Intrinsic Network Changes in Bilateral Tinnitus Patients with Cognitive Impairment: A Resting-State Functional MRI Study
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Audiological and Psychoacoustic Evaluation
2.3. Image Acquisition
2.4. Data Preprocessing
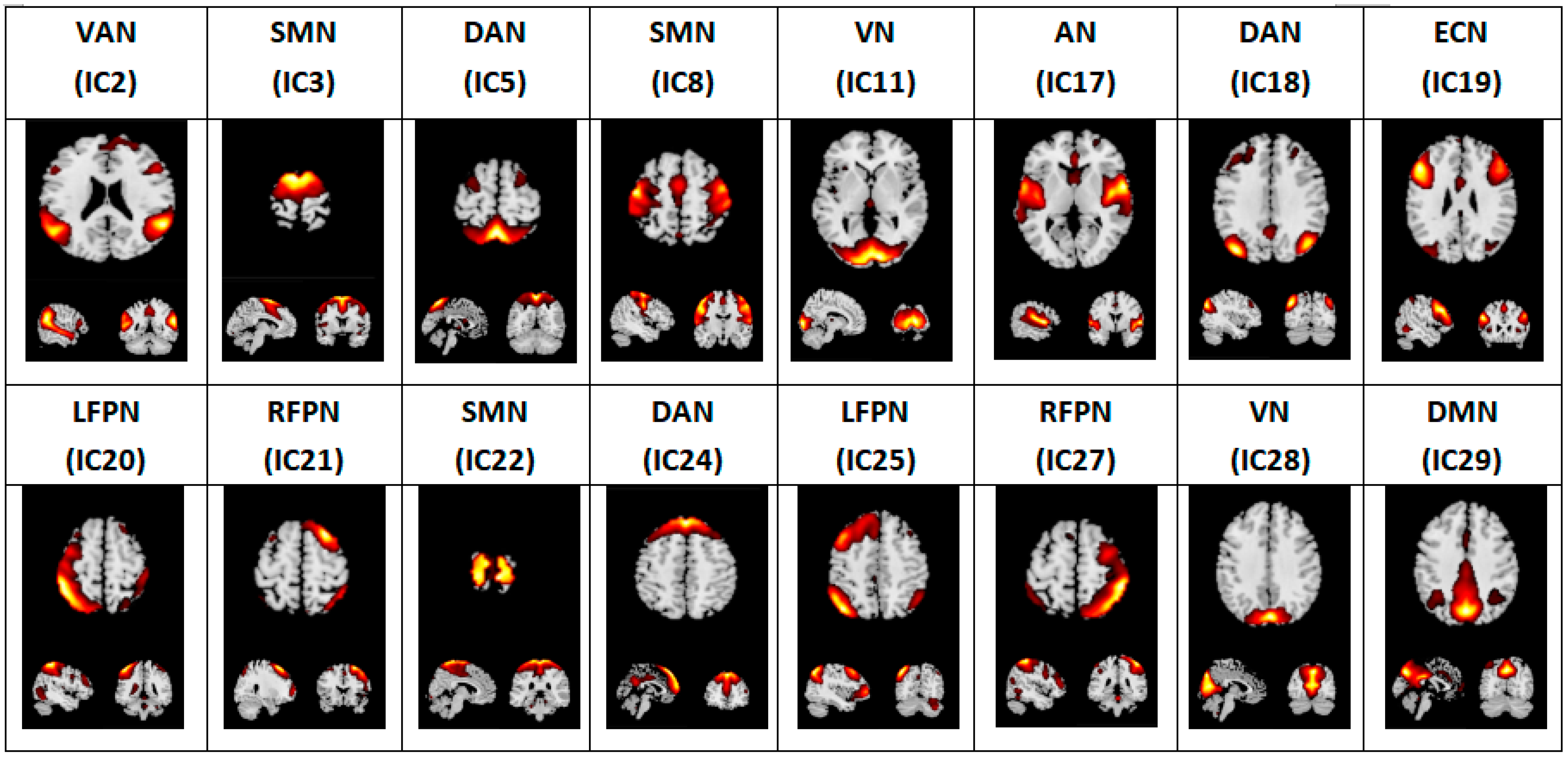
2.5. Independent Component Analysis
2.6. Statistical Analysis
3. Results
3.1. Aberrant Intrinsic Connectivity
3.2. Correlation between FC of RSNs and Clinical Characteristics
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baguley, D.; McFerran, D.; Hall, D. Tinnitus. Lancet 2013, 382, 1600–1607. [Google Scholar] [CrossRef]
- Tunkel, D.E.; Bauer, C.A.; Sun, G.H.; Rosenfeld, R.M.; Chandrasekhar, S.S.; Cunningham, E.R.; Archer, S.M.; Blakley, B.W.; Carter, J.M.; Granieri, E.C.; et al. Clinical Practice Guideline. Otolaryngol. Head Neck Surg. 2014, 151, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Hébert, S.; Canlon, B.; Hasson, D. Emotional Exhaustion as a Predictor of Tinnitus. Psychother. Psychosom. 2012, 81, 324–326. [Google Scholar] [CrossRef] [PubMed]
- Kujawa, S.G.; Liberman, M.C. Adding Insult to Injury: Cochlear Nerve Degeneration after “Temporary” Noise-Induced Hearing Loss. The Journal of Neuroscience: The Official. J. Soc. Neurosci. 2009, 29, 14077–14085. [Google Scholar] [CrossRef] [PubMed]
- Eggermont, J.J.; Roberts, L.E. Tinnitus: Animal Models and Findings in Humans. Cell Tissue Res. 2015, 361, 311–336. [Google Scholar] [CrossRef]
- Henton, A.; Tzounopoulos, T. What’s the Buzz? The Neuroscience and the Treatment of Tinnitus. Physiol. Rev. 2021, 101, 1609–1632. [Google Scholar] [CrossRef]
- Leaver, A.M.; Renier, L.; Chevillet, M.A.; Morgan, S.; Kim, H.J.; Rauschecker, J.P. Dysregulation of Limbic and Auditory Networks in Tinnitus. Neuron 2011, 69, 33–43. [Google Scholar] [CrossRef]
- Seydell-Greenwald, A.; Leaver, A.M.; Turesky, T.K.; Morgan, S.; Kim, H.J.; Rauschecker, J.P. Functional MRI Evidence for a Role of Ventral Prefrontal Cortex in Tinnitus. Brain Res. 2012, 1485, 22–39. [Google Scholar] [CrossRef]
- Roberts, L.E.; Husain, F.T.; Eggermont, J.J. Role of Attention in the Generation and Modulation of Tinnitus. Neurosci. Biobehav. Rev. 2013, 37, 1754–1773. [Google Scholar] [CrossRef]
- Larson, P.S.; Cheung, S.W. Deep Brain Stimulation in Area LC Controllably Triggers Auditory Phantom Percepts. Neurosurgery 2011, 70, 398–406. [Google Scholar] [CrossRef]
