Assessing the Utility of Neonatal Screening Assessments in Early Diagnosis of Cerebral Palsy in Preterm Infants †
Abstract
:1. Introduction
2. Methods
2.1. Study Design
- Normal = 0;
- PR = 1;
- PR/CS = 2;
- CS on one occasion = 3;
- CS on two or more occasions = 4.
- Present = 0;
- Sporadic = 1;
- Abnormal = 2;
- Absent = 3.
2.2. Statistical Analysis
3. Results
3.1. Demographics
3.2. Primary Outcome—Univariate Analysis—Early Neonatal Assessments vs. CP/High Risk Diagnosis
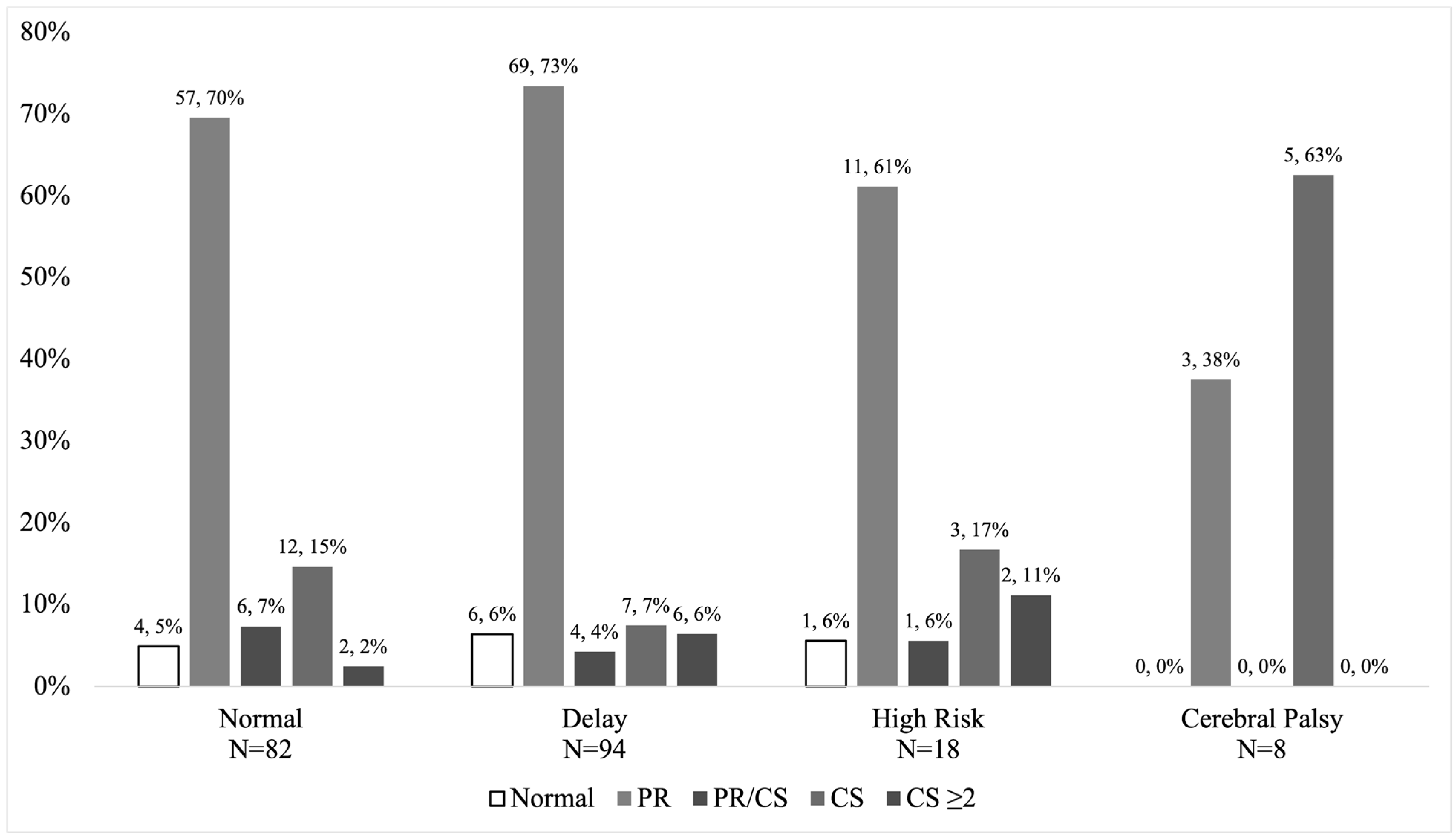
3.3. Secondary Outcome—Early Neonatal vs. Infant Assessments
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jette, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Egister, A.C.P. Report of the Australian Cerebral Palsy Register. Birth Years 1995–2012; Cerebral Palsy Alliance: Sydney, Australia, 2018. [Google Scholar]
- Register, A.C.P. Australian Cerebral Palsy Register Bulletin: Birth Years 1995–2014; Cerebral Palsy Alliance: Sydney, Australia, 2020. [Google Scholar]
- Hadders-Algra, M.; Boxum, A.G.; Hielkema, T.; Hamer, E.G. Effect of early intervention in infants at very high risk of cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2017, 59, 246–258. [Google Scholar] [CrossRef] [PubMed]
- King, A.R.; Machipisa, C.; Finlayson, F.; Fahey, M.C.; Novak, I.; Malhotra, A. Early detection of cerebral palsy in high-risk infants: Translation of evidence into practice in an Australian hospital. J. Paediatr. Child Health 2021, 57, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A systematic review of tests to predict cerebral palsy in young children. Dev. Med. Child Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef]
- Harpster, K.; Merhar, S.; Priyanka Illapani, V.S.; Peyton, C.; Kline-Fath, B.; Parikh, N.A. Associations Between Early Structural Magnetic Resonance Imaging, Hammersmith Infant Neurological Examination, and General Movements Assessment in Infants Born Very Preterm. J. Pediatr. 2021, 232, 80–86. [Google Scholar] [CrossRef]
- Aizawa, C.Y.P.; Einspieler, C.; Genovesi, F.F.; Ibidi, S.M.; Hasue, R.H. The general movement checklist: A guide to the assessment of general movements during preterm and term age. J. Pediatr. 2021, 97, 445–452. [Google Scholar] [CrossRef]
- Einspieler, C.; Prechtl, H.F. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 61–67. [Google Scholar] [CrossRef]
- Einspieler, C.; Prechtl, H.F.; Ferrari, F.; Cioni, G.; Bos, A.F. The qualitative assessment of general movements in preterm, term and young infants--review of the methodology. Early Hum. Dev. 1997, 50, 47–60. [Google Scholar] [CrossRef]
- Øberg, G.K.; Jacobsen, B.K.; Jørgensen, L. Predictive Value of General Movement Assessment for Cerebral Palsy in Routine Clinical Practice. Phys. Ther. 2015, 95, 1489–1495. [Google Scholar] [CrossRef] [Green Version]
