A Short Functional Neuroimaging Assay Using Attachment Scenes to Recruit Neural Correlates of Social Cognition—A Replication Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
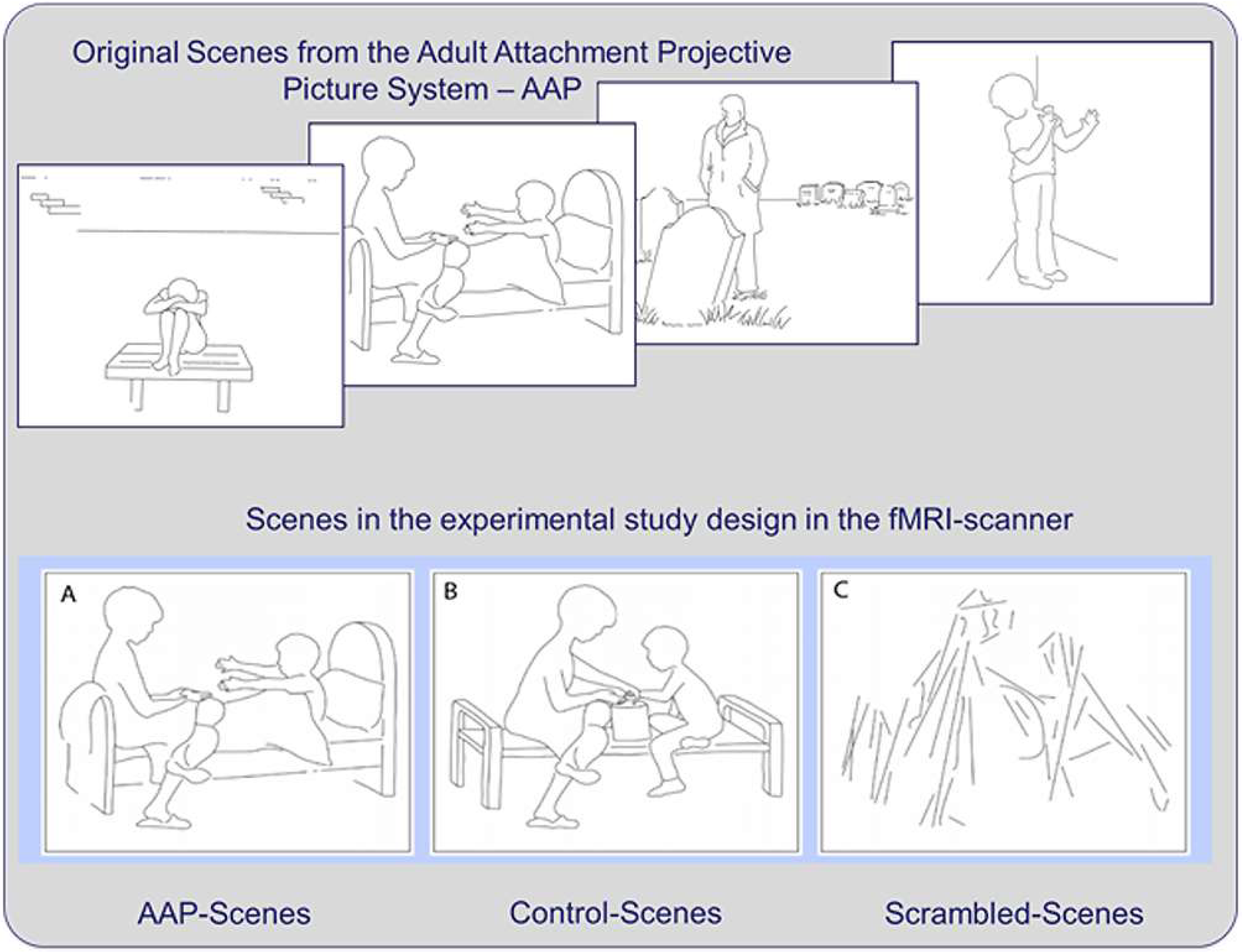
2.2. Stimulus Material
2.3. MRI Data Acquisition, Preprocessing and Statistical Modeling
2.4. Overlap with Other Functional Activation Maps
3. Results
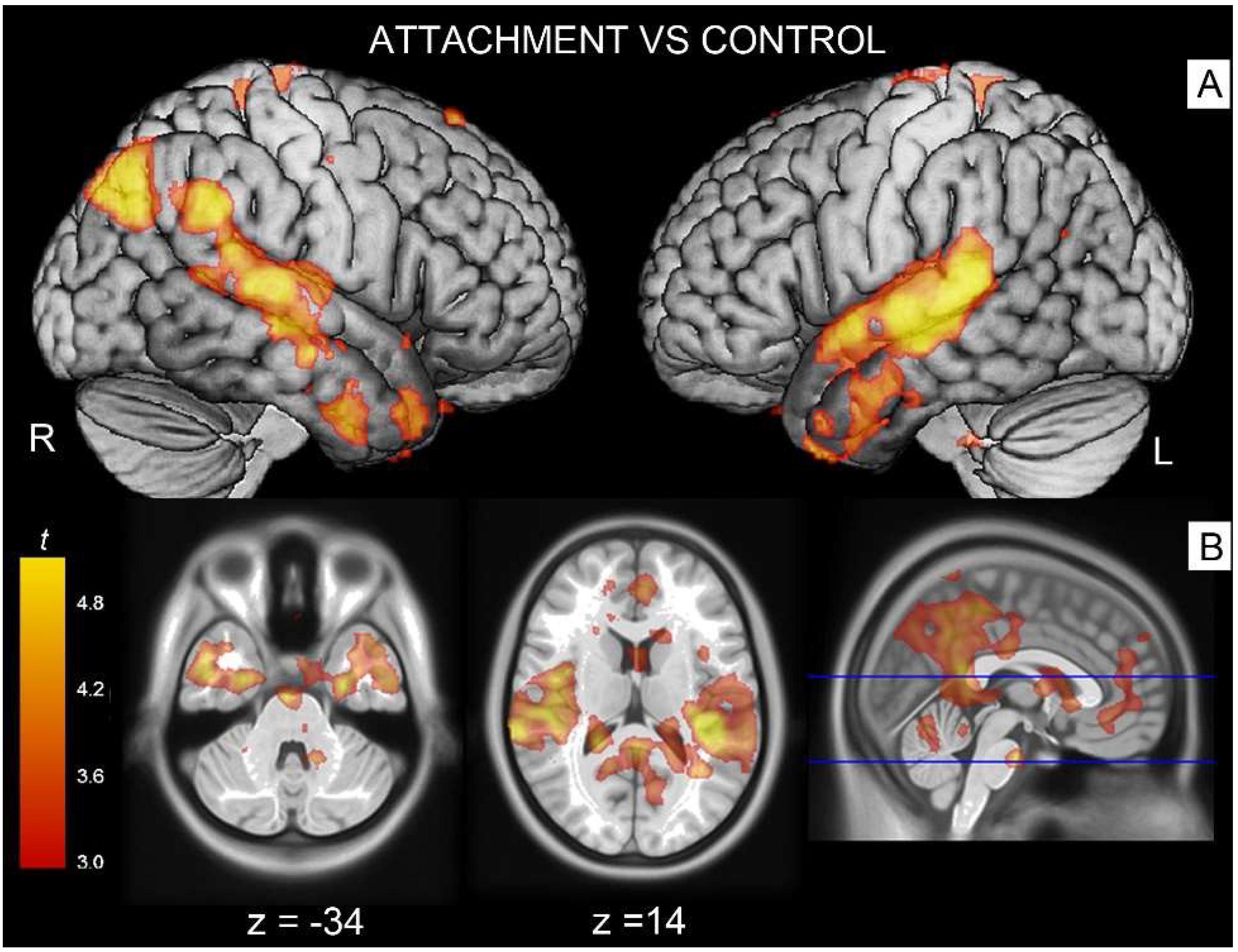
3.1. Attachment-Related Pictures vs. Neutral Pictures
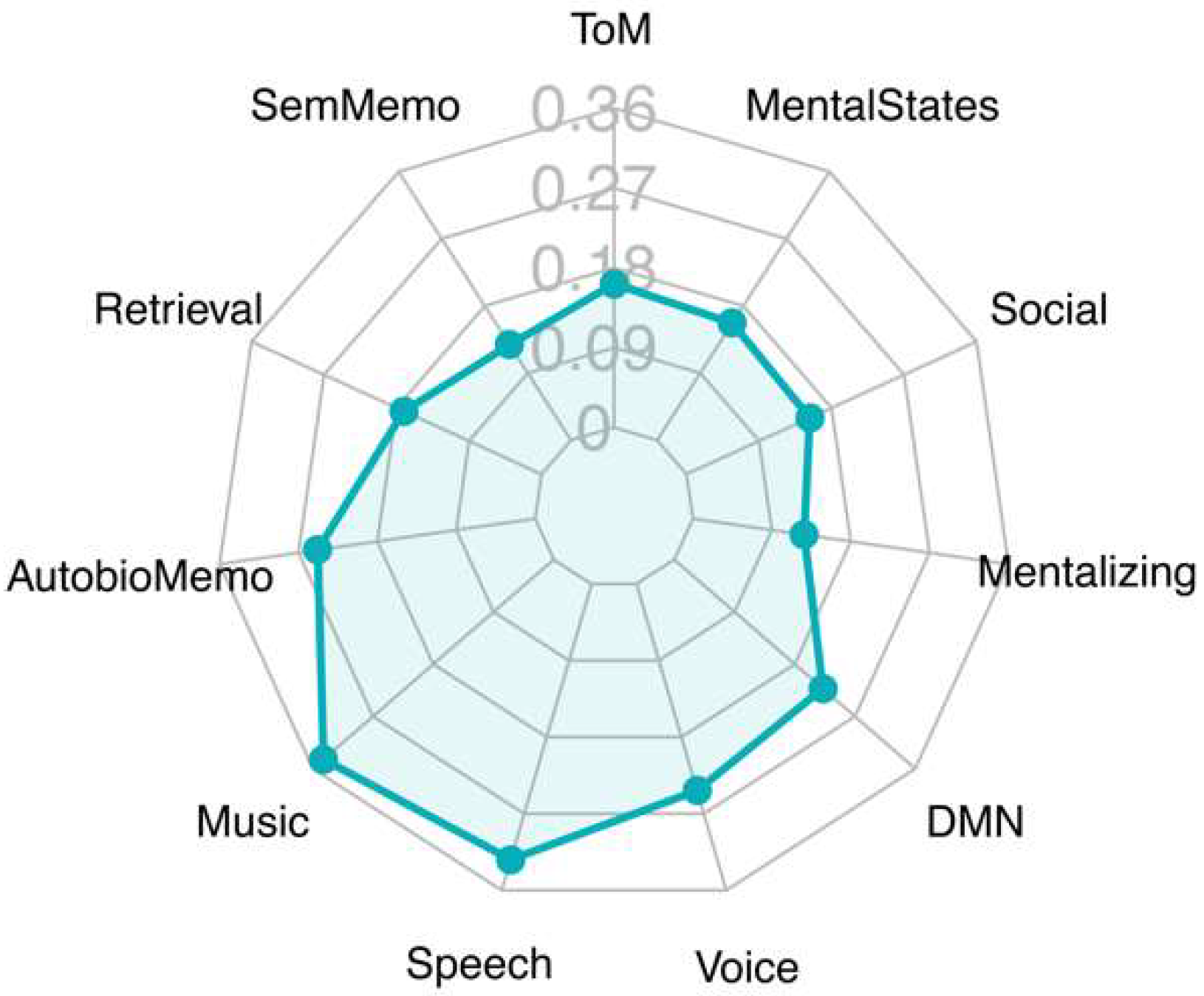
3.2. Decoding Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchheim, A.; Horz-Sagstetter, S.; Doering, S.; Rentrop, M.; Schuster, P.; Buchheim, P.; Pokorny, D.; Fischer-Kern, M. Change of Unresolved Attachment in Borderline Personality Disorder: RCT Study of Transference-Focused Psychotherapy. Psychother. Psychosom. 2017, 86, 314–316. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, A.; George, C.; Gündel, H.; Viviani, R. Editorial: Neuroscience of Human Attachment. Front. Hum. Neurosci. 2017, 11, 136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchheim, A.; Erk, S.; George, C.; Kächele, H.; Kircher, T.; Martius, P.; Pokorny, D.; Ruchsow, M.; Spitzer, M.; Walter, H. Neural correlates of attachment trauma in borderline personality disorder: A functional magnetic resonance imaging study. Psychiatry Res. 2008, 163, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Verbeke, W.; Ein-Dor, T.; Vrtička, P. A functional neuro-anatomical model of human attachment (NAMA): Insights from first- and second-person social neuroscience. Cortex 2020, 126, 281–321. [Google Scholar] [CrossRef] [PubMed]
- Vrticka, P.; Vuilleumier, P. Neuroscience of human social interactions and adult attachment style. Front. Hum. Neurosci. 2012, 6, 212. [Google Scholar] [CrossRef]
- Fonagy, P.; Luyten, P. Attachment, mentalizing, and the self. In Handbook of Personality Disorders; Livesley, W.J., Larstone, R., Eds.; Guilford Press: New York, NY, USA, 2018. [Google Scholar]
