Epidemiology of Cerebral Palsy among Children and Adolescents in Arabic-Speaking Countries: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection
2.3. Quality Assessment (Risk of Bias Assessment)
2.4. Data Extraction
2.5. Data Analysis
3. Results
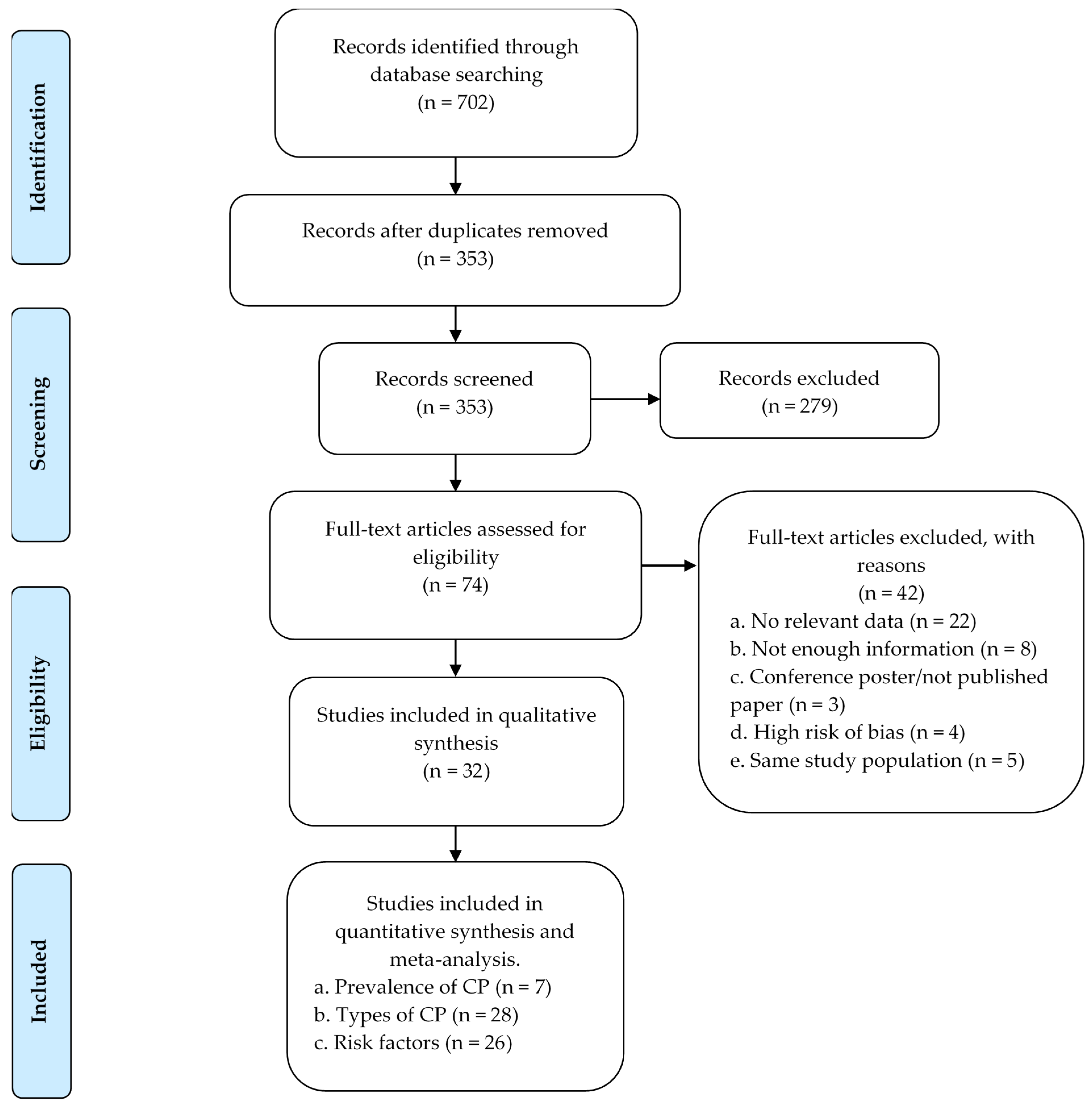
3.1. Study Selection and Eligibility
3.2. Study Characteristics of Included Publications
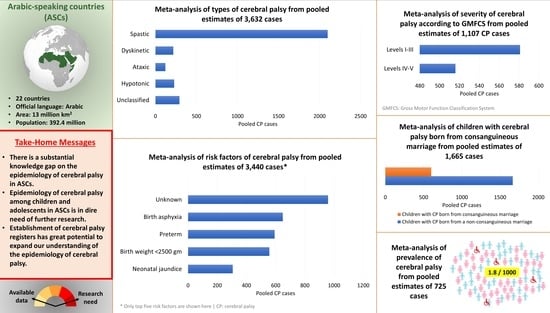
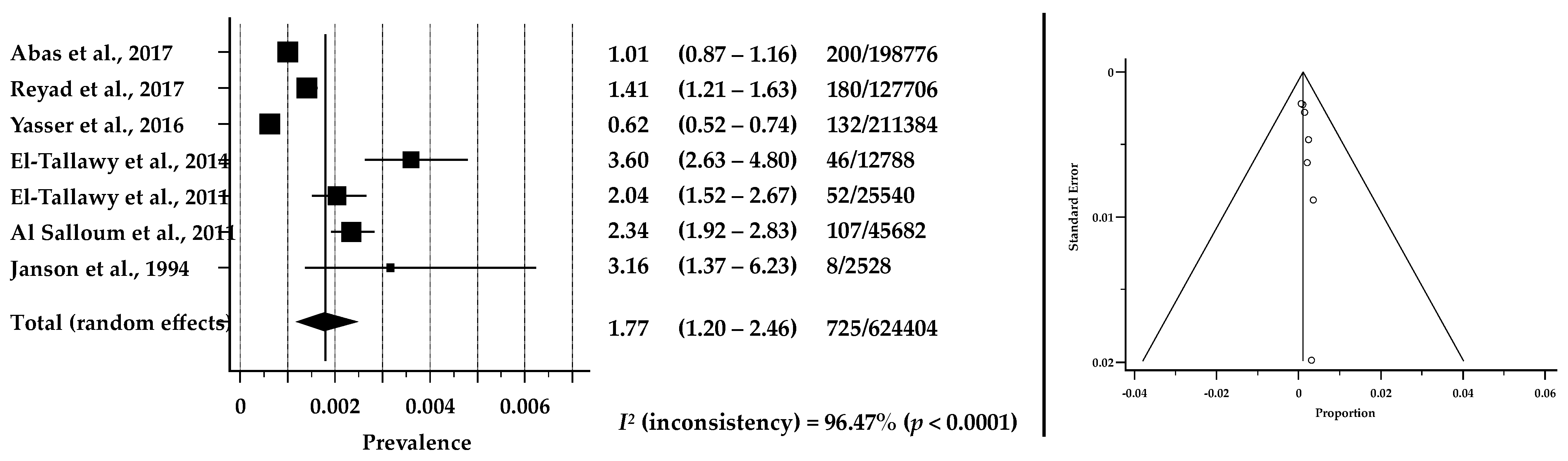
3.3. Prevalence of CP in ASCs
3.4. Motor Types and Severity of CP in ASCs
3.5. Risk Factors of CP in ASCs
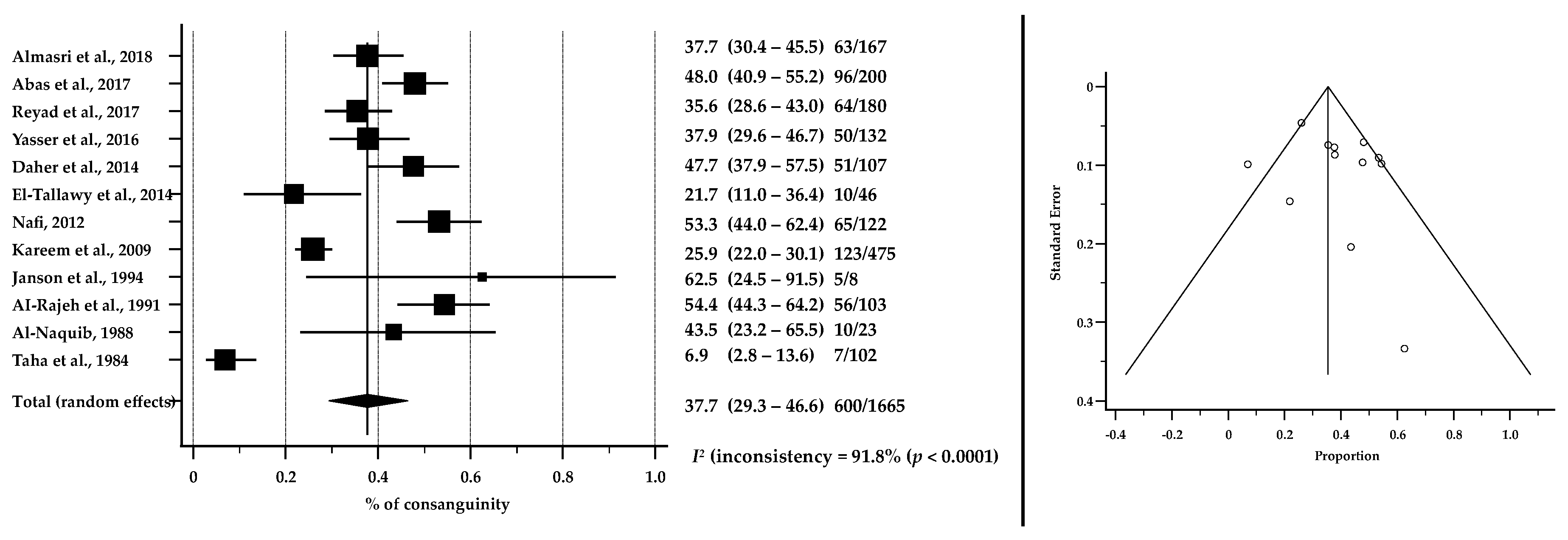
3.6. Consanguinity as a Risk Factor for CP in ASCs
3.7. Rehabilitation Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wimalasundera, N.; Stevenson, V.L. Cerebral palsy. Pract. Neurol. 2016, 16, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Badawi, N.; McIntyre, S.; Hunt, R.W. Perinatal care with a view to preventing cerebral palsy. Dev. Med. Child. Neurol. 