- Cai, W.-W.; Li, Z.; Yang, Q.; Zhang, T. Abnormal Spontaneous Neural Activity of the Central Auditory System Changes the Functional Connectivity in the Tinnitus Brain: A Resting-State Functional MRI Study. Front. Neurosci. 2019, 13, 1314. [Google Scholar] [CrossRef] [PubMed]
- Smitha, K.; Akhil Raja, K.; Arun, K.; Rajesh, P.; Thomas, B.; Kapilamoorthy, T.; Kesavadas, C. Resting State FMRI: A Review on Methods in Resting State Connectivity Analysis and Resting State Networks. Neuroradiol. J. 2017, 30, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Gander, P.E.; Andrews, M.; Hall, D.A. Auditory Network Connectivity in Tinnitus Patients: A Resting-State FMRI Study. Int. J. Audiol. 2013, 53, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.A.; Carpenter-Thompson, J.; Husain, F.T. Connectivity of Precuneus to the Default Mode and Dorsal Attention Networks: A Possible Invariant Marker of Long-Term Tinnitus. NeuroImage Clin. 2017, 16, 196–204. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Weisz, N.; Londero, A.; Schlee, W.; Elgoyhen, A.B.; Langguth, B. An Integrative Model of Auditory Phantom Perception: Tinnitus as a Unified Percept of Interacting Separable Subnetworks. Neurosci. Biobehav. Rev. 2014, 44, 16–32. [Google Scholar] [CrossRef]
- Jafari, Z.; Kolb, B.E.; Mohajerani, M.H. Age-Related Hearing Loss and Tinnitus, Dementia Risk, and Auditory Amplification Outcomes. Ageing Res. Rev. 2019, 56, 100963. [Google Scholar] [CrossRef]
- Mohamad, N.; Hoare, D.J.; Hall, D.A. The Consequences of Tinnitus and Tinnitus Severity on Cognition: A Review of the Behavioural Evidence. Hear. Res. 2016, 332, 199–209. [Google Scholar] [CrossRef]
- Tegg-Quinn, S.; Bennett, R.J.; Eikelboom, R.H.; Baguley, D.M. The Impact of Tinnitus upon Cognition in Adults: A Systematic Review. Int. J. Audiol. 2016, 55, 533–540. [Google Scholar] [CrossRef]
- Hallam, R.S.; Mckenna, L.; Shurlock, L. Tinnitus Impairs Cognitive Efficiency. Int. J. Audiol. 2004, 43, 218–226. [Google Scholar] [CrossRef]
- Laureano, M.R.; Onishi, E.T.; Bressan, R.A.; Castiglioni, M.L.V.; Batista, I.R.; Reis, M.A.; Garcia, M.V.; de Andrade, A.N.; de Almeida, R.R.; Garrido, G.J.; et al. Memory Networks in Tinnitus: A Functional Brain Image Study. PLoS ONE 2014, 9, e87839. [Google Scholar] [CrossRef]