- Pires, C.D.S.; Marba, S.T.M.; Caldas, J.P.S.; Stopiglia, M.C.S. Predictive Value of the General Movements Assessment in Preterm Infants: A Meta-Analysis. Rev. Paul. Pediatr. 2020, 38, e2018286. [Google Scholar] [CrossRef] [PubMed]
- Kwong, A.K.L.; Fitzgerald, T.L.; Doyle, L.W.; Cheong, J.L.Y.; Spittle, A.J. Predictive validity of spontaneous early infant movement for later cerebral palsy: A systematic review. Dev. Med. Child Neurol. 2018, 60, 480–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olsen, J.E.; Allinson, L.G.; Doyle, L.W.; Brown, N.C.; Lee, K.J.; Eeles, A.L.; Cheong, J.L.Y.; Spittle, A.J. Preterm and term-equivalent age general movements and 1-year neurodevelopmental outcomes for infants born before 30 weeks’ gestation. Dev. Med. Child Neurol. 2018, 60, 47–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spittle, A.J.; Boyd, R.N.; Inder, T.E.; Doyle, L.W. Predicting motor development in very preterm infants at 12 months’ corrected age: The role of qualitative magnetic resonance imaging and general movements assessments. Pediatrics 2009, 123, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.M.; Ricci, D.; Brogna, C.; Mercuri, E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: A critical review of the literature. Dev. Med. Child Neurol. 2016, 58, 240–245. [Google Scholar] [CrossRef]
- Romeo, D.M.; Cioni, M.; Scoto, M.; Mazzone, L.; Palermo, F.; Romeo, M.G. Neuromotor development in infants with cerebral palsy investigated by the Hammersmith Infant Neurological Examination during the first year of age. Eur. J. Paediatr. Neurol. 2008, 12, 24–31. [Google Scholar] [CrossRef]
- Dubowitz, L.; Mercuri, E.; Dubowitz, V. An optimality score for the neurologic examination of the term newborn. J. Pediatr. 1998, 133, 406–416. [Google Scholar] [CrossRef]
- Eeles, A.L.; Olsen, J.E.; Walsh, J.M.; McInnes, E.K.; Molesworth, C.M.; Cheong, J.L.; Doyle, L.W.; Spittle, A.J. Reliability of Neurobehavioral Assessments from Birth to Term Equivalent Age in Preterm and Term Born Infants. Phys. Occup. Ther. Pediatr. 2017, 37, 108–119. [Google Scholar] [CrossRef]
- Spittle, A.J.; Walsh, J.; Olsen, J.E.; McInnes, E.; Eeles, A.L.; Brown, N.C.; Anderson, P.J.; Doyle, L.W.; Cheong, J.L.Y. Neurobehaviour and neurological development in the first month after birth for infants born between 32–42 weeks’ gestation. Early Hum. Dev. 2016, 96, 7–14. [Google Scholar] [CrossRef]
- Ricci, D.; Romeo, D.M.; Haataja, L.; van Haastert, I.C.; Cesarini, L.; Maunu, J.; Pane, M.; Gallini, F.; Luciano, R.; Romagnoli, C.; et al. Neurological examination of preterm infants at term equivalent age. Early Hum. Dev. 2008, 84, 751–761. [Google Scholar] [CrossRef]