- Labek, K.; Viviani, R.; Gizewski, E.R.; Verius, M.; Buchheim, A. Neural Correlates of the Appraisal of Attachment Scenes in Healthy Controls and Social Cognition—An fMRI Study. Front. Hum. Neurosci. 2016, 10, 345. [Google Scholar] [CrossRef][Green Version]
- Buchheim, A.; Labek, K.; Taubner, S.; Kessler, H.; Pokorny, D.; Kachele, H.; Cierpka, M.; Roth, G.; Pogarell, O.; Karch, S. Modulation of Gamma Band Activity and Late Positive Potential in Patients with Chronic Depression after Psychodynamic Psychotherapy. Psychother. Psychosom. 2018, 87, 252–254. [Google Scholar] [CrossRef]
- Buchheim, A.; Viviani, R.; Kessler, H.; Kächele, H.; Cierpka, M.; Roth, G.; George, C.; Kernberg, O.F.; Bruns, G.; Taubner, S. Changes in Prefrontal-Limbic Function in Major Depression after 15 Months of Long-Term Psychotherapy. PLoS ONE 2012, 7, e33745. [Google Scholar] [CrossRef]
- George, C.; West, M.L. The Adult Attachment Projective Picture System: Attachment Theory and Assessment in Adults; Guilford Press: New York, NY, USA, 2012. [Google Scholar]
- Frith, U.; Frith, C.D. Development and neurophysiology of mentalizing. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2003, 358, 459–473. [Google Scholar] [CrossRef]
- Gündel, H.; O’Connor, M.-F.F.; Littrell, L.; Fort, C.; Lane, R.D. Functional neuroanatomy of grief: An FMRI study. Am. J. Psychiatry 2003, 160, 1946–1953. [Google Scholar] [CrossRef]
- Kersting, A.; Ohrmann, P.; Pedersen, A.; Kroker, K.; Samberg, D.; Bauer, J.; Kugel, H.; Koelkebeck, K.; Steinhard, J.; Heindel, W.; et al. Neural Activation Underlying Acute Grief in Women After the Loss of an Unborn Child. Am. J. Psychiatry 2009, 166, 1402–1410. [Google Scholar] [CrossRef] [PubMed]
- Labek, K.; Berger, S.; Buchheim, A.; Bosch, J.; Spohrs, J.; Dommes, L.; Beschoner, P.; Stingl, J.; Viviani, R. The iconography of mourning and its neural correlates: A functional neuroimaging study. Soc. Cogn. Affect. Neurosci. 2017, 12, 1303–1313. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M.; Radua, J.J.; Tholen, M.G.; Maliske, L.; Margulies, D.S.; Mars, R.B.; Sallet, J.J.; Kanske, P.P. Toward a hierarchical model of social cognition: A neuroimaging meta-analysis and integrative review of empathy and theory of mind. Psychol. Bull. 2021, 147, 293–327. [Google Scholar] [CrossRef] [PubMed]