- Araneda, R.; De Volder, A.G.; Deggouj, N.; Philippot, P.; Heeren, A.; Lacroix, E.; Decat, M.; Rombaux, P.; Renier, L. Altered Top-down Cognitive Control and Auditory Processing in Tinnitus: Evidences from Auditory and Visual Spatial Stroop. Restor. Neurol. Neurosci. 2015, 33, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-M.; Jiao, Y.; Tang, T.-Y.; Lu, C.-Q.; Zhang, J.; Salvi, R.; Teng, G.-J. Altered Spatial and Temporal Brain Connectivity in the Salience Network of Sensorineural Hearing Loss and Tinnitus. Front. Neurosci. 2019, 13, 246. [Google Scholar] [CrossRef] [PubMed]
- Lanting, C.; WoźAniak, A.; van Dijk, P.; Langers, D.R.M. Tinnitus- and Task-Related Differences in Resting-State Networks. Adv. Exp. Med. Biol. 2016, 175–187. [Google Scholar] [CrossRef]
- Burton, H.; Wineland, A.; Bhattacharya, M.; Nicklaus, J.; Garcia, K.S.; Piccirillo, J.F. Altered Networks in Bothersome Tinnitus: A Functional Connectivity Study. BMC Neurosci. 2012, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Peelle, J.E.; Wingfield, A. The Neural Consequences of Age-Related Hearing Loss. Trends Neurosci. 2016, 39, 486–497. [Google Scholar] [CrossRef]
- Shore, S.E.; Roberts, L.E.; Langguth, B. Maladaptive Plasticity in Tinnitus—Triggers, Mechanisms and Treatment. Nat. Rev. Neurol. 2016, 12, 150–160. [Google Scholar] [CrossRef]
- Cole, D.M.; Smith, S.M.; Beckmann, C.F. Advances and Pitfalls in the Analysis and Interpretation of Resting-State FMRI Data. Front. Syst. Neurosci. 2010, 4, 1–15. [Google Scholar] [CrossRef]
- Gentil, A.; Deverdun, J.; Menjot de Champfleur, N.; Puel, J.-L.; Le Bars, E.; Venail, F. Alterations in Regional Homogeneity in Patients with Unilateral Chronic Tinnitus. Trends Hear. 2019, 23, 233121651983023. [Google Scholar] [CrossRef]
- Adamchic, I.; Hauptmann, C.; Tass, P.A. Changes of Oscillatory Activity in Pitch Processing Network and Related Tinnitus Relief Induced by Acoustic CR Neuromodulation. Front. Syst. Neurosci. 2012, 6, 18. [Google Scholar] [CrossRef]
- Newman, C.W.; Jacobson, G.P.; Spitzer, J.B. Development of the Tinnitus Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1996, 122, 143–148. [Google Scholar] [CrossRef]