- Manacero, S.A.; Marschik, P.B.; Nunes, M.L.; Einspieler, C. Is it possible to predict the infant’s neurodevelopmental outcome at 14 months of age by means of a single preterm assessment of General Movements? Early Hum. Dev. 2012, 88, 39–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snider, L.; Majnemer, A.; Mazer, B.; Campbell, S.; Bos, A.F. Prediction of motor and functional outcomes in infants born preterm assessed at term. Pediatr. Phys. Ther. 2009, 21, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Spittle, A.J.; Walsh, J.M.; Potter, C.; McInnes, E.; Olsen, J.E.; Lee, K.J.; Anderson, P.J.; Doyle, L.W.; Cheong, J.L. Neurobehaviour at term-equivalent age and neurodevelopmental outcomes at 2 years in infants born moderate-to-late preterm. Dev. Med. Child Neurol. 2017, 59, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Brogna, C.; Romeo, D.M.; Cervesi, C.; Scrofani, L.; Romeo, M.G.; Mercuri, E.; Guzzetta, A. Prognostic value of the qualitative assessments of general movements in late-preterm infants. Early Hum. Dev. 2013, 89, 1063–1066. [Google Scholar] [CrossRef]
- Ferrari, F.; Plessi, C.; Lucaccioni, L.; Bertoncelli, N.; Bedetti, L.; Ori, L.; Berardi, A.; Della Casa, E.; Iughetti, L.; D’Amico, R. Motor and Postural Patterns Concomitant with General Movements Are Associated with Cerebral Palsy at Term and Fidgety Age in Preterm Infants. J. Clin. Med. 2019, 8, 1189. [Google Scholar] [CrossRef] [Green Version]
- Venkata, S.; Pournami, F.; Prabhakar, J.; Nandakumar, A.; Jain, N. Disability Prediction by Early Hammersmith Neonatal Neurological Examination: A Diagnostic Study. J. Child Neurol. 2020, 35, 731–736. [Google Scholar] [CrossRef]
- Pascal, A.; Govaert, P.; Oostra, A.; Naulaers, G.; Ortibus, E.; Van den Broeck, C. Neurodevelopmental outcome in very preterm and very-low-birthweight infants born over the past decade: A meta-analytic review. Dev. Med. Child Neurol. 2018, 60, 342–355. [Google Scholar] [CrossRef] [Green Version]
- Himpens, E.; Van den Broeck, C.; Oostra, A.; Calders, P.; Vanhaesebrouck, P. Prevalence, type, distribution, and severity of cerebral palsy in relation to gestational age: A meta-analytic review. Dev. Med. Child Neurol. 2008, 50, 334–340. [Google Scholar] [CrossRef]


| Patient Characteristics | No Cerebral Palsy Diagnosis | Cerebral Palsy or High-Risk Diagnosis | p-Value |
|---|---|---|---|
| N = 176 | N = 26 | ||
| Sex, n (%) | |||
| Male | 83 (47.2) | 15 (57.7) | 0.32 |
| Female | 93 (52.8) | 11 (42.3) | |
| Gestational Age (w + d), median (IQR) | |||
| 27 + 4 (25 + 5, 28 + 4) | 26 + 2 (26, 27 + 2) | 0.018 | |
| Birth weight (g), mean (SD) | |||
| 883.63 (242.03) | 839.62 (237.44) | 0.39 | |
| SGA/IUGR, n (%) | |||
| 62 (35.2) | 8 (30.8) | 0.66 | |
| Writhing GMA, n (%) | |||
| Normal | 10 (5.8) | 1 (3.8) | 0.078 |
| PR | 126 (72.8) | 14 (53.8) | |
| PR/CS | 10 (5.8) | 1 (3.8) | |
| CS | 19 (11.0) | 8 (30.8) | |
| CS ≥ 2 | 8 (4.6) | 2 (7.7) | |
| HNNE score, median (IQR) | |||
| N = 147 | N = 23 | ||
| 28.5 (26.5, 30) | 27 (24.5, 29) | 0.018 | |
| Fidgety GMA, n (%) | |||
