning Verbal Fluency Test | Mean ± SD | 16.0 ± 4.7 | 19.2 ± 2.5 | 4.23 a (<0.001 **) |
| Median [Range] | 16.0 [5.0–27.0] | 19.0 [15.0–25.0] | ||
| Executive Functioning Trail Making Test | Mean ± SD | 217.7 ± 70.5 | 97.0 ± 24.1 | 11.45 a (<0.001 **) |
| Median [Range] | 240.0 [79.0–301.0] | 92.5 [76.0–235.0] | ||
| Hospital Anxiety and Depression Scale-Anxiety | Yes (≥8) No | 17 (32.7) 35 (67.3) | 23 (53.5) 20 (46.5) | 4.18 b (0.041 *) |
| Hospital Anxiety and Depression Scale-Depression | Yes (≥8) No | 13 (25.0) 39 (75.0) | 8 (18.6) 35 (81.4) | 0.56 b (0.455) |
| Intellectual and Cognitive Functioning | Univariate | |||
|---|---|---|---|---|
| Inadequate HL (N = 18) | Adequate HL (N = 34) | Statistics a (p-Value) | ||
| Current Reasoning (Raven’s Progressive Matrices) | Mean ± SD | 32.8 ± 9.6 | 31.3 ± 11.5 | 244.50 (0.196) |
| Median [Range] | 30.0 [25.0–60.0] | 25.0 [24.0–75.0] | ||
| Attention and Concentration Digit Span | Mean ± SD | 15.6 ± 2.7 | 17.4 ± 2.4 | 186.00 (0.017 *) |
| Median [Range] | 16.0 [12.0–21.0] | 18.0 [12.0–23.0] | ||
| Memory (Long Delay Free Recall) California Verbal Learning Test | Mean ± SD | 8.6 ± 2.3 | 9.0 ± 2.6 | 271.00 (0.495) |
| Median [Range] | 8.0 [6.0–14.0] | 9.0 [5.0–15.0] | ||
| Executive Functioning Verbal Fluency Test | Mean ± SD | 15.2 ± 4.2 | 16.4 ± 4.9 | 252.50 (0.301) |
| Median [Range] | 15.0 [7.0–21.0] | 16.5 [5.0–27.0] | ||
| Executive Functioning Trail Making Test | Mean ± SD | 215.2 ± 75.4 | 218.9 ± 69.0 | 277.50 (0.818) |
| Median [Range] | 240 [102.0–301.0] | 238.0 [79.0–301.0] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chan, M.F.; Ganesh, A.; Mahadevan, S.; Shamli, S.A.; Al-Waili, K.; Al-Mukhaini, S.; Al-Rasadi, K.; Al-Adawi, S. A Comprehensive Neuropsychological Study of Familial Hypercholesterolemia and Its Relationship with Psychosocial Functioning: A Biopsychosocial Approach. Brain Sci. 2022, 12, 1127. https://doi.org/10.3390/brainsci12091127
Chan MF, Ganesh A, Mahadevan S, Shamli SA, Al-Waili K, Al-Mukhaini S, Al-Rasadi K, Al-Adawi S. A Comprehensive Neuropsychological Study of Familial Hypercholesterolemia and Its Relationship with Psychosocial Functioning: A Biopsychosocial Approach. Brain Sciences. 2022; 12(9):1127. https://doi.org/10.3390/brainsci12091127
Chicago/Turabian StyleChan, Moon Fai, Aishwarya Ganesh, Sangeetha Mahadevan, Siham Al Shamli, Khalid Al-Waili, Suad Al-Mukhaini, Khalid Al-Rasadi, and Samir Al-Adawi. 2022. "A Comprehensive Neuropsychological Study of Familial Hypercholesterolemia and Its Relationship with Psychosocial Functioning: A Biopsychosocial Approach" Brain Sciences 12, no. 9: 1127. https://doi.org/10.3390/brainsci12091127
APA StyleChan, M. F., Ganesh, A., Mahadevan, S., Shamli, S. A., Al-Waili, K., Al-Mukhaini, S., Al-Rasadi, K., & Al-Adawi, S. (2022). A Comprehensive Neuropsychological Study of Familial Hypercholesterolemia and Its Relationship with Psychosocial Functioning: A Biopsychosocial Approach. Brain Sciences, 12(9), 1127. https://doi.org/10.3390/brainsci12091127