- Hu, J.; Zhou, W.; Hu, S.; Huang, M.; Wei, N.; Qi, H.; Huang, J.; Xu, Y. Cross-Cultural Difference and Validation of the Chinese Version of Montreal Cognitive Assessment in Older Adults Residing in Eastern China: Preliminary Findings. Arch. Gerontol. Geriatr. 2013, 56, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, Y.; Yu, C.; Li, L. Reliability and validity of depression scales of Chinese version: A systematic review. Chin. Zhonghua Liu Xing Bing Xue Za Zhi 2017, 38, 110–116. [Google Scholar] [CrossRef]
- He, J.; Liu, Y.; Cheng, C.; Fang, S.; Wang, X.; Yao, S. Psychometric Properties of the Chinese Version of the 10-Item Ruminative Response Scale among Undergraduates and Depressive Patients. Front. Psychiatry 2021, 12, e626859. [Google Scholar] [CrossRef]
- Mueller, S.; Costa, A.; Keeser, D.; Pogarell, O.; Berman, A.; Coates, U.; Reiser, M.F.; Riedel, M.; Möller, H.-J.; Ettinger, U.; et al. The Effects of Methylphenidate on Whole Brain Intrinsic Functional Connectivity. Hum. Brain Mapp. 2014, 35, 5379–5388. [Google Scholar] [CrossRef]
- Di, X.; Biswal, B.B. Dynamic Brain Functional Connectivity Modulated by Resting-State Networks. Brain Struct. Funct. 2013, 220, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qin, W.; Zhang, J.; Tian, T.; Li, Y.; Meng, L.; Zhang, X.; Yu, C. Altered Functional Organization within and between Resting-State Networks in Chronic Subcortical Infarction. J. Cereb. Blood Flow Metab. 2014, 34, 597–605. [Google Scholar] [CrossRef]
- Liu, L.; Jiang, H.; Wang, D.; Zhao, X. A Study of Regional Homogeneity of Resting-State Functional Magnetic Resonance Imaging in Mild Cognitive Impairment. Behav. Brain Res. 2021, 402, 113103. [Google Scholar] [CrossRef]
- Justen, C.; Herbert, C. The Spatio-Temporal Dynamics of Deviance and Target Detection in the Passive and Active Auditory Oddball Paradigm: A SLORETA Study. BMC Neurosci. 2018, 19, 25. [Google Scholar] [CrossRef]
- Mannarelli, D.; Pauletti, C.; Mancini, P.; Fioretti, A.; Greco, A.; De Vincentiis, M.; Fattapposta, F. Selective Attentional Impairment in Chronic Tinnitus: Evidence from an Event-Related Potentials Study. Clin. Neurophysiol. 2017, 128, 411–417. [Google Scholar] [CrossRef]
- Sadaghiani, S.; Kleinschmidt, A. Brain Networks and α-Oscillations: Structural and Functional Foundations of Cognitive Control. Trends Cogn. Sci. 2016, 20, 805–817. [Google Scholar] [CrossRef]
- Suo, X.; Ding, H.; Li, X.; Zhang, Y.; Liang, M.; Zhang, Y.; Yu, C.; Qin, W. Anatomical and Functional Coupling between the Dorsal and Ventral Attention Networks. NeuroImage 2021, 232, 117868. [Google Scholar] [CrossRef]