| Fidgety Present | 144 (82.8) | 2 (8.0) | |
| Fidgety Present (sporadic) | 20 (11.5) | 4 (16.0) | |
| Abnormal Fidgety | 2 (1.1) | 0 (0.0) | |
| Fidgety Absent | 8 (4.6) | 19 (76.0) | |
| HINE score, median (IQR) | |||
| N = 168 | N = 26 | ||
| 57 (52.5, 62) | 43 (39, 48.5) | ||
| Diagnosis at 3–4 months’ CA, n (%) | |||
| Normal | 82 (46.6) | 0 (0.0) | |
| Delay | 94 (53.4) | 0 (0.0) | |
| High Risk | 0 (0.0) | 18 (69.2) | |
| CP | 0 (0.0) | 8 (30.8) | |
| N | OR | Std. Err. | p-Value | 95% CI | |
|---|---|---|---|---|---|
| Sex | 202 | 0.65 | 0.28 | 0.318 | 0.28–1.50 |
| GA | 202 | 0.78 | 0.09 | 0.029 | 0.62–0.97 |
| BW | 202 | 1.00 | 0.00 | 0.385 | 1.00–1.00 |
| SGA | 202 | 0.82 | 0.37 | 0.656 | 0.34–1.99 |
| Writhing GMs | 199 | 1.56 | 0.30 | 0.019 | 1.07–2.27 |
| HNNE | 169 | 0.88 | 0.06 | 0.05 | 0.78–1.00 |
| N | OR | Std. Error | p Value | 95% CI | |
|---|---|---|---|---|---|
| Sex | 169 | 0.87 | 0.43 | 0.776 | 0.33–2.31 |
| GA | 169 | 0.67 | 0.15 | 0.068 | 0.44–1.03 |
| BW | 169 | 1.00 | 0.00 | 0.619 | 1.00–1.00 |
| SGA | 169 | 1.42 | 1.04 | 0.633 | 0.34–5.94 |
| Writhing | 169 | 1.44 | 0.31 | 0.087 | 0.95–2.20 |
| HNNE | 169 | 0.91 | 0.06 | 0.142 | 0.79–1.03 |
| Fidgety GMs | HINE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | rs | Std. Err. | p-Value | 95% CI | N | rs | Std. Err. | p-Value | 95% CI | |
| Writhing GMs | 196 | 0.03 | 0.07 | 0.723 | −0.12–0.17 | 191 | −0.10 | 0.07 | 0.173 | −0.24–0.04 |
| HNNE | 167 | −0.18 | 0.07 | 0.012 | −0.32–0.04 | 161 | 0.43 | 0.06 | <0.001 | 0.31–0.56 |
| R | Std. Err. | p-Value | 95% CI | |
|---|---|---|---|---|
| HNNE | 0.80 | 0.17 | <0.001 | 0.47–1.13 |
| GA | 0.81 | 0.29 | 0.005 | 0.26–1.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Connors, R.; Sackett, V.; Machipisa, C.; Tan, K.; Pharande, P.; Zhou, L.; Malhotra, A. Assessing the Utility of Neonatal Screening Assessments in Early Diagnosis of Cerebral Palsy in Preterm Infants. Brain Sci. 2022, 12, 847. https://doi.org/10.3390/brainsci12070847
Connors R, Sackett V, Machipisa C, Tan K, Pharande P, Zhou L, Malhotra A. Assessing the Utility of Neonatal Screening Assessments in Early Diagnosis of Cerebral Palsy in Preterm Infants. Brain Sciences. 2022; 12(7):847. https://doi.org/10.3390/brainsci12070847
Chicago/Turabian StyleConnors, Rebecca, Vathana Sackett, Catherine Machipisa, Kenneth Tan, Pramod Pharande, Lindsay Zhou, and Atul Malhotra. 2022. "Assessing the Utility of Neonatal Screening Assessments in Early Diagnosis of Cerebral Palsy in Preterm Infants" Brain Sciences 12, no. 7: 847. https://doi.org/10.3390/brainsci12070847
APA StyleConnors, R., Sackett, V., Machipisa, C., Tan, K., Pharande, P., Zhou, L., & Malhotra, A. (2022). Assessing the Utility of Neonatal Screening Assessments in Early Diagnosis of Cerebral Palsy in Preterm Infants. Brain Sciences, 12(7), 847. https://doi.org/10.3390/brainsci12070847