2021, 63, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Larsen, M.L.; Rackauskaite, G.; Greisen, G.; Laursen, B.; Uldall, P.; Krebs, L.; Hoei-Hansen, C.E. Declining prevalence of cerebral palsy in children born at term in Denmark. Dev. Med. Child. Neurol. 2021, 64, 715–722. [Google Scholar] [CrossRef]
- Galea, C.; McIntyre, S.; Smithers-Sheedy, H.; Reid, S.M.; Gibson, C.; Delacy, M.; Watson, L.; Goldsmith, S.; Badawi, N.; Blair, E. Cerebral palsy trends in Australia (1995–2009): A population-based observational study. Dev. Med. Child. Neurol. 2019, 61, 186–193. [Google Scholar] [CrossRef]
- Sellier, E.; Platt, M.J.; Andersen, G.L.; Krägeloh-Mann, I.; De La Cruz, J.; Cans, C. Decreasing prevalence in cerebral palsy: A multi-site European population-based study, 1980 to 2003. Dev. Med. Child. Neurol. 2016, 58, 85–92. [Google Scholar] [CrossRef]
- Stanley, F.J.; Blair, E.; Alberman, E. Cerebral Palsies: Epidemiology and Causal Pathways; Cambridge University Press: Cambridge, UK, 2000. [Google Scholar]
- Cerebral Palsy Alliance. Types of Cerebral Palsy. Available online: https://cerebralpalsy.org.au/our-research/about-cerebral-palsy/what-is-cerebral-palsy/types-of-cerebral-palsy/ (accessed on 8 January 2022).
