Feasibility of EEG Phase-Amplitude Coupling to Stratify Encephalopathy Severity in Neonatal HIE Using Short Time Window
Abstract
:1. Introduction
2. Methods
2.1. Participants
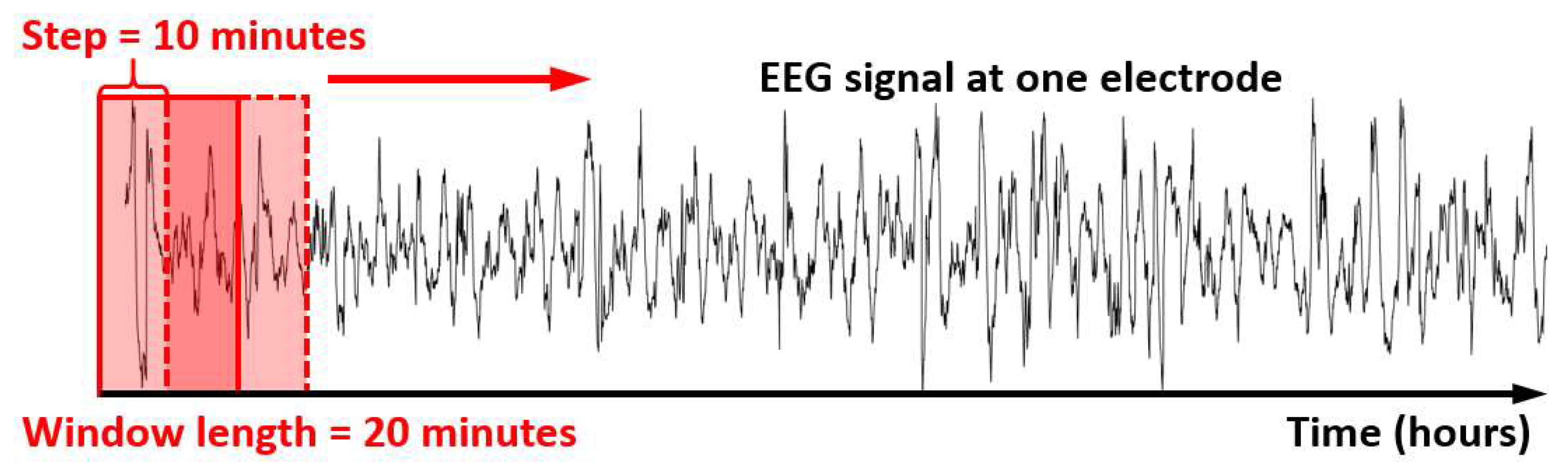
2.2. EEG Data Acquisition and Pre-Processing
2.3. Time-Dependent PACm Quantification
