Neuromotor Development in the Shank3 Mouse Model of Autism Spectrum Disorder
Abstract
:1. Introduction
2. Methods
2.1. Animals
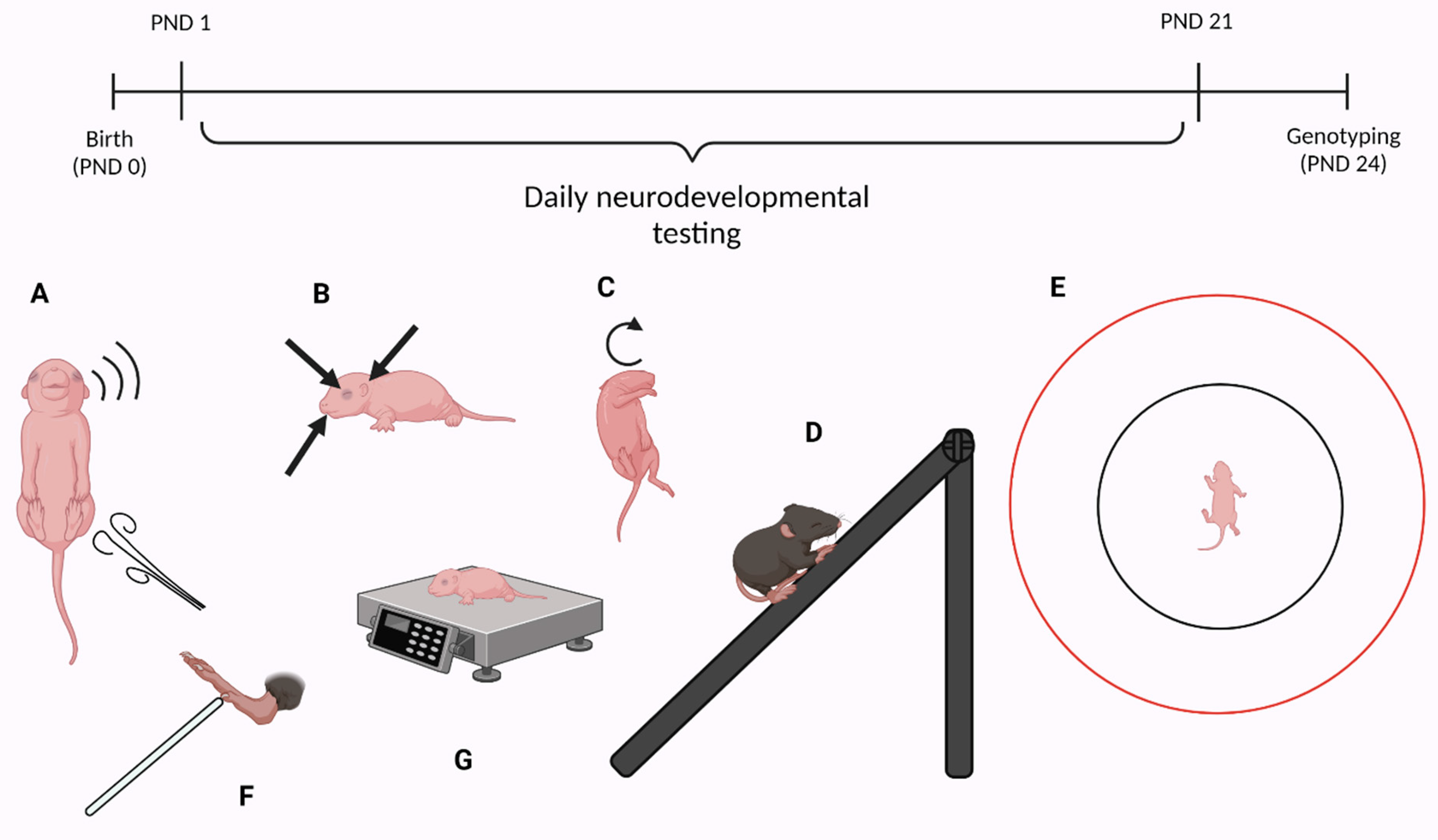
2.2. Neurodevelopment
2.3. Physical and Morphological Landmarks of Development
2.4. Reflexes
2.5. Motor Skills
2.6. Statistical Analysis
3. Results
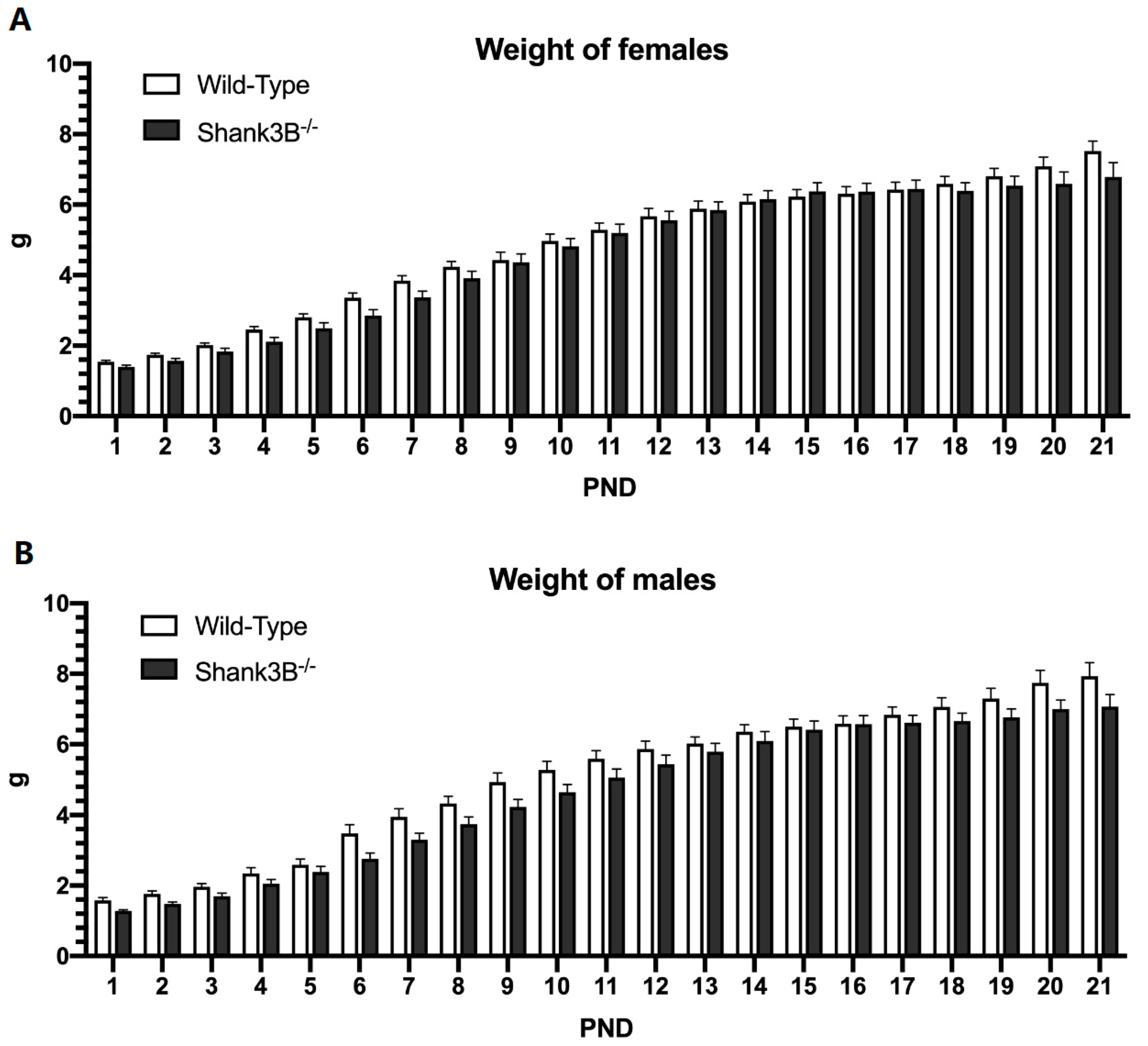
3.1. Body Weight
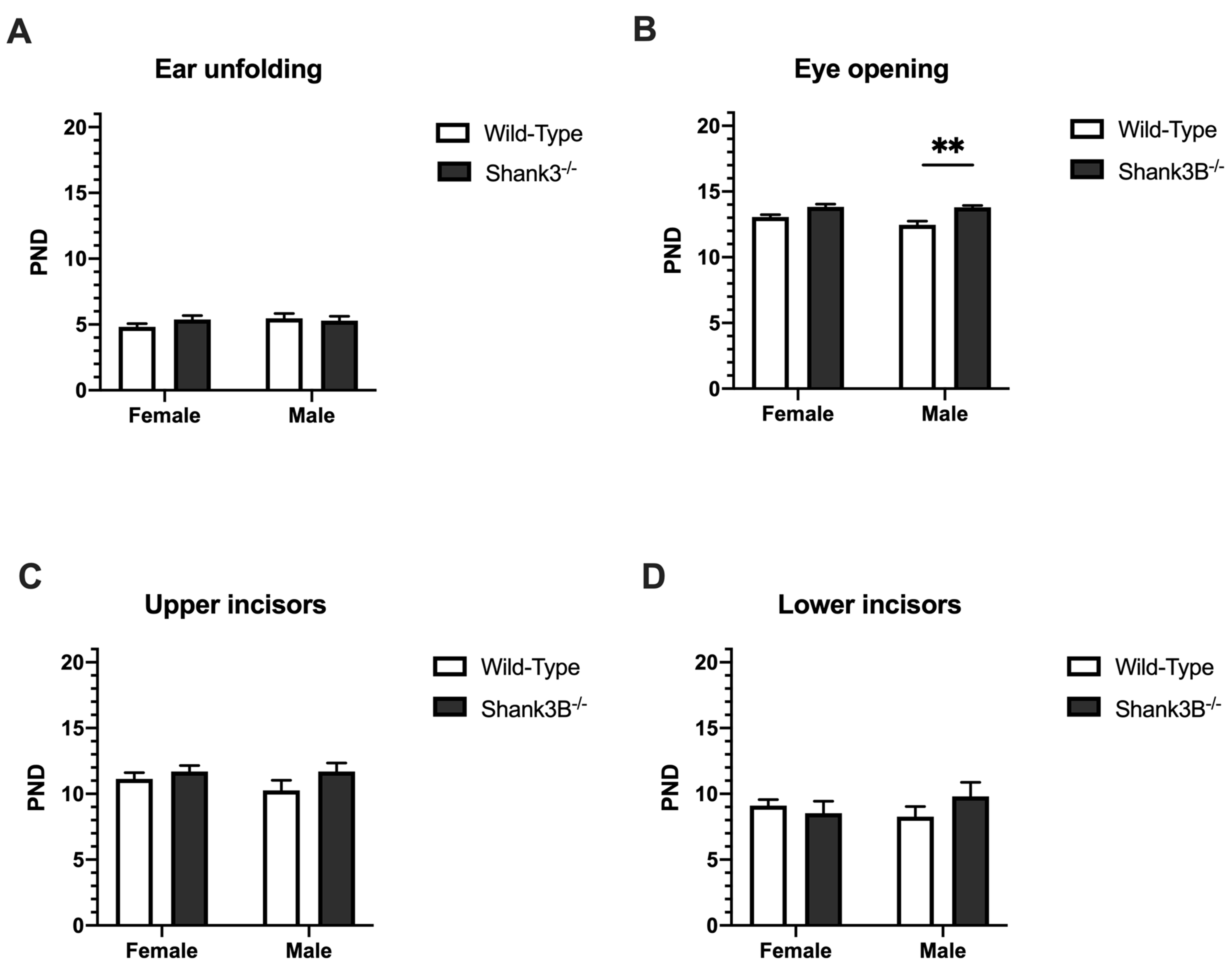
3.2. Physical and Morphological Landmarks of Development
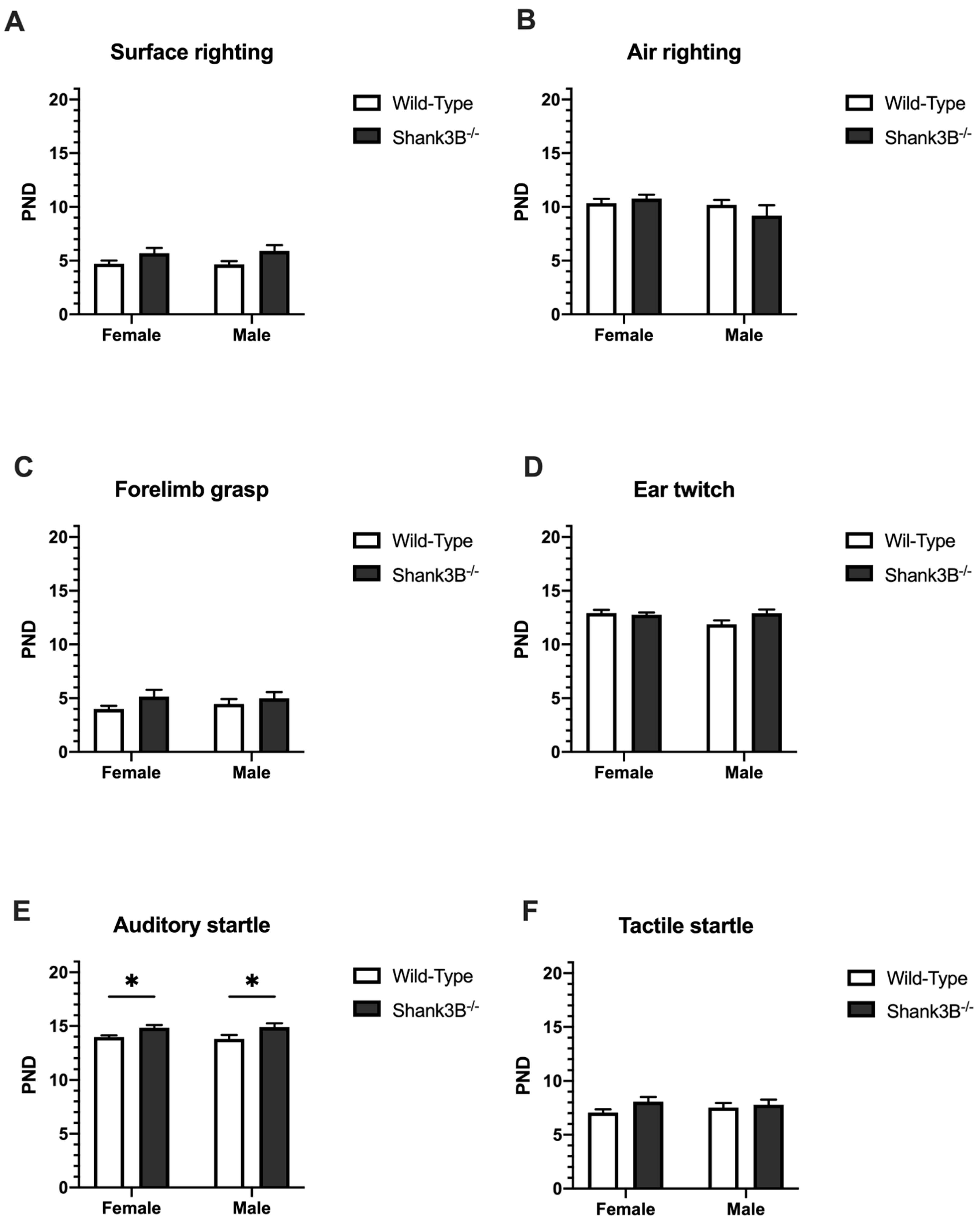
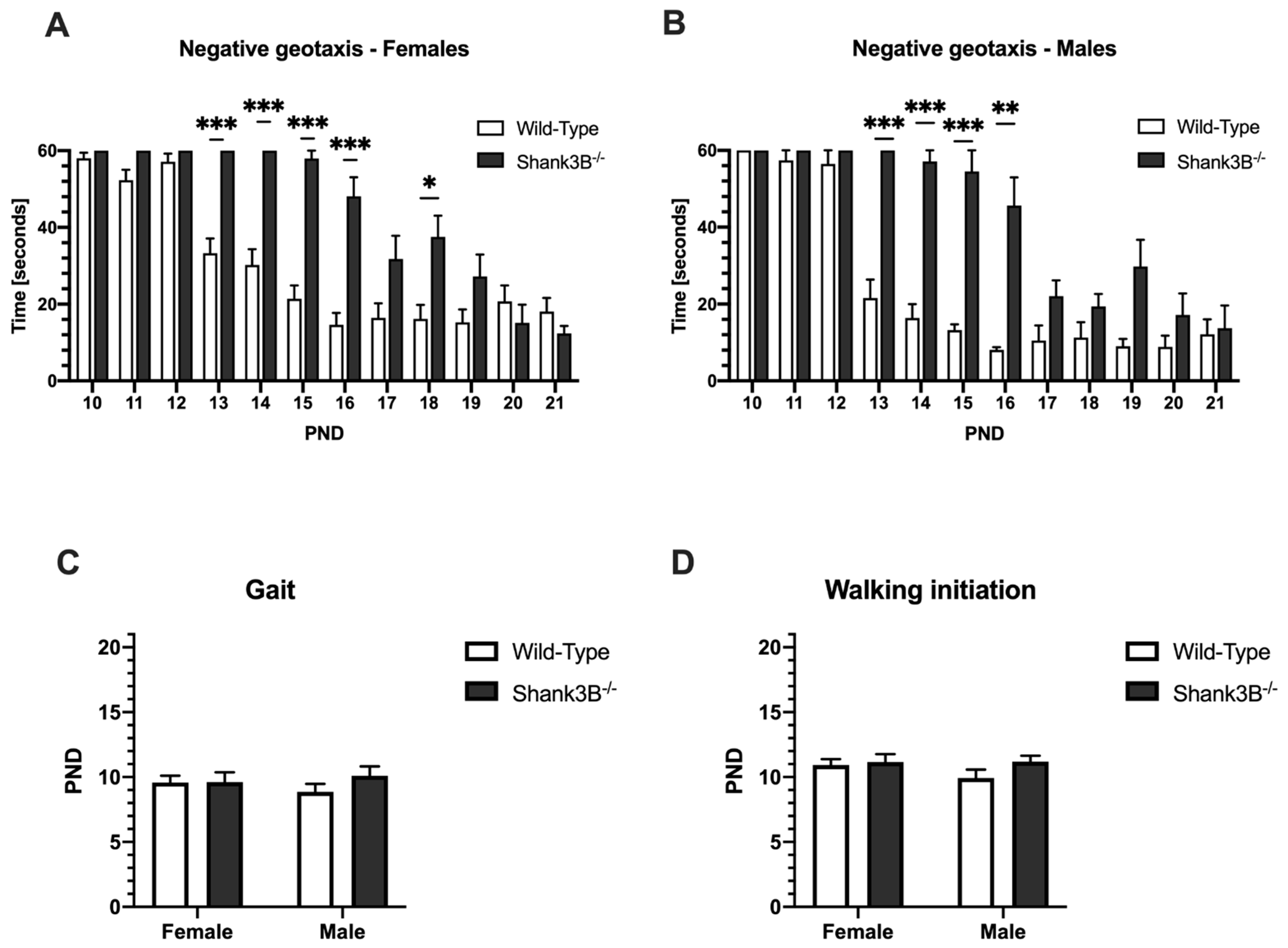
3.3. Reflexes
3.4. Motor Skills
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fournier, K.A.; Hass, C.J.; Naik, S.K.; Lodha, N.; Cauraugh, J.H. Motor coordination in autism spectrum disorders: A synthesis and meta-analysis. J. Autism Dev. Disord. 2010, 40, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Gidley Larson, J.C.; Bastian, A.J.; Donchin, O.; Shadmehr, R.; Mostofsky, S.H. Acquisition of internal models of motor tasks in children with autism. Brain 2008, 131, 2894–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Moraes, Í.A.P.; Massetti, T.; Crocetta, T.B.; da Silva, T.D.; de Menezes, L.D.C.; de Mello Monteiro, C.B.; Magalhães, F.H. Motor learning characterization in people with autism spectrum disorder: A systematic review. Dement. Neuropsychol. 2017, 11, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Tick, B.; Bolton, P.; Happé, F.; Rutter, M.; Rijsdijk, F. Heritability of autism spectrum disorders: A meta-analysis of twin studies. J. Child Psychol. Psychiatry 2016, 57, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Halladay, A.K.; Bishop, S.; Constantino, J.N.; Daniels, A.M.; Koenig, K.; Palmer, K.; Messinger, D.; Pelphrey, K.; Sanders, S.J.; Singer, A.T.; et al. Sex