- Hidecker, M.J.; Paneth, N.; Rosenbaum, P.L.; Kent, R.D.; Lillie, J.; Eulenberg, J.B.; Chester, K., Jr.; Johnson, B.; Michalsen, L.; Evatt, M.; et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev. Med. Child. Neurol. 2011, 53, 704–710. [Google Scholar] [CrossRef]
- Palisano, R.; Rosenbaum, P.; Walter, S.; Russell, D.; Wood, E.; Galuppi, B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev. Med. Child. Neurol. 1997, 39, 214–223. [Google Scholar] [CrossRef]
- Öhrvall, A.M.; Krumlinde-Sundholm, L.; Eliasson, A.C. The stability of the Manual Ability Classification System over time. Dev. Med. Child. Neurol. 2014, 56, 185–189. [Google Scholar] [CrossRef]
- Paulson, A.; Vargus-Adams, J. Overview of Four Functional Classification Systems Commonly Used in Cerebral Palsy. Children 2017, 4, 30. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Causes and Risk Factors of Cerebral Palsy. Available online: https://www.cdc.gov/ncbddd/cp/causes.html (accessed on 8 January 2022).
- Cerebral Palsy Alliance. What Causes Cerebral Palsy? Available online: https://cerebralpalsy.org.au/our-research/about-cerebral-palsy/what-is-cerebral-palsy/causes/ (accessed on 8 January 2022).
- Nelson, K.B.; Grether, J.K. Causes of cerebral palsy. Curr. Opin. Pediatrics 1999, 11, 487–491. [Google Scholar] [CrossRef] [PubMed]
- UNDP—UN’s Global Development Network. The Regional Bureau for Arab States (RBAS). Available online: https://www.arabstates.undp.org/content/rbas/en/home/about-us.html (accessed on 25 January 2022).
- The World Bank Group. Surface Area (sq. km)—Arab World. Available online: https://data.worldbank.org/indicator/AG.SRF.TOTL.K2?end=2018&locations=1A&start=1961&view=chart (accessed on 25 January 2022).
- Al-Gazali, L.; Hamamy, H. Consanguinity and Dysmorphology in Arabs. Hum. Hered. 2014, 77, 93–107. [Google Scholar] [CrossRef] [PubMed]
- UK Government. The Arab World: An Introduction To Cultural Appreciation. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/16871/arab_world_introduction_cultural_appreciation_booklet.pdf (accessed on 25 January 2022).
- The World Bank. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519#High_income (accessed on 16 January 2022).
- United Nations—Department of Economic and Social Affairs. Population by Age Groups—Both Sexes. Available online: https://population.un.org/wpp/Download/Standard/Population/ (accessed on 20 November 2021).
- The World Bank Group. Arab World. Available online: https://data.worldbank.org/country/1A (accessed on 25 January 2022).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Mushta, S.; McIntyre, S.; King, C.; Goldsmith, S.; Smithers-Sheedy, H.; Badahdah, A.; Rashid, H.; Badawi, N.; Khandaker, G. Epidemiology of Cerebral Palsy in the Arabic-Speaking Countries: A Systematic Review and Meta-Analysis. PROSPERO 2020 CRD42020172159. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020172159 (accessed on 7 January 2022).
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 29 August 2021).
- Murad, M.H.; Sultan, S.; Haffar, S.; Bazerbachi, F. Methodological quality and synthesis of case series and case reports. BMJ Evid. Based Med. 2018, 23, 60–63. [Google Scholar] [CrossRef]
- MedCalc Software Ltd. MedCalc® Statistical Software, 20.110; MedCalc Software Ltd.: Ostend, Belgium, 2022. [Google Scholar]
- Almuneef, A.R.; Almajwal, A.; Alam, I.; Abulmeaty, M.; Bader, B.A.; Badr, M.F.; Almuammar, M.; Razak, S. Malnutrition is common in children with cerebral palsy in Saudi Arabia—A cross-sectional clinical observational study. BMC Neurol. 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Abolfotouh, M.A.; Al Saif, S.; Altwaijri, W.A.; Al Rowaily, M.A. Prospective study of early and late outcomes of extremely low birthweight in Central Saudi Arabia. BMC Pediatr. 2018, 18, 1–9. [Google Scholar] [CrossRef]
- Al Salloum, A.A.; El Mouzan, M.I.; Al Omar, A.A.; Al Herbish, A.S.; Qurashi, M.M. The prevalence of neurological disorders in saudi children: A community-based study. J. Child Neurol. 2011, 26, 21–24. [Google Scholar] [CrossRef]
- Al-Asmari, A.; Al Moutaery, K.; Akhdar, F.; Al Jadid, M. Cerebral palsy: Incidence and clinical features in Saudi Arabia. Disabil. Rehabil. 2006, 28, 1373–1377. [Google Scholar] [CrossRef]
- Al-Sulaiman, A.A.; Bademosi, O.F.; Ismail, H.M.; Al-Quliti, K.W.; Al-Shammary, S.F.; Abumadini, M.S.; Al-Umran, K.U.; Magbool, G.M. Cerebral palsy in Saudi children. Neurosciences 2003, 8, 26–29. [Google Scholar]
- Izuora, G.I.; Anis, A.S. Neurological disorders in Saudi children at the Al-Majardah General Hospital, Asir Region. Ann. Saudi Med. 1992, 12, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajeh, S.; Bademosi, O.; Awada, A.; Ismail, H.; Al-Shammasi, S.; Dawodu, A. CEREBRAL PALSY IN SAUDI ARABIA: A CASE-CONTROL STUDY OF RISK FACTORS. Dev. Med. Child Neurol. 1991, 33, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Al-naquib, N. Neuro-developmental problems in children in riyadh, saudi arabia: 1-year’s experience in a family practice centre. J. Trop. Pediatr. 1988, 34, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Alfrayh, A.; Naquib, N.A. The pattern of central nervous disease in children in king khalid university hospital in Riyadh, Saudi Arabia. J. Trop. Pediatr. 1987, 33, 124–130. [Google Scholar] [CrossRef]
- Taha, S.A.; Mahdi, A.H. Cerebral palsy in Saudi Arabia: A clinical study of 102 cases. Ann. Trop. Paediatr. 1984, 4, 155–158. [Google Scholar] [CrossRef]