- Yarkoni, T.; Poldrack, R.A.; Nichols, T.E.; Van Essen, D.C.; Wager, T.D. Large-scale automated synthesis of human functional neuroimaging data. Nat. Methods 2011, 8, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Gorgolewski, K.J.; Varoquaux, G.; Rivera, G.; Schwarz, Y.; Ghosh, S.S.; Maumet, C.; Sochat, V.V.; Nichols, T.E.; Poldrack, R.A.; Poline, J.-B.; et al. NeuroVault.org: A web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinformatics 2015, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Gorgolewski, K.J.; Varoquaux, G.; Rivera, G.; Schwartz, Y.; Sochat, V.V.; Ghosh, S.S.; Maumet, C.; Nichols, T.E.; Poline, J.-B.; Yarkoni, T.; et al. NeuroVault.org: A repository for sharing unthresholded statistical maps, parcellations, and atlases of the human brain. NeuroImage 2016, 124, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The CES-D scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Bailer, M.; Hautzinger, M.; Hofmeister, D.; Keller, F. Allgemeine Depressionsskala (ADS). Psychiatr. Prax. 2012, 39, 302–304. [Google Scholar]
- Spielberger, C.D.; Gorsuch, L.; Laux, L.; Glanzmann, P.; Schaffner, P. Das State-Trait-Angstinventar: STAI; Beltz Test: Göttingen, Germany, 2001. [Google Scholar]
- Laux, L.; Glanzmann, P.; Schaffner, P.; Spielberger, C.D. Das State-Trait-Angstinventar (STAI); Manual Beltz: Weinheim, Germany, 1981. [Google Scholar]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–33+34–57. [Google Scholar]
- Ackenheil, M.; Stotz-Ingenlath, G.; Dietz-Bauer, R.; Vossen, A. MINI Mini International Neuropsychiatric Interview, German Version 5.0. 0 DSM IV; Psychiatric University Clinic: Munich, Germany, 1999. [Google Scholar]
- Winer, B.J.; Brown, D.R.; Michels, K.M. Statistical Principles in Experimental Design; McGraw-Hill: New York, NY, USA, 1991. [Google Scholar]