and gender differences in autism spectrum disorder: Summarizing evidence gaps and identifying emerging areas of priority. Mol. Autism 2015, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mashayekhi, F.; Mizban, N.; Bidabadi, E.; Salehi, Z. The association of SHANK3 gene polymorphism and autism. Minerva Pediatrica 2021, 73, 251–255. [Google Scholar] [CrossRef]
- Arons, M.H.; Lee, K.; Thynne, C.J.; Kim, S.A.; Schob, C.; Kindler, S.; Montgomery, J.M.; Garner, C.C. Shank3 Is Part of a Zinc-Sensitive Signaling System That Regulates Excitatory Synaptic Strength. J. Neurosci. 2016, 36, 9124–9134. [Google Scholar] [CrossRef] [Green Version]
- Herbert, M.R. SHANK3, the synapse, and autism. N. Engl. J. Med. 2011, 365, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Guo, B.; Chen, J.; Chen, Q.; Ren, K.; Feng, D.; Mao, H.; Yao, H.; Yang, J.; Liu, H.; Liu, Y.; et al. Anterior cingulate cortex dysfunction underlies social deficits in Shank3 mutant mice. Nat. Neurosci. 2019, 22, 1223–1234. [Google Scholar] [CrossRef] [PubMed]
- Yoo, T.; Cho, H.; Lee, J.; Park, H.; Yoo, Y.-E.; Yang, E.; Kim, J.Y.; Kim, H.; Kim, E. GABA Neuronal Deletion of Shank3 Exons 14-16 in Mice Suppresses Striatal Excitatory Synaptic Input and Induces Social and Locomotor Abnormalities. Front. Cell. Neurosci. 2018, 12, 341. [Google Scholar] [CrossRef]
- De Sena Cortabitarte, A.; Degenhardt, F.; Strohmaier, J.; Lang, M.; Weiss, B.; Roeth, R.; Giegling, I.; Heilmann-Heimbach, S.; Hofmann, A.; Rujescu, D.; et al. Investigation of SHANK3 in schizophrenia. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2017, 174, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Ohba, C.; Yamashita, Y.; Saitsu, H.; Matsumoto, N.; Matsuishi, T. De novo SHANK3 mutation causes Rett syndrome-like phenotype in a female patient. Am. J. Med. Genet. Part A 2015, 167, 1593–1596. [Google Scholar] [CrossRef] [PubMed]