- Almasri, N.A.; Dunst, C.J.; Saleh, M.; Okasheh, R. Determinants of Utilization of Health Services Provided for Children with Cerebral Palsy in Jordan. J. Dev. Phys. Disabil. 2019, 31, 205–217. [Google Scholar] [CrossRef]
- Almasri, N.A.; Saleh, M.; Abu-Dahab, S.; Malkawi, S.H.; Nordmark, E. Development of a Cerebral Palsy Follow-up Registry in Jordan (CPUP-Jordan). Child Care Health Dev. 2018, 44, 131–139. [Google Scholar] [CrossRef]
- Almasri, N.A.; Saleh, M.; Abu-Dahab, S.; Malkawi, S.H.; Nordmark, E. Functional profiles of children with cerebral palsy in Jordan based on the association between gross motor function and manual ability. BMC Pediatr. 2018, 18, 1–8. [Google Scholar] [CrossRef]
- Saleh, M.; Almasri, N.A. Use of the Measure of Processes of Care (MPOC-20) to evaluate health service delivery for children with cerebral palsy and their families in Jordan: Validation of Arabic-translated version (AR- MPOC-20). Child Care Health Dev. 2014, 40, 680–688. [Google Scholar] [CrossRef]
- Nafi, O.A. Clinical spectrum of cerebral palsy in South Jordan: Analysis of 122 cases. Jordan Med. J. 2012, 46, 210–215. [Google Scholar] [CrossRef][Green Version]
- Al-Ajlouni, S.; Alagrabawi, M.; Al-Balas, H.; Alawneh, M.; Daoud, A. Cerebral palsy in Jordan: Clinical and neuroimaging characteristics. Jordan Med. J. 2008, 42, 162–169. [Google Scholar]
- Al Ajlouni, S.F.; Aqrabawi, M.; Oweis, N.; Daoud, A.S. Clinical spectrum of cerebral palsy in Jordanian children: An analysis of 200 cases. J. Pediatr. Neurol. 2006, 4, 251–255. [Google Scholar] [CrossRef]
- Janson, S.; Dawani, H. Chronic illness in preschool Jordanian children. Ann. Trop. Paediatr. 1994, 14, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Reyad, A.; Abdelaziem, F.H.; Kilany, A. Physical Therapy Registry for Establishment of Cerebral Palsy in Alexandria City (Almontazah District), Egypt. IOSR J. Nurs. Health Sci. 2017, 6, 20–24. [Google Scholar] [CrossRef]
- Abas, O.; Abdelaziem, F.; Kilany, A. Clinical spectrum of cerebral palsy and associated disability in South Egypt: A local survey study. Maced. J. Med. Sci. 2017, 5, 37–41. [Google Scholar] [CrossRef]
- Yasser, S.; Abdelaziem, F.; Eltallawy, H. Establish Registry of Cerebral Palsy in Mit-Ghamer City, Egypt. Ph.D. Thesis, Cairo University, Giza, Egypt, 2016. [Google Scholar]
- El-Tallawy, H.N.; Farghaly, W.M.A.; Shehata, G.A.; Rageh, T.A.; Metwally, N.A.; Badry, R.; Sayed, M.A.M.; El Hamed, M.A.; Abd-Elwarth, A.; Kandil, M.R. Cerebral palsy in Al-Quseir City, Egypt: Prevalence, subtypes, and risk factors. Neuropsychiatr. Dis. Treat. 2014, 10, 1267–1272. [Google Scholar] [CrossRef]
- El-Tallawy, H.N.; Farghaly, W.M.A.; Shehata, G.A.; Badry, R.; Rageh, T.A. Epileptic and cognitive changes in children with cerebral palsy: An Egyptian study. Neuropsychiatr. Dis. Treat. 2014, 10, 971–975. [Google Scholar] [CrossRef]
- El-Tallawy, H.N.; Farghaly, W.M.A.; Shehata, G.A.; Metwally, N.A.; Rageh, T.A.; Abo-Elfetoh, N. Epidemiology of cerebral palsy in El-Kharga District-New Valley (Egypt). Brain Dev. 2011, 33, 406–411. [Google Scholar] [CrossRef]
- Khadir, S.; Issa, S.A. Magnetic resonance imaging findings in patients with cerebral palsy in Duhok, Iraq: Case series. J. Surg. Med. 2020, 4, 1–4. [Google Scholar] [CrossRef]
- Ahmed, A.; Salman, M.N.A.; Sarhat, A.R. Cerebral palsy epidemiology in Tikrit-Iraq. Indian J. Public Health Res. Dev. 2019, 10, 942–947. [Google Scholar] [CrossRef]
- Kareem, A.A.; Kamel, M.A.S. Risk factors and clinical profiles in Iraqi children with cerebral palsy. New Iraqi J. Med. 2009, 5, 64–68. [Google Scholar]
- Hassan, K.H. Cerebral palsy among Kurdish children in the city of Dohuk: A case-series study. Jordan Med. J. 2009, 43, 205–211. [Google Scholar]
- Salih, K. Pattern of Cerebral Palsy Among Sudanese Children Less Than 15 Years of Age. Cureus 2020, 12, e7232. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, H.; Satti, M.; Rayis, D.A.; Imam, A.M.; Adam, I. Intra-partum fever and cerebral palsy in Khartoum, Sudan. BMC Res. Notes 2013, 6, 1–4. [Google Scholar] [CrossRef]
- Khan, M.A. Intellectual and developmental assessment of cerebral palsy cases in Libyan city. Indian J. Med. Sci. 1992, 46, 235–238. [Google Scholar]
- Daher, S.; El-Khairy, L. Association of cerebral palsy with consanguineous parents and other risk factors in a Palestinian population. East. Mediterr. Health J. 2014, 20, 459–468. [Google Scholar] [CrossRef]
- O’Shea, T.M. Diagnosis, treatment, and prevention of cerebral palsy. Clin Obs. Gynecol 2008, 51, 816–828. [Google Scholar] [CrossRef]
- Strickland, A.D. Prevention of cerebral palsy, autism spectrum disorder, and attention deficit—Hyperactivity disorder. Med. Hypotheses 2014, 82, 522–528. [Google Scholar] [CrossRef]
- Conde-Agudelo, A.; Romero, R. Antenatal magnesium sulfate for the prevention of cerebral palsy in preterm infants less than 34 weeks’ gestation: A systematic review and metaanalysis. Am. J. Obstet. Gynecol. 2009, 200, 595–609. [Google Scholar] [CrossRef]
- Stavsky, M.; Mor, O.; Mastrolia, S.A.; Greenbaum, S.; Than, N.G.; Erez, O. Cerebral Palsy-Trends in Epidemiology and Recent Development in Prenatal Mechanisms of Disease, Treatment, and Prevention. Front. Pediatr. 2017, 5, 21. [Google Scholar] [CrossRef]
- Centrers for Disease Control and Prevention. Data and Statisticss for Cerebral Palsy, Prevalence and Characteristics. Available online: https://www.cdc.gov/ncbddd/cp/data.html (accessed on 12 January 2022).