- Fox, M.D.; Corbetta, M.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Spontaneous Neuronal Activity Distinguishes Human Dorsal and Ventral Attention Systems. Proc. Natl. Acad. Sci. USA 2006, 103, 10046–10051. [Google Scholar] [CrossRef] [PubMed]
- Corbetta, M.; Shulman, G.L. Control of Goal-Directed and Stimulus-Driven Attention in the Brain. Nature reviews. Neuroscience 2002, 3, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, C.J.; Valera, E.M.; Schmahmann, J.D. Functional Topography of the Cerebellum for Motor and Cognitive Tasks: An FMRI Study. NeuroImage 2012, 59, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Bubb, E.J.; Metzler-Baddeley, C.; Aggleton, J.P. The Cingulum Bundle: Anatomy, Function, and Dysfunction. Neurosci. Biobehav. Rev. 2018, 92, 104–127. [Google Scholar] [CrossRef]
- Meyer, M.; Neff, P.; Liem, F.; Kleinjung, T.; Weidt, S.; Langguth, B.; Schecklmann, M. Differential Tinnitus-Related Neuroplastic Alterations of Cortical Thickness and Surface Area. Hear. Res. 2016, 342, 1–12. [Google Scholar] [CrossRef]
- Raya, J.G.; Horng, A.; Dietrich, O.; Krasnokutsky, S.; Beltran, L.S.; Storey, P.; Reiser, M.F.; Recht, M.P.; Sodickson, D.K.; Glaser, C. Articular Cartilage: In Vivo Diffusion-Tensor Imaging. Radiology 2012, 262, 550–559. [Google Scholar] [CrossRef]
- De Ridder, D.; Vanneste, S.; Congedo, M. The Distressed Brain: A Group Blind Source Separation Analysis on Tinnitus. PLoS ONE 2011, 6, e24273. [Google Scholar] [CrossRef]
- Dosenbach, N.U.F.; Fair, D.A.; Miezin, F.M.; Cohen, A.L.; Wenger, K.K.; Dosenbach, R.A.T.; Fox, M.D.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E.; et al. Distinct Brain Networks for Adaptive and Stable Task Control in Humans. Proc. Natl. Acad. Sci. USA 2007, 104, 11073–11078. [Google Scholar] [CrossRef]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Smith, S.M.; Fox, P.T.; Miller, K.L.; Glahn, D.C.; Fox, P.M.; Mackay, C.E.; Filippini, N.; Watkins, K.E.; Toro, R.; Laird, A.R.; et al. Correspondence of the Brain’s Functional Architecture during Activation and Rest. Proc. Natl. Acad. Sci. USA 2009, 106, 13040–13045. [Google Scholar] [CrossRef] [PubMed]