- Soorya, L.; Kolevzon, A.; Zweifach, J.; Lim, T.; Dobry, Y.; Schwartz, L.; Frank, Y.; Wang, A.T.; Cai, G.; Parkhomenko, E. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol. Autism 2013, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boccuto, L.; Lauri, M.; Sarasua, S.M.; Skinner, C.D.; Buccella, D.; Dwivedi, A.; Orteschi, D.; Collins, J.S.; Zollino, M.; Visconti, P.; et al. Prevalence of SHANK3 variants in patients with different subtypes of autism spectrum disorders. Eur. J. Hum. Genet. 2013, 21, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Leblond, C.S.; Nava, C.; Polge, A.; Gauthier, J.; Huguet, G.; Lumbroso, S.; Giuliano, F.; Stordeur, C.; Depienne, C.; Mouzat, K.; et al. Meta-analysis of SHANK Mutations in Autism Spectrum Disorders: A gradient of severity in cognitive impairments. PLoS Genet. 2014, 10, e1004580. [Google Scholar] [CrossRef] [Green Version]
- Sarasua, S.M.; Dwivedi, A.; Boccuto, L.; Rollins, J.D.; Chen, C.-F.; Rogers, R.C.; Phelan, K.; DuPont, B.R.; Collins, J.S. Association between deletion size and important phenotypes expands the genomic region of interest in Phelan–McDermid syndrome (22q13 deletion syndrome). J. Med. Genet. 2011, 48, 761–766. [Google Scholar] [CrossRef]
- Wilson, H.; Wong, A.; Shaw, S.; Tse, W.; Stapleton, G.; Phelan, M.; Hu, S.; Marshall, J.; McDermid, H. Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J. Med. Genet. 2003, 40, 575–584. [Google Scholar] [CrossRef] [Green Version]
- Fox, W.M. Reflex-ontogeny and behavioural development of the mouse. Anim. Behav. 1965, 13, 234–235. [Google Scholar] [CrossRef]