- Khandaker, G.; Muhit, M.; Karim, T.; Smithers-Sheedy, H.; Novak, I.; Jones, C.; Badawi, N. Epidemiology of cerebral palsy in Bangladesh: A population-based surveillance study. Dev. Med. Child Neurol. 2019, 61, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Muhit, M.; Hardianto, D.; Laryea, F.; Chhetri, A.B.; Smithers-Sheedy, H.; McIntyre, S.; Badawi, N.; Khandaker, G. Epidemiology of cerebral palsy in low-and middle-income countries: Preliminary findings from an international multi-centre cerebral palsy register. Dev. Med. Child Neurol. 2021, 63, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Kakooza-Mwesige, A.; Andrews, C.; Peterson, S.; Wabwire Mangen, F.; Eliasson, A.C.; Forssberg, H. Prevalence of cerebral palsy in Uganda: A population-based study. Lancet Glob. Health 2017, 5, e1275–e1282. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke. Cerebral Palsy: Hope Through Research. Available online: https://www.ninds.nih.gov/Disorders/Patient-Caregiver-Education/Hope-Through-Research/Cerebral-Palsy-Hope-Through-Research (accessed on 12 January 2022).
- Naeye, R.L.; Peters, E.C.; Bartholomew, M.; Landis, J.R. Origins of Cerebral Palsy. Am. J. Dis. Child. 1989, 143, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Badawi, N.; Felix, J.F.; Kurinczuk, J.J.; Dixon, G.; Watson, L.; Keogh, J.M.; Valentine, J.; Stanley, F.J. Cerebral palsy following term newborn encephalopathy: A population-based study. Dev. Med. Child Neurol. 2005, 47, 293–298. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, S.; Badawi, N.; Brown, C.; Blair, E. Population Case-Control Study of Cerebral Palsy: Neonatal Predictors for Low-Risk Term Singletons. Pediatrics 2011, 127, e667–e673. [Google Scholar] [CrossRef]
- Al-Ghanim, K.A. Consanguineous marriage in the Arab societies. J. Psychol. Clin. Psychiatry 2020, 11, 166–168. [Google Scholar] [CrossRef]
- Al-Kandari, Y.Y.; Al-Kandari, Y.Y. Consanguineous Marriage and its Relationship with Sociocultural Variables in Urban and Bedouin Geographical Regions in Kuwait. Arab. Humanit. 2019, 10. [Google Scholar] [CrossRef]
- Alharbi, O.A.; Al-Shaia, W.A.; Al-Hamam, A.A.; Al-Marzoug, H.M.; Ahmed, A.E.; Bagha, M. Attitude of Saudi Arabian adults towards consanguineous marriage. Qatar Med. J. 2015, 2015, 12. [Google Scholar] [CrossRef]
- Tadmouri, G.O.; Nair, P.; Obeid, T.; Al Ali, M.T.; Al Khaja, N.; Hamamy, H.A. Consanguinity and reproductive health among Arabs. Reprod. Health 2009, 6, 17. [Google Scholar] [CrossRef]
- Khlat, M. Endogamy in the Arab world. Oxf. Monogr. Med. Genet. 1997, 30, 63–82. [Google Scholar]
- Jabbour, S.; Yamout, R. Public Health in the Arab World; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Novak, I.; Morgan, C.; Fahey, M.; Finch-Edmondson, M.; Galea, C.; Hines, A.; Langdon, K.; Namara, M.M.; Paton, M.C.B.; Popat, H.; et al. State of the Evidence Traffic Lights 2019: Systematic Review of Interventions for Preventing and Treating Children with Cerebral Palsy. Curr. Neurol. Neurosci. Rep. 2020, 20, 3. [Google Scholar] [CrossRef] [PubMed]
- The Cerebral Palsy Registry in Jordan—Cpup. Available online: http://sites.ju.edu.jo/en/cpup/Home.aspx (accessed on 20 May 2022).