- Fiehler, K.; Rösler, F. Plasticity of Multisensory Dorsal Stream Functions: Evidence from Congenitally Blind and Sighted Adults. Restor. Neurol. Neurosci. 2010, 28, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Weisz, N.; Voss, S.; Berg, P.; Elbert, T. Abnormal Auditory Mismatch Response in Tinnitus Sufferers with High-Frequency Hearing Loss Is Associated with Subjective Distress Level. BMC Neurosci. 2004, 5, 8. [Google Scholar] [CrossRef][Green Version]
- Cheng, S.; Xu, G.; Zhou, J.; Qu, Y.; Li, Z.; He, Z.; Yin, T.; Ma, P.; Sun, R.; Liang, F. A Multimodal Meta-Analysis of Structural and Functional Changes in the Brain of Tinnitus. Front. Hum. Neurosci. 2020, 14, 28. [Google Scholar] [CrossRef]
- Chen, Q.; Lv, H.; Wang, Z.; Wei, X.; Liu, J.; Zhao, P.; Yang, Z.; Gong, S.; Wang, Z. Pretreatment Intranetwork Connectivity Can Predict the Outcomes in Idiopathic Tinnitus Patients Treated with Sound Therapy. Hum. Brain Mapp. 2021, 42, 4762–4776. [Google Scholar] [CrossRef] [PubMed]
- Puche Sarmiento, A.C.; Bocanegra García, Y.; Ochoa Gómez, J.F. Active Information Storage in Parkinson’s Disease: A Resting State FMRI Study over the Sensorimotor Cortex. Brain Imaging Behav. 2019, 14, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Panouillères, M.T.N.; Möttönen, R. Decline of Auditory-Motor Speech Processing in Older Adults with Hearing Loss. Neurobiol. Aging 2018, 72, 89–97. [Google Scholar] [CrossRef]
- Job, A.; Pons, Y.; Lamalle, L.; Jaillard, A.; Buck, K.; Segebarth, C.; Delon-Martin, C. Abnormal Cortical Sensorimotor Activity during “Target” Sound Detection in Subjects with Acute Acoustic Trauma Sequelae: An FMRI Study. Brain Behav. 2012, 2, 187–199. [Google Scholar] [CrossRef]
- Zhou, G.-P.; Shi, X.-Y.; Wei, H.-L.; Qu, L.-J.; Yu, Y.-S.; Zhou, Q.-Q.; Yin, X.; Zhang, H.; Tao, Y.-J. Disrupted Intraregional Brain Activity and Functional Connectivity in Unilateral Acute Tinnitus Patients with Hearing Loss. Front. Neurosci. 2019, 13, 1010. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Y.-C.; Lv, H.; Xia, W.; Mao, C.-N.; Bo, F.; Chen, H.; Xu, J.-J.; Yin, X. Increased Resting-State Cerebellar-Cerebral Functional Connectivity Underlying Chronic Tinnitus. Front. Aging Neurosci. 2018, 10, 59. [Google Scholar] [CrossRef]
- Lange, I.; Kasanova, Z.; Goossens, L.; Leibold, N.; De Zeeuw, C.I.; van Amelsvoort, T.; Schruers, K. The Anatomy of Fear Learning in the Cerebellum: A Systematic Meta-Analysis. Neurosci. Biobehav. Rev. 2015, 59, 83–91. [Google Scholar] [CrossRef] [PubMed]