- Margulies, D.S.; Ghosh, S.S.; Goulas, A.; Falkiewicz, M.; Huntenburg, J.M.; Langs, G.; Bezgin, G.; Eickhoff, S.B.; Castellanos, F.X.; Petrides, M.; et al. Situating the default-mode network along a principal gradient of macroscale cortical organization. Proc. Natl. Acad. Sci. USA 2016, 113, 12574. [Google Scholar] [CrossRef]
- Singer, T.; Seymour, B.; O’doherty, J.; Kaube, H.; Dolan, R.J.; Frith, C.D. Empathy for pain involves the affective but not sensory components of pain. Science 2004, 303, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Blakemore, S.-J.; Frith, U. How does the brain deal with the social world? Neuroreport 2004, 15, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Lamm, C.; Majdandžić, J. The role of shared neural activations, mirror neurons, and morality in empathy—A critical comment. Neurosci. Res. 2015, 90, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, H.L.; Frith, C. Functional imaging of ‘theory of mind’. Trends Cogn. Sci. 2003, 7, 77–83. [Google Scholar] [CrossRef]
- Sosic-Vasic, Z.; Eberhardt, J.; Bosch, J.E.; Dommes, L.; Labek, K.; Buchheim, A.; Viviani, R. Mirror neuron activations in encoding of psychic pain in borderline personality disorder. NeuroImage Clin. 2019, 22, 101737. [Google Scholar] [CrossRef] [PubMed]
- Ripoll, L.H.; Snyder, R.; Steele, H.; Siever, L.J. The Neurobiology of Empathy in Borderline Personality Disorder. Curr. Psychiatry Rep. 2013, 15, 344. [Google Scholar] [CrossRef] [PubMed]
- Schoett, M.J.S.; Basten, U.; Deichmann, R.; Fiebach, C.J.; Fischmann, T. Brain responses to social cues of attachment in mid-childhood. Attach. Hum. Dev. 2021. [Google Scholar] [CrossRef] [PubMed]
- Buchheim, A.; Labek, K.; Walter, S.; Viviani, R. A clinical case study of a psychoanalytic psychotherapy monitored with functional neuroimaging. Front. Hum. Neurosci. 2013, 7, 677. [Google Scholar]
- Eickhoff, S.B.; Amunts, K.; Mohlberg, H.; Zilles, K. The Human Parietal Operculum. II. Stereotaxic Maps and Correlation with Functional Imaging Results. Cereb. Cortex 2005, 16, 268–279. [Google Scholar] [CrossRef]
- Penfield, W.; Jasper, H. Epilepsy and the Functional Anatomy of the Human Brain; Little, Brown & Co., Ltd.: Oxford, UK, 1954. [Google Scholar]