- El Rifai, M.R.; Ramia, S.; Moore, V. Cerebral palsy in Riyadh, Saudi Arabia: I. Aetiological factors. Ann. Trop. Paediatr. 1984, 4, 7–12. [Google Scholar] [CrossRef]
- El Rifai, M.R.; Ramia, S.; Moore, V. Cerebral palsy in Riyadh, Saudi Arabia: II. Associations between gestational age, birthweight and cerebral palsy. Ann. Trop. Paediatr. 1984, 4, 13–17. [Google Scholar] [CrossRef]
- Al-Wazna, T.; Bamgboye, E.A. Characteristics of institutionalized disabled in Saudi Arabia. Saudi Med. J. 1997, 18, 70–73. [Google Scholar]
- Al-Sulaiman, A.A. Neurological disorders in institutionalized patients in the Eastern Province of Saudi Arabia. Saudi Med. J. 1997, 18, 387–389. [Google Scholar]
- El Tallawy, H.N.A.; Farghaly, W.M.A.; Rageh, T.A.; Shehata, G.A.; Badry, R.; Metwally, N.A.; El Moselhy, E.A.; Hassan, M.; Sayed, M.A.; Waris, A.A.; et al. Door-to-door survey of major neurological disorders (project) in Al Quseir City, Red Sea Governorate, Egypt. Neuropsychiatr. Dis. Treat. 2013, 9, 767–771. [Google Scholar] [CrossRef]
- El Tallawy, H.N.A.; Farghaly, W.M.A.; Rageh, T.A.; Shehata, G.A.; Metwaly, N.A.; Elftoh, N.A.; Hegazy, A.M.; El Moselhy, E.A.; Rayan, I.; Al Fawal, B.M.A.; et al. Epidemiology of major neurological disorders project in Al Kharga District, New Valley, Egypt. Neuroepidemiology 2010, 35, 291–297. [Google Scholar] [CrossRef]
- Al Rajeh, S.; Bademosi, O.; Ismail, H.; Awada, A.; Dawodu, A.; Al-Freihi, H.; Assuhaimi, S.; Borollosi, M.; Al-Shammasi, S. A community survey of neurological disorders in Saudi Arabia: The thugbah study. Neuroepidemiology 1993, 12, 164–178. [Google Scholar] [CrossRef]
- Al-Tunaiki, M.H.; Al-Falahi, L.A. Specialized seating program in riyadh. J. Prosthet. Orthot. 1994, 6, 52–56. [Google Scholar] [CrossRef]
- Saleh, M.; Almasri, N.A. Cerebral palsy in Jordan: Demographics, medical characteristics, and access to services. Children’s Health Care 2017, 46, 49–65. [Google Scholar] [CrossRef]


| # | Country/State 1 | Classification by Income | Children and Adolescents Aged 0–19 Years 2 N (% of Total Population) | Studies Included (N) |
|---|---|---|---|---|
| 1 | Algeria | LMIC | 16,409,237 (37) | – |
| 2 | Bahrain | HIC | 399,990 (24) | – |
| 3 | Comoros | LMIC | 428,906 (49) | – |
| 4 | Djibouti | LMIC | 376,430 (38) | – |
| 5 | Egypt | LMIC | 43,413,971 (42) | 6 |
| 6 | Emirates | HIC | 1,854,704 (19) | – |
| 7 | Iraq | UMIC | 19,320,987 (48) | 4 |
| 8 | Jordan | UMIC | 4,392,416 (43) | 8 |
| 9 | Kuwait | HIC | 1,141,552 (27) | – |
| 10 | Lebanon | UMIC | 2,287,154 (34) | – |
| 11 | Libya | UMIC | 2,471,165 (36) | 1 |
| 12 | Mauritania | LMIC | 2,315,383 (50) | – |
| 13 | Morocco | LMIC | 12,849,811 (35) | – |
| 14 | Oman | HIC | 1,362,877 (27) | – |
| 15 | Palestine 3 | LMIC | 2,474,021 (48) | 1 |
| 16 | Qatar | HIC | 498,936 (17) | – |
| 17 | Saudi Arabia | HIC | 10,816,497 (31) | 10 |
| 18 | Somalia | LIC | 9,152,954 (58) | – |
| 19 | Sudan | LIC | 22,252,463 (51) | 2 |
| 20 | Syria | LIC | 6,961,028 (40) | – |
| 21 | Tunisia | LMIC | 3,657,697 (31) | – |
| 22 | Yemen | LIC | 14,783,682 (50) | – |
| Total (N) studies | 32 | |||
| First Author, Year | Country (Economic Profile) 1 | Design | Setting | Population | Study Period (Year) 2 | Children with CP (N) | Participants’ Age in Years (Mean) Range 3 | Male % | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Reyad et al., 2017 [47] | Egypt (LMIC) | Cross-sectional | Hospitals and rehabilitation centres | Children with CP | 7 months | 180 | (NR) 0.5–15 | 58.0 |
| 2 | Abas et al., 2017 [48] | Egypt (LMIC) | Prospective cross-sectional; individual assessment | Rehabilitation centres | Children with CP | 7 months (2015) | 200 | (NR) 0.25–18 | 55.0 |
| 3 | Yasser et al., 2016 [49] | Egypt (LMIC) | Cross-sectional | Hospitals and rehabilitation centres | Children with CP | No information | 132 | (NR) 0.5–15 | 59.1 |
| 4 | El-Tallawy et al., 2014 [50] | Egypt (LMIC) | Cross-sectional; door-to-door; with comparison group | Community | All residents | No information | 46 | (10.3) NR | 66.7 |
| 5 | El-Tallawy et al., 2014 [51] | Egypt (LMIC) | Cross-sectional, descriptive, population-based, and case-control | Community | Children with CP | No information | 98 | (7.9) NR | 53.1 |
| 6 | El-Tallawy et al., 2011 [52] | Egypt (LMIC) | Cross-sectional; door-to-door | Community | All residents | 17 years (1990–2007) | 52 | (7.17) NR | 44.2 |