| Demographic (Mean ± SD) | PA | HC | T-Value | p-Value |
|---|---|---|---|---|
| Gender (M/F) | 10/7 | 7/14 | 2.404 | 0.121 a |
| Age (years) | 54.76 ± 8.92 | 51.24 ± 6.33 | 1.424 | 0.163 b |
| Education (years) | 11.82 ± 2.19 | 12.90 ± 2.81 | −1.299 | 0.202 b |
| Handedness(right/left) | 17/0 | 21/0 | N/A | 1.000 a |
| Duration (years) | 2.61 ± 2.50 | |||
| THI | 48.12 ± 30.50 | |||
| Pure Tone Average (dB) | 33.50 ± 10.18 | |||
| MoCA | 23.41 ± 2.43 | |||
| BDI | 11.53 ± 11.41 | |||
| S-AI | 38.12 ± 11.68 | |||
| T-AI | 37.47 ± 9.29 |
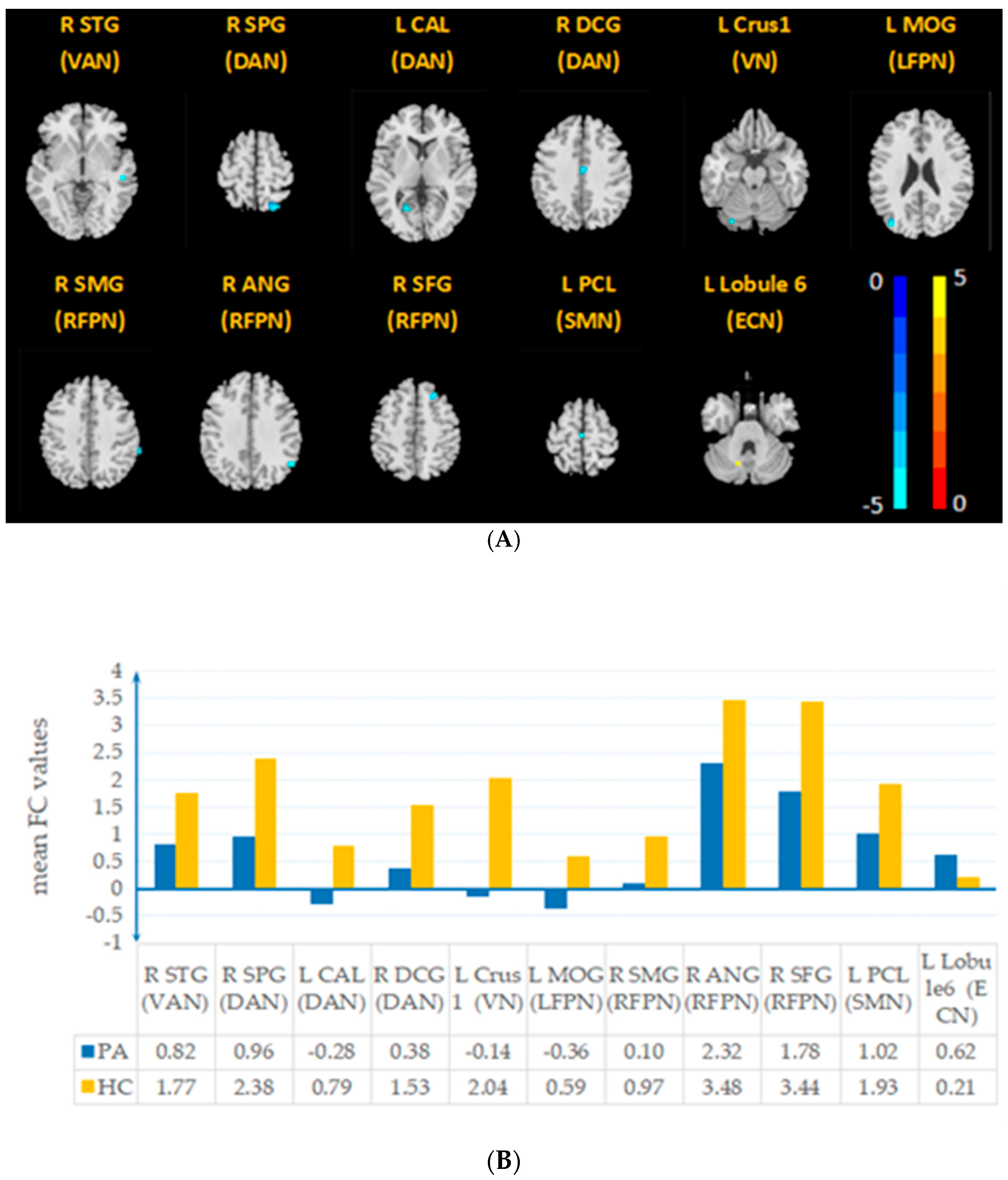
| RSN | Brain Region | Cluster Size, mm³ | Peak MNI, mm | Peak t Value | |
|---|---|---|---|---|---|
| x y z | |||||
| PA < NC | VAN (IC2) | R STG | 16 | 51 −27 −3 | −4.4865 |
| DAN (IC5) | R SPG | 69 | 24 −69 63 | −6.9585 | |
| DAN (IC18) | L CAL | 14 | −18 −63 6 | −4.4156 | |
| DAN (IC24) | R DCG | 16 | 3 −12 39 | −4.9841 | |
| VN (IC11) | L Crus1 | 10 | −27 −81 −21 | −5.2585 | |
| LFPN (IC20) | L MOG | 11 | −39 −81 24 | −5.7306 | |
| RFPN (IC22) | R ANG | 12 | 54 −57 36 | −4.8083 | |
| RFPN(IC27) | R SMG | 11 | 66 −39 39 | −4.7568 | |
| SMN (IC21) | L PCL | 12 | −3 −18 66 | −4.2251 | |
| R SFG | 20 | 24 27 51 | −5.0179 | ||
| PA > NC | ECN (IC19) | L Lobule 6 | 10 | −15 −63 −30 | 4.7252 |
| Brian Regions | MoCA Scores | BDI Scores | S-AI Scores | T-AI Scores | |||||
|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | ||
| VAN | R STG | 0.093 | 0.722 | 0.161 | 0.537 | 0.200 | 0.441 | 0.198 | 0.446 |
| R SPG | 0.474 | 0.055 | −0.137 | 0.601 | 0.357 | 0.159 | 0.305 | 0.234 | |
| DAN | L CAL | −0.607 * | 0.010 | 0.000 | 1.000 | 0.301 | 0.241 | 0.152 | 0.559 |
| R DCG | 0.252 | 0.330 | −0.241 | 0.351 | −0.430 | 0.085 | −0.036 | 0.892 | |
| VN | L Crus1 | 0.071 | 0.787 | −0.549 * | 0.022 | −0.151 | 0.563 | −0.127 | 0.626 |
| LFPN | L MOG | 0.406 | 0.106 | −0.233 | 0.369 | −0.247 | 0.339 | −0.329 | 0.197 |
| R SMG | −0.405 | 0.107 | 0.134 | 0.608 | −0.108 | 0.680 | 0.181 | 0.488 | |
| RFPN | R ANG | 0.324 | 0.204 | −0.165 | 0.527 | −0.076 | 0.772 | 0.042 | 0.873 |
| R SFG | 0.080 | 0.759 | −0.235 | 0.364 | −0.377 | 0.136 | −0.479 | 0.052 | |
| SMN | L PCL | 0.012 | 0.965 | −0.120 | 0.646 | −0.198 | 0.445 | −0.271 | 0.293 |
| ECN | L Lobule_6 | −0.014 | 0.957 | 0.085 | 0.746 | 0.080 | 0.761 | −0.004 | 0.989 |