- Woolsey, C.N.; Erickson, T.C.; Gilson, W.E. Localization in somatic sensory and motor areas of human cerebral cortex as determined by direct recording of evoked potentials and electrical stimulation. J. Neurosurg. 1979, 51, 476–506. [Google Scholar] [CrossRef]
- Peyron, R.; García-Larrea, L.; Grégoire, M.-C.; Costes, N.; Convers, P.; Lavenne, F.; Mauguière, F.; Michel, D.; Laurent, B. Haemodynamic brain responses to acute pain in humans: Sensory and attentional networks. Brain 1999, 122, 1765–1780. [Google Scholar] [CrossRef] [PubMed]
- Keysers, C.; Kaas, J.H.; Gazzola, V. Somatosensation in social perception. Nature reviews. Neuroscience 2010, 11, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Bufalari, I.; Ionta, S. The social and personality neuroscience of empathy for pain and touch. Front. Hum. Neurosci. 2013, 7, 393. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, F.Å.; Balslev, D.; Hansen, L.K. Mining the posterior cingulate: Segregation between memory and pain components. NeuroImage 2005, 27, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2013, 137, 12–32. [Google Scholar] [CrossRef]
- Schneck, N.; Tu, T.; Michel, C.A.; Bonanno, G.A.; Sajda, P.; Mann, J.J. Attentional Bias to Reminders of the Deceased as Compared With a Living Attachment in Grieving. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Schneck, N.; Haufe, S.; Tu, T.; Bonanno, G.A.; Ochsner, K.N.; Sajda, P.; Mann, J.J. Tracking Deceased-Related Thinking With Neural Pattern Decoding of a Cortical-Basal Ganglia Circuit. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2017, 2, 421–429. [Google Scholar] [CrossRef]
- Adolphs, R.; Damasio, H.; Tranel, D.; Cooper, G.; Damasio, A.R. A role for somatosensory cortices in the visual recognition of emotion as revealed by three-dimensional lesion mapping. J. Neurosci. 2000, 20, 2683–2690. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V. The vicarious brain. In Mechanisms of Social Connection: From Brain to Group; Mikulincer, M., Shaver, P.R., Eds.; American Psychological Association: Worcester, MA, USA, 2014; pp. 71–88. [Google Scholar] [CrossRef]