- Heyser, C.J. Assessment of Developmental Milestones in Rodents. Curr. Protoc. Neurosci. 2003, 25, 11–15. [Google Scholar] [CrossRef]
- Peça, J.; Feliciano, C.; Ting, J.T.; Wang, W.; Wells, M.F.; Venkatraman, T.N.; Lascola, C.D.; Fu, Z.; Feng, G. Shank3 mutant mice display autistic-like behaviours and striatal dysfunction. Nature 2011, 472, 437–442. [Google Scholar] [CrossRef] [Green Version]
- Foley, K.A.; Ossenkopp, K.-P.; Kavaliers, M.; Macfabe, D.F. Pre- and neonatal exposure to lipopolysaccharide or the enteric metabolite, propionic acid, alters development and behavior in adolescent rats in a sexually dimorphic manner. PLoS ONE 2014, 9, e87072. [Google Scholar] [CrossRef] [Green Version]
- Ruhela, R.K.; Soni, S.; Sarma, P.; Prakash, A.; Medhi, B. Negative geotaxis: An early age behavioral hallmark to VPA rat model of autism. Ann. Neurosci. 2019, 26, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, A.; Hammerschmidt, K.; Tantra, M.; Krueger, D.; Brose, N.; Ehrenreich, H. Juvenile manifestation of ultrasound communication deficits in the neuroligin-4 null mutant mouse model of autism. Behav. Brain Res. 2014, 270, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Sadigurschi, N.; Golan, H.M. Maternal and offspring methylenetetrahydrofolate-reductase genotypes interact in a mouse model to induce autism spectrum disorder-like behavior. Genes Brain Behav. 2019, 18, e12547. [Google Scholar] [CrossRef] [Green Version]
- Al Sagheer, T.; Haida, O.; Balbous, A.; Francheteau, M.; Matas, E.; Fernagut, P.-O.; Jaber, M. Motor Impairments Correlate with Social Deficits and Restricted Neuronal Loss in an Environmental Model of Autism. Int. J. Neuropsychopharmacol. 2018, 21, 871–882. [Google Scholar] [CrossRef] [Green Version]