| Hearing | THI | Duration | Tinnitus-Hz | Tinnitus-dB | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | ||
| VAN | R STG | 0.012 | 0.963 | 0.120 | 0.646 | 0.402 | 0.123 | 0.189 | 0.484 | 0.041 | 0.879 |
| R SPG | 0.166 | 0.525 | 0.100 | 0.701 | 0.420 | 0.106 | −0.048 | 0.859 | 0.482 | 0.059 | |
| DAN | L CAL | −0.426 | 0.089 | −0.125 | 0.633 | −0.394 | 0.131 | −0.045 | 0.868 | −0.279 | 0.295 |
| R DCG | −0.013 | 0.959 | −0.184 | 0.480 | 0.321 | 0.225 | −0.069 | 0.798 | −0.155 | 0.566 | |
| VN | L Crus 1 | −0.015 | 0.955 | −0.416 | 0.097 | −0.039 | 0.887 | −0.041 | 0.881 | 0.152 | 0.573 |
| LFPN | L MOG | 0.309 | 0.227 | −0.219 | 0.397 | 0.450 | 0.080 | 0.289 | 0.277 | 0.371 | 0.157 |
| R SMG | 0.262 | 0.309 | 0.020 | 0.940 | −0.146 | 0.590 | −0.486 | 0.056 | −0.167 | 0.537 | |
| RFPN | R ANG | 0.152 | 0.560 | −0.140 | 0.593 | 0.202 | 0.452 | −0.470 | 0.066 | 0.021 | 0.939 |
| R SFG | −0.235 | 0.363 | −0.279 | 0.277 | −0.283 | 0.289 | −0.056 | 0.837 | −0.131 | 0.627 | |
| SMN | L PCL | −0.531 | 0.028 * | −0.055 | 0.833 | −0.107 | 0.693 | −0.101 | 0.709 | −0.378 | 0.149 |
| ECN | L Lobule_6 | −0.042 | 0.874 | 0.456 | 0.066 | −0.028 | 0.917 | −0.133 | 0.624 | −0.186 | 0.490 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Ma, X.; Wang, Q.; He, X.; Qu, X.; Zhang, L.; Chen, L.; Liu, Z. Intrinsic Network Changes in Bilateral Tinnitus Patients with Cognitive Impairment: A Resting-State Functional MRI Study. Brain Sci. 2022, 12, 1049. https://doi.org/10.3390/brainsci12081049
Li W, Ma X, Wang Q, He X, Qu X, Zhang L, Chen L, Liu Z. Intrinsic Network Changes in Bilateral Tinnitus Patients with Cognitive Impairment: A Resting-State Functional MRI Study. Brain Sciences. 2022; 12(8):1049. https://doi.org/10.3390/brainsci12081049
Chicago/Turabian StyleLi, Wei, Xiaobo Ma, Qian Wang, Xueying He, Xiaoxia Qu, Lirong Zhang, Lanyue Chen, and Zhaohui Liu. 2022. "Intrinsic Network Changes in Bilateral Tinnitus Patients with Cognitive Impairment: A Resting-State Functional MRI Study" Brain Sciences 12, no. 8: 1049. https://doi.org/10.3390/brainsci12081049
APA StyleLi, W., Ma, X., Wang, Q., He, X., Qu, X., Zhang, L., Chen, L., & Liu, Z. (2022). Intrinsic Network Changes in Bilateral Tinnitus Patients with Cognitive Impairment: A Resting-State Functional MRI Study. Brain Sciences, 12(8), 1049. https://doi.org/10.3390/brainsci12081049