- Viviani, R.; Dommes, L.; Bosch, J.E.; Labek, K. Segregation, connectivity, and gradients of deactivation in neural correlates of evidence in social decision making. NeuroImage 2020, 223, 117339. [Google Scholar] [CrossRef]
- Frith, C.D.; Frith, U. The neural basis of mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef]
- Saxe, R. Uniquely human social cognition. Curr. Opin. Neurobiol. 2006, 16, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Saxe, R. The right temporo-parietal junction: A specific brain region for thinking about thoughts. In Handbook of Theory of Mind; Lesilie, A., German, T., Eds.; Psychology Press: Oxford, UK, 2010. [Google Scholar]
- Olson, I.R.; McCoy, D.; Klobusicky, E.; Ross, L.A. Social cognition and the anterior temporal lobes: A review and theoretical framework. Soc. Cogn. Affect. Neurosci. 2013, 8, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Ross, L.A.; Olson, I.R. Social cognition and the anterior temporal lobes. NeuroImage 2010, 49, 3452–3462. [Google Scholar] [CrossRef] [PubMed]
- Ralph, M.A.L.; Jefferies, E.; Patterson, K.; Rogers, T.T. The neural and computational bases of semantic cognition. Nat. Rev. Neurosci. 2017, 18, 42–55. [Google Scholar] [CrossRef]
- Snowden, J.S.; Harris, J.M.; Thompson, J.C.; Kobylecki, C.; Jones, M.; Richardson, A.M.; Neary, D. Semantic dementia and the left and right temporal lobes. Cortex 2018, 107, 188–203. [Google Scholar] [CrossRef]
- Irish, M.; Hodges, J.R.; Piguet, O. Right anterior temporal lobe dysfunction underlies theory of mind impairments in semantic dementia. Brain 2014, 137, 1241–1253. [Google Scholar] [CrossRef] [PubMed]
- Zahn, R.; Moll, J.; Krueger, F.; Huey, E.D.; Garrido, G.; Grafman, J. Social concepts are represented in the superior anterior temporal cortex. Proc. Natl. Acad. Sci. USA 2007, 104, 6430. [Google Scholar] [CrossRef]
- Northoff, G.; Heinzel, A.; de Greck, M.; Bermpohl, F.; Dobrowolny, H.; Panksepp, J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. NeuroImage 2006, 31, 440–457. [Google Scholar] [CrossRef]