- Yang, E.-J.; Ahn, S.; Lee, K.; Mahmood, U.; Kim, H.-S. Early behavioral abnormalities and perinatal alterations of PTEN/AKT pathway in valproic acid autism model mice. PLoS ONE 2016, 11, e0153298. [Google Scholar]
- Laugeray, A.; Herzine, A.; Perche, O.; Hébert, B.; Aguillon-Naury, M.; Richard, O.; Menuet, A.; Mazaud-Guittot, S.; Lesné, L.; Briault, S. Pre-and postnatal exposure to low dose glufosinate ammonium induces autism-like phenotypes in mice. Front. Behav. Neurosci. 2014, 8, 390. [Google Scholar] [CrossRef] [Green Version]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; d’Amato, F.R.; Crusio, W.E. Genetic-background modulation of core and variable autistic-like symptoms in Fmr1 knock-out mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Tan, J.; Guo, J.; Zheng, Y.; Han, Q.; So, K.-F.; Yu, J.; Zhang, L. Aberrant Development and Synaptic Transmission of Cerebellar Cortex in a VPA Induced Mouse Autism Model. Front. Cell. Neurosci. 2018, 12, 500. [Google Scholar] [CrossRef] [Green Version]
- Hou, Q.; Wang, Y.; Li, Y.; Chen, D.; Yang, F.; Wang, S. A Developmental Study of Abnormal Behaviors and Altered GABAergic Signaling in the VPA-Treated Rat Model of Autism. Front. Behav. Neurosci. 2018, 12, 182. [Google Scholar] [CrossRef] [Green Version]
- Kiss, P.; Tamas, A.; Lubics, A.; Szalai, M.; Szalontay, L.; Lengvari, I.; Reglodi, D. Development of neurological reflexes and motor coordination in rats neonatally treated with monosodium glutamate. Neurotox. Res. 2005, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Wagner, G.C.; Reuhl, K.R.; Cheh, M.; McRae, P.; Halladay, A.K. A New Neurobehavioral Model of Autism in Mice: Pre- and Postnatal Exposure to Sodium Valproate. J. Autism Dev. Disord. 2006, 36, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Drapeau, E.; Riad, M.; Kajiwara, Y.; Buxbaum, J.D. Behavioral Phenotyping of an Improved Mouse Model of Phelan-McDermid Syndrome with a Complete Deletion of the Shank3 Gene. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrovolsky, A.P.; Gedzun, V.R.; Bogin, V.I.; Ma, D.; Ichim, T.E.; Sukhanova, I.A.; Malyshev, A.V.; Dubynin, V.A. Beneficial effects of xenon inhalation on behavioral changes in a valproic acid-induced model of autism in rats. J. Transl. Med. 2019, 17, 400. [Google Scholar] [CrossRef] [Green Version]
- Morakotsriwan, N.; Wattanathorn, J.; Kirisattayakul, W.; Chaisiwamongkol, K. Autistic-Like Behaviors, Oxidative Stress Status, and Histopathological Changes in Cerebellum of Valproic Acid Rat Model of Autism Are Improved by the Combined Extract of Purple Rice and Silkworm Pupae. Oxid. Med. Cell. Longev. 2016, 2016, 3206561. [Google Scholar] [CrossRef] [Green Version]
- Naisbitt, S.; Kim, E.; Tu, J.C.; Xiao, B.; Sala, C.; Valtschanoff, J.; Weinberg, R.J.; Worley, P.F.; Sheng, M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron 1999, 23, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Boeckers, T.M.; Winter, C.; Smalla, K.-H.; Kreutz, M.R.; Bockmann, J.; Seidenbecher, C.; Garner, C.C.; Gundelfinger, E.D. Proline-rich synapse-associated proteins ProSAP1 and ProSAP2 interact with synaptic proteins of the SAPAP/GKAP family. Biochem. Biophys. Res. Commun. 1999, 264, 247–252. [Google Scholar] [CrossRef]
- Sheng, M.; Kim, E. The Shank family of scaffold proteins. J. Cell Sci. 2000, 113, 1851–1856. [Google Scholar] [CrossRef]