| 7 | Khadir et al., 2020 [53] | Iraq (UMIC) | Case-series | Hospital | Children with CP | 8 months (2016–2017) | 48 | (1.8) <1–5 | 70.8 |
| 8 | Salman et al., 2019 [54] | Iraq (UMIC) | Case-control | Hospital | Children with CP | 5 months (2012–2013) | 100 | (NR) 4 0.25–13 | 58.0 |
| 9 | Kareem et al., 2009 [55] | Iraq (UMIC) | Case-series | Hospital | Children with CP | 40 months (2005–2008) | 475 | (6.67) 0.83–12.5 | 63.8 |
| 10 | Hassan, 2009 [56] | Iraq (UMIC) | Case-series | Rehabilitation centre | Children with CP | 1 year (2002–2003) | 100 | (NR) 0–1.5 | 57.0 |
| 11 | Almasri et al., 2019 [39] | Jordan (UMIC) | Cross-sectional | Hospital | Children with CP | No information | 116 | (4.6) NR | 53.4 |
| 12 | Almasri et al., 2018 [40] | Jordan (UMIC) | Population-based case-series | Hospitals, rehabilitation centres, and schools for children with CP | Children with CP | 2 years (2013–2015) | 167 | (3.6) NR | 58.3 |
| 13 | Almasri et al., 2018 [41] | Jordan (UMIC) | Population-based case-series | Hospitals, rehabilitation centres, and schools | Children with CP | No information | 124 | 2–16 (4.5) | 55.6 |
| 14 | Saleh et al., 2013 [42] | Jordan (UMIC) | Case-series | Hospitals | Children with CP and their families | No information | 114 | (NR) 0.08–17 | 53.5 |
| 15 | Nafi, 2012 [43] | Jordan (UMIC) | Case-series | Early Diagnostic Centre | Children with CP | 32 months (2007–2010) | 122 | (6.3) 0.58–17 | 54.1 |
| 16 | Al-Ajlouni et al., 2008 [44] | Jordan (UMIC) | Case-series | Hospital | Children with CP | 2 years (2006–2007) | 158 | No information | 53.0 |
| 17 | Al Ajlouni et al., 2006 [45] | Jordan (UMIC) | Cross-sectional; individual assessment | Hospital | Children with CP | 15 years (1990–2005) | 200 | (3.19) 0.08–15 | 69.0 |
| 18 | Janson et al., 1994 [46] | Jordan (UMIC) | Cross-sectional | Community | All children | No information | 8 | (NR) 0–7 | 50.6 |
| 19 | Khan, 1992 [59] | Libya (UMIC) | Case-control | Hospital | Children with CP | 4 years (1983–1987) | 60 | (NR) 1–15 | 65.0 |
| 20 | Daher et al., 2014 [60] | Palestine (LMIC) | Case-control | Hospitals and rehabilitation centres | Children with CP | 8 months (2011) | 107 | (3.87) 1–15 | 54.2 |
| 21 | Almuneef et al., 2019 [29] | Saudi Arabia (HIC) | Cross-sectional; individual assessment | Rehabilitation centre | Children with CP | 8 months (2015) | 74 | (5.6) 1–12 | 59.5 |
| 22 | Abolfotouh et al., 2018 [30] | Saudi Arabia (HIC) | Retrospective/prospective cohort | Hospital | New-borns with extreme low birth weight (ELBW) | 3 years (2005–2007) | 23 | Infants | 56.4 |
| 23 | Al Salloum et al., 2011 [31] | Saudi Arabia (HIC) | Cross-sectional | Community | All residents | 2 years (2004–2005) | 107 | (NR) 0–19 | 61.7 |
| 24 | Al-Asmari et al., 2006 [32] | Saudi Arabia (HIC) | Case-series | Hospital | All children | 20 years (1984–2003) | 412 | (NR) 1–10 | 62.6 |
| 25 | Al-Sulaiman et al., 2003 [33] | Saudi Arabia (HIC) | Case-series | Hospital | Children with CP | 1 year (2000) | 187 | (1.7) 1–3 | 58.3 |
| 26 | Izuora et al., 1992 [34] | Saudi Arabia (HIC) | Case-series | Hospital | Children with neurological problems | 18 months (1988–1990) | 34 | (4.4) 0–12 5 | 57.2 |
| 27 | Al-Rajeh et al., 1991 [35] | Saudi Arabia (HIC) | Case-control | Hospital | Children with CP + control group | 5 years (1984–1988) | 103 | (3.8) 1–12 | 55.3 |
| 28 | Al-Naquib, 1988 [36] | Saudi Arabia (HIC) | Case-series; individual assessment | Hospital | Children with neurological problems | 1 year (1984–1985) | 23 | (NR) 0.08–14 | 35.0 |
| 29 | Alfrayh et al., 1987 [37] | Saudi Arabia (HIC) | Retrospective chart review | Hospital | Children with neurological problems | 18 months (1982–1983) | 52 | (NR) 0.25–15 | 36.5 |
| 30 | Taha et al., 1984 [38] | Saudi Arabia (HIC) | Case-series | Hospital | Children with CP | 3 years (1980–1983) | 102 | (NR) 2–9 | 59.8 |
| 31 | Salih, 2020 [57] | Sudan (LIC) | Retrospective hospital-based | Hospital | Children with CP | 3 years | 108 | No information | 54.6 |
| 32 | Abdullahi et al., 2013 [58] | Sudan (LIC) | Case-control | Hospital | Children with CP | 6 months (2012) | 111 | (4.1) 1–11 | 53.2 |
| Types of CP | Number of Included Children 1 | Total % (Random Effects) | 95% CI | Heterogeneity I2 (Inconsistency) % | Publication Bias (Kendall’s Tau) |
|---|---|---|---|---|---|
| Spastic (all) | 2099 | 59.8 | 46.2–72.7 | 98.6 | −0.2523; p = 0.0595 |
| Quadriplegic | 1024 | 25.1 | 18.2–32.8 | 96.2 | −0.01328; p = 0.9210 |