- Lou, H.C.; Luber, B.; Crupain, M.; Keenan, J.P.; Nowak, M.; Kjaer, T.W.; Sackeim, H.A.; Lisanby, S.H. Parietal cortex and representation of the mental Self. Proc. Natl. Acad. Sci. USA 2004, 101, 6827. [Google Scholar] [CrossRef]
- Sperduti, M.; Martinelli, P.; Kalenzaga, S.; Devauchelle, A.-D.; Lion, S.; Malherbe, C.; Gallarda, T.; Amado, I.; Krebs, M.-O.; Oppenheim, C.; et al. Don’t be Too Strict with Yourself! Rigid Negative Self-Representation in Healthy Subjects Mimics the Neurocognitive Profile of Depression for Autobiographical Memory. Front. Behav. Neurosci. 2013, 7, 41. [Google Scholar] [CrossRef]
- Adolphs, R. How should neuroscience study emotions? by distinguishing emotion states, concepts, and experiences. Soc. Cogn. Affect. Neurosci. 2017, 12, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zachlod, D.; Rüttgers, B.; Bludau, S.; Mohlberg, H.; Langner, R.; Zilles, K.; Amunts, K. Four new cytoarchitectonic areas surrounding the primary and early auditory cortex in human brains. Cortex 2020, 128, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Luyten, P.; Campbell, C.; Allison, E.; Fonagy, P. The Mentalizing Approach to Psychopathology: State of the Art and Future Directions. Annu. Rev. Clin. Psychol. 2020, 16, 297–325. [Google Scholar] [CrossRef] [PubMed]
- Kampa, M.; Schick, A.; Sebastian, A.; Wessa, M.; Tüscher, O.; Kalisch, R.; Yuen, K. Replication of fMRI group activations in the neuroimaging battery for the Mainz Resilience Project (MARP). NeuroImage 2020, 204, 116223. [Google Scholar] [CrossRef]
| n = 19 | |
|---|---|
| Age mean (std. dev.) | 24.16 (4.66) |
| female (%) | 14 (66.7) |
| male (%) | 5 (23.8) |
| ADS mean (std. dev.) | 8.84 (6.59) |
| STAI-S mean (std. dev.) | 37.89 (8.77) |
| STAI-T mean (std. dev.) | 39.21 (7.18) |
| Cluster Level | Peak Level | |||||
|---|---|---|---|---|---|---|
| Cluster # | Region (Side) | Voxel | p | t | p | MNI Coordinates |
| Count | Clust | Peak | (x, y, z) | |||
| (k) | ||||||
| Contrast Attachment > Neutral | ||||||