- Roussignol, G.; Ango, F.; Romorini, S.; Tu, J.C.; Sala, C.; Worley, P.F.; Bockaert, J.; Fagni, L. Shank expression is sufficient to induce functional dendritic spine synapses in aspiny neurons. J. Neurosci. 2005, 25, 3560–3570. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Knight, M.J.; Proepper, C.; Bockmann, J.; Joubert, M.; Rowan, M.; Nienhaus, G.U.; Garner, C.C.; Bowie, J.U.; Kreutz, M.R. Concerted action of zinc and ProSAP/Shank in synaptogenesis and synapse maturation. EMBO J. 2011, 30, 569–581. [Google Scholar] [CrossRef] [Green Version]
- Durand, C.M.; Perroy, J.; Loll, F.; Perrais, D.; Fagni, L.; Bourgeron, T.; Montcouquiol, M.; Sans, N. SHANK3 mutations identified in autism lead to modification of dendritic spine morphology via an actin-dependent mechanism. Mol. Psychiatry 2012, 17, 71–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffney Lara, J.; Zhong, P.; Wei, J.; Matas, E.; Cheng, J.; Qin, L.; Ma, K.; Dietz David, M.; Kajiwara, Y.; Buxbaum Joseph, D.; et al. Autism-like Deficits in Shank3-Deficient Mice Are Rescued by Targeting Actin Regulators. Cell Rep. 2015, 11, 1400–1413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bey, A.L.; Wang, X.; Yan, H.; Kim, N.; Passman, R.L.; Yang, Y.; Cao, X.; Towers, A.J.; Hulbert, S.W.; Duffney, L.J.; et al. Brain region-specific disruption of Shank3 in mice reveals a dissociation for cortical and striatal circuits in autism-related behaviors. Transl. Psychiatry 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Bidinosti, M.; Botta, P.; Krüttner, S.; Proenca, C.C.; Stoehr, N.; Bernhard, M.; Fruh, I.; Mueller, M.; Bonenfant, D.; Voshol, H.; et al. CLK2 inhibition ameliorates autistic features associated with SHANK3 deficiency. Science 2016, 351, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Minakova, E.; Lang, J.; Medel-Matus, J.-S.; Gould, G.G.; Reynolds, A.; Shin, D.; Mazarati, A.; Sankar, R. Melanotan-II reverses autistic features in a maternal immune activation mouse model of autism. PLoS ONE 2019, 14, e0210389. [Google Scholar] [CrossRef] [Green Version]