| Diplegic | 778 | 16.9 | 11.4–23.3 | 95.7 | −0.1301; p = 0.3311 |
| Hemiplegic | 436 | 10.4 | 7.3–13.8 | 90.1 | −0.03453; p = 0.7965 |
| Dyskinetic/Athetoid | 218 | 4.4 | 2.9–6.2 | 82.1 | −0.2899; p = 0.0304 |
| Ataxic | 121 | 2.7 | 1.5–4.2 | 83.2 | −0.04515; p = 0.7360 |
| Hypotonic/Atonic | 228 | 4.1 | 1.7–7.3 | 94.4 | 0.1089; p = 0.4161 |
| Mixed | 171 | 4.6 | 2.3–7.7 | 93.0 | 0.3958; p = 0.0031 |
| Unclassified | 292 | 3.7 | 1.5–6.9 | 94.8 | 0.1463; p = 0.2747 |
| Country (Economic Profile) | Setting | GMFCS N (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Level I | Level II | Level III | Level I–III | Level IV | Level V | Level IV–V | Unclassified | |||
| Almuneef et al., 2019 [29] | Saudi Arabis (HIC) | Rehabilitation centre | - | - | - | 22 (29.7) | - | - | 52 (70.3) | - |
| Almasri et al., 2019 [39] | Jordan (UMIC) | Cross-sectional | 19 (16.4) | 14 (12.1) | 24 (20.7) | 57 (49.1) | 31 (26.7) | 25 (21.6) | 56 (48.3) | 3 (2.6) |
| Almasri et al., 2018 [40] | Jordan (UMIC) | Population-based case-series | 18 (10.7) | 33 (19.6) | 19 (11.3) | 70 (41.7) | 51 (30.4) | 44 (26.2) | 95 (56.6) | - |
| Almasri et al., 2018 [41] | Jordan (UMIC) | Population-based case-series | 16 (13.1) | 28 (23.0) | 15 (12.3) | 59 (48.4) | 41 (33.6) | 22 (18.0) | 63 (51.6) | - |
| Abas et al., 2017 [48] | Egypt (LMIC) | Prospective cross-sectional; individual assessment | 19 (9.5) | 45 (22.5) | 66 (33.0) | 130 (65.0) | 39 (19.5) | 31 (15.5) | 70 (35.0) | - |
| Reyad et al., 2017 [47] | Egypt (LMIC) | Cross-sectional | 11 (6.1) | 40 (22.2) | 64 (35.6) | 115 (63.9) | 55 (30.5) | 10 (5.5) | 65 (36.1) | - |
| Yasser et al., 2016 [49] | Egypt (LMIC) | Cross-sectional | 14 (10.6) | 23 (17.4) | 36 (27.3) | 73 (55.3) | 20 (15.2) | 39 (29.6) | 59 (44.7) | - |
| Saleh et al., 2013 [42] | Jordan (UMIC) | Case-series | 18 (15.8) | 14 (12.3) | 24 (21.1) | 56 (49.1) | 31 (27.2) | 25 (21.9) | 56 (49.1) | 2 (1.8) |
| Risk Factors | Total % (Random Effects) | 95% CI | Heterogeneity I2 (Inconsistency) % | Publication Bias (Kendall’s Tau) |
|---|---|---|---|---|
| Consanguinity 1 | 37.7 | 29.3–46.6 | 91.8 | 0.09091; p = 0.6808 |
| Unknown | 30.7 | 20.3–42.2 | 98.0 | 0.1113; p = 0.4253 |
| Birth asphyxia | 16.0 | 8.8–24.9 | 97.6 | 0.07419; p = 0.5951 |
| Preterm | 12.2 | 6.9–18.7 | 96.5 | −0.1731; p = 0.2150 |
| Birth weight <2500 gm | 9.7 | 4.4–16.8 | 97.4 | 0.006182; p = 0.9647 |
| Neonatal Jaundice | 5.9 | 1.9–11.9 | 97.5 | 0.3277; p = 0.0189 |
| Acquired infections | 3.3 | 1.4–6.1 | 93.3 | 0.2040; p = 0.1439 |
| NICU admission | 2.5 | 0.4–6.5 | 97.0 | 0.6749; p < 0.0001 |
| Family history of CP 2 | 2.2 | 1.0–4.1 | 89.0 | 0.5209; p = 0.0002 |
| Multiple birth 3 | 1.9 | 0.6–3.9 | 91.5 | 0.3158; p = 0.0237 |
| Medical condition of mother 4 | 1.8 | 0.5–3.9 | 93.2 | 0.5139; p = 0.0002 |
| Congenital infections 5 | 1.3 | 0.4–2.8 | 89.3 | 0.6059; p < 0.0001 |
| Injury/trauma | 0.9 | 0.3–2.0 | 84.7 | 0.6502; p < 0.0001 |
| Assisted reproduction | 0.2 | 0.1–0.4 | 0.0 | 0.9068; p < 0.0001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mushta, S.M.; King, C.; Goldsmith, S.; Smithers-Sheedy, H.; Badahdah, A.-M.; Rashid, H.; Badawi, N.; Khandaker, G.; McIntyre, S. Epidemiology of Cerebral Palsy among Children and Adolescents in Arabic-Speaking Countries: A Systematic Review and Meta-Analysis. Brain Sci. 2022, 12, 859. https://doi.org/10.3390/brainsci12070859
Mushta SM, King C, Goldsmith S, Smithers-Sheedy H, Badahdah A-M, Rashid H, Badawi N, Khandaker G, McIntyre S. Epidemiology of Cerebral Palsy among Children and Adolescents in Arabic-Speaking Countries: A Systematic Review and Meta-Analysis. Brain Sciences. 2022; 12(7):859. https://doi.org/10.3390/brainsci12070859
Chicago/Turabian StyleMushta, Sami Mukhdari, Catherine King, Shona Goldsmith, Hayley Smithers-Sheedy, Al-Mamoon Badahdah, Harunor Rashid, Nadia Badawi, Gulam Khandaker, and Sarah McIntyre. 2022. "Epidemiology of Cerebral Palsy among Children and Adolescents in Arabic-Speaking Countries: A Systematic Review and Meta-Analysis" Brain Sciences 12, no. 7: 859. https://doi.org/10.3390/brainsci12070859
APA StyleMushta, S. M., King, C., Goldsmith, S., Smithers-Sheedy, H., Badahdah, A.-M., Rashid, H., Badawi, N., Khandaker, G., & McIntyre, S. (2022). Epidemiology of Cerebral Palsy among Children and Adolescents in Arabic-Speaking Countries: A Systematic Review and Meta-Analysis. Brain Sciences, 12(7), 859. https://doi.org/10.3390/brainsci12070859