| 1 | Superior Temporal Gyrus (R) | 3534 | <0.001 | 8.09 | 0.013 | 52, −26, 4 |
| Thalamus (R) | 7.24 | 0.043 | 54, −18, 0 | |||
| Parahippocampal Gyrus | 6.81 | 0.079 | 20, −10, −22 | |||
| 2 | Fusiform Gyrus (L) | 498 | <0.001 | 7.5 | 0.029 | −28, −32, −16 |
| 3 | Superior Temporal Gyrus/(L) | 2313 | <0.001 | 7.28 | 0.04 | −44, −32, 10 |
| Supramarginal Gyrus (L) | 6.15 | 0.204 | −58, −48, 32 | |||
| Mid Temp Gyrus | 5.82 | 0.318 | −62, −30, −4 | |||
| 4 | Angular Gyrus (L) | 301 | 0.009 | 5.2 | 0.643 | −44, −74, 44 |
| 5 | Post Cingulum/Precuneus | 217 | 0.036 | 4.34 | 0.982 | 12, −40, 10 |
| 6 | Mid Cingulum/Precuneus (R) | 180 | 0.069 | 4.97 | 0.772 | 8, −38, 52 |
| Mid Cingulum (L) | 4.7 | 0.896 | −8, −30, 52 | |||
| Contrast Neutral > Attachment | ||||||
| 7 | Lingual/Calcarine (R) | 357 | 0.004 | 5.29 | 0.589 | 8, −82, −8 |
| Calcarine (R) | 4.57 | 0.939 | 18, −78, −12 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labek, K.; Dommes, L.; Bosch, J.E.; Schurz, M.; Viviani, R.; Buchheim, A. A Short Functional Neuroimaging Assay Using Attachment Scenes to Recruit Neural Correlates of Social Cognition—A Replication Study. Brain Sci. 2022, 12, 855. https://doi.org/10.3390/brainsci12070855
Labek K, Dommes L, Bosch JE, Schurz M, Viviani R, Buchheim A. A Short Functional Neuroimaging Assay Using Attachment Scenes to Recruit Neural Correlates of Social Cognition—A Replication Study. Brain Sciences. 2022; 12(7):855. https://doi.org/10.3390/brainsci12070855
Chicago/Turabian StyleLabek, Karin, Lisa Dommes, Julia Eva Bosch, Matthias Schurz, Roberto Viviani, and Anna Buchheim. 2022. "A Short Functional Neuroimaging Assay Using Attachment Scenes to Recruit Neural Correlates of Social Cognition—A Replication Study" Brain Sciences 12, no. 7: 855. https://doi.org/10.3390/brainsci12070855
APA StyleLabek, K., Dommes, L., Bosch, J. E., Schurz, M., Viviani, R., & Buchheim, A. (2022). A Short Functional Neuroimaging Assay Using Attachment Scenes to Recruit Neural Correlates of Social Cognition—A Replication Study. Brain Sciences, 12(7), 855. https://doi.org/10.3390/brainsci12070855