- Tatavarty, V.; Torrado Pacheco, A.; Groves Kuhnle, C.; Lin, H.; Koundinya, P.; Miska, N.J.; Hengen, K.B.; Wagner, F.F.; Van Hooser, S.D.; Turrigiano, G.G. Autism-Associated Shank3 Is Essential for Homeostatic Compensation in Rodent V1. Neuron 2020, 106, 769–777.e4. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Qiu, T.; Ke, X.; Xiao, X.; Xiao, T.; Liang, F.; Zou, B.; Huang, H.; Fang, H.; Chu, K.; et al. Autism spectrum disorder as early neurodevelopmental disorder: Evidence from the brain imaging abnormalities in 2–3 years old toddlers. J. Autism Dev. Disord. 2014, 44, 1633–1640. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pillerová, M.; Drobná, D.; Szabó, J.; Renczés, E.; Borbélyová, V.; Ostatníková, D.; Celec, P.; Tóthová, Ľ. Neuromotor Development in the Shank3 Mouse Model of Autism Spectrum Disorder. Brain Sci. 2022, 12, 872. https://doi.org/10.3390/brainsci12070872
Pillerová M, Drobná D, Szabó J, Renczés E, Borbélyová V, Ostatníková D, Celec P, Tóthová Ľ. Neuromotor Development in the Shank3 Mouse Model of Autism Spectrum Disorder. Brain Sciences. 2022; 12(7):872. https://doi.org/10.3390/brainsci12070872
Chicago/Turabian StylePillerová, Miriam, Diana Drobná, Jakub Szabó, Emese Renczés, Veronika Borbélyová, Daniela Ostatníková, Peter Celec, and Ľubomíra Tóthová. 2022. "Neuromotor Development in the Shank3 Mouse Model of Autism Spectrum Disorder" Brain Sciences 12, no. 7: 872. https://doi.org/10.3390/brainsci12070872
APA StylePillerová, M., Drobná, D., Szabó, J., Renczés, E., Borbélyová, V., Ostatníková, D., Celec, P., & Tóthová, Ľ. (2022). Neuromotor Development in the Shank3 Mouse Model of Autism Spectrum Disorder. Brain Sciences, 12(7), 872. https://doi.org/10.3390/brainsci12070872