2.4. Statistical Analysis for Repeated Measures Using the Linear Mixed-Effects Models
2.5. Statistical ROC Analysis
3. Results
3.1. Determination of Time-Dependent tPACm from Both HIE Neonate Groups
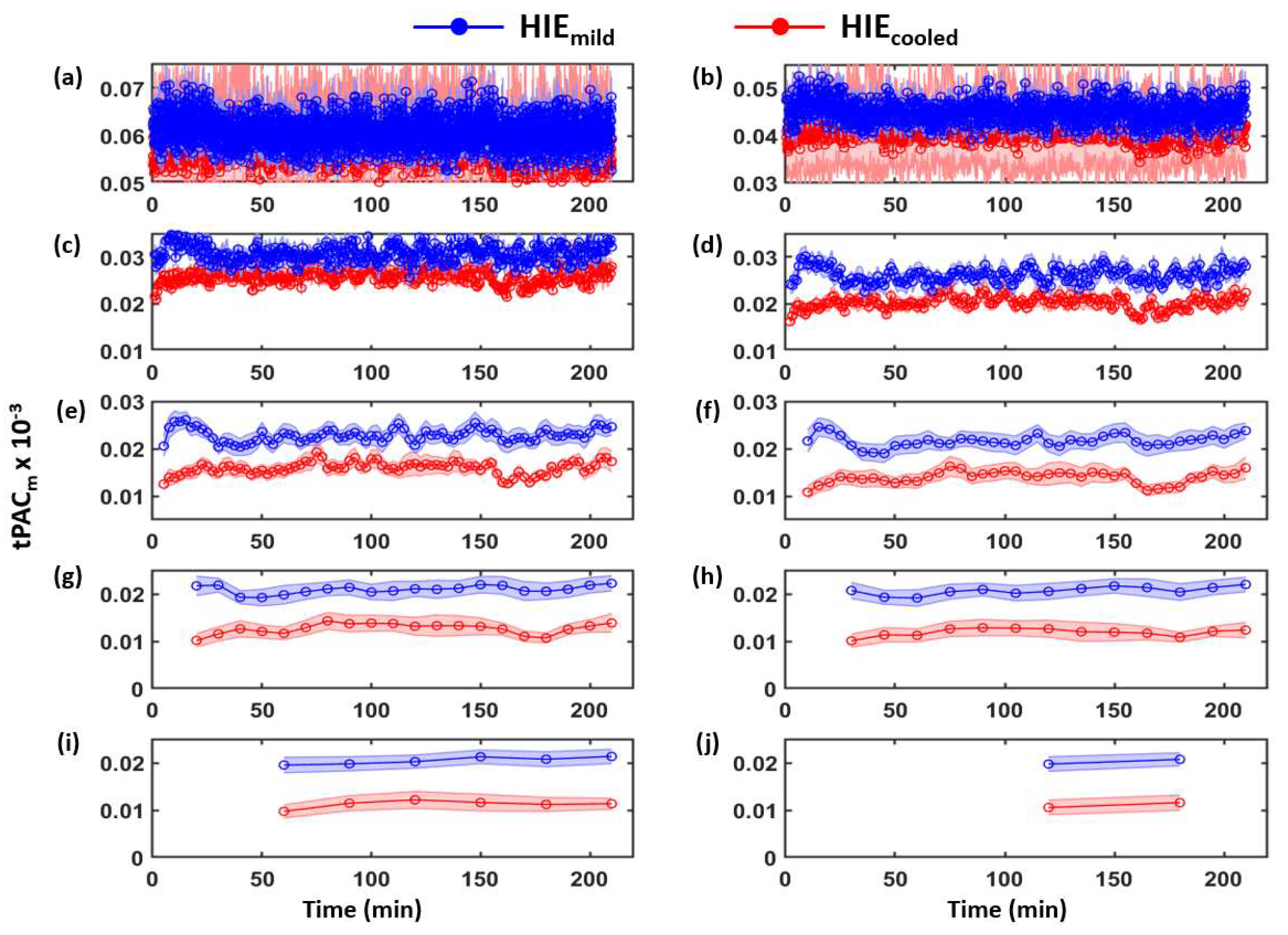
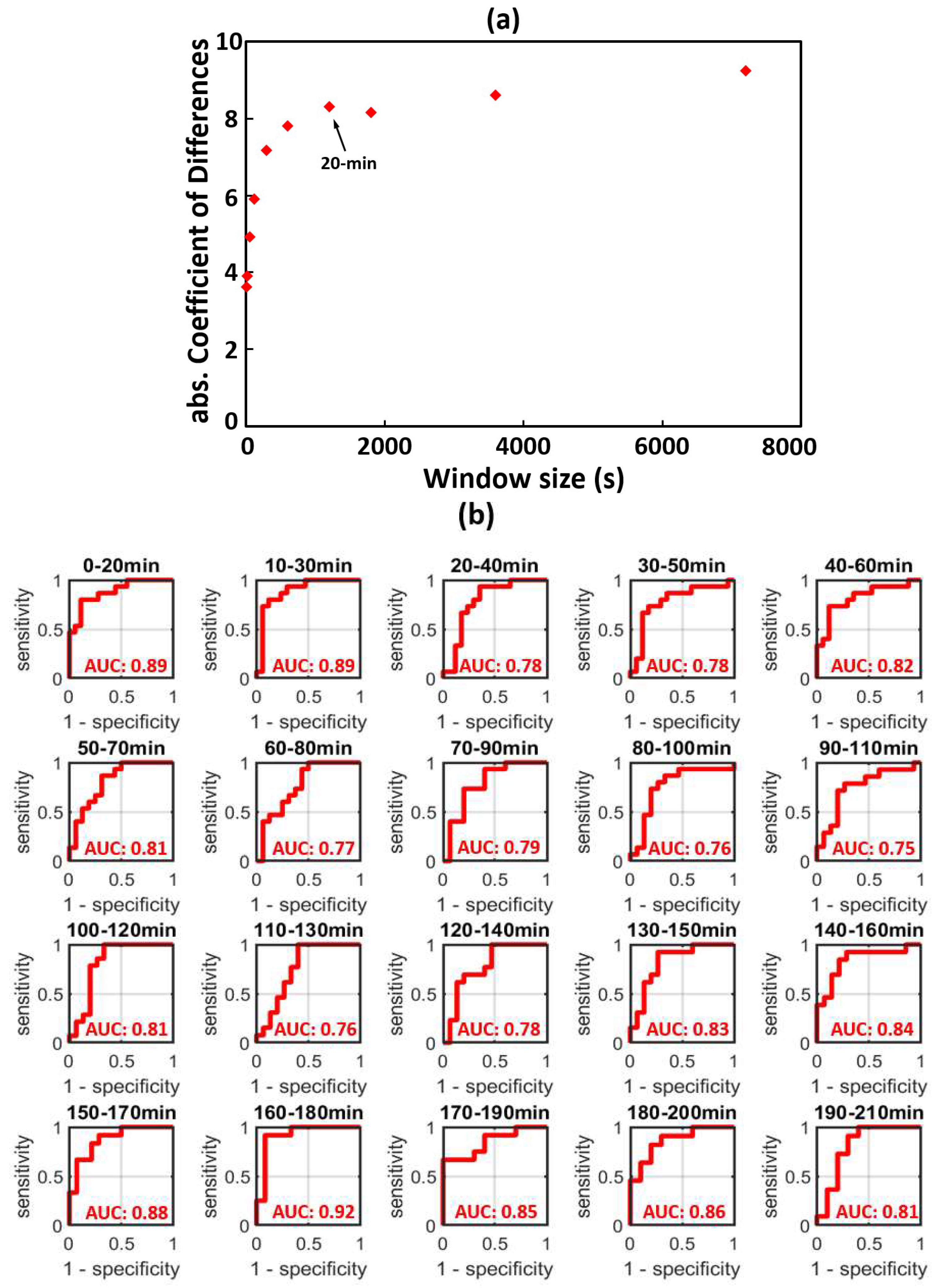
3.2. Time-Dependent ROC Classification Using 20 min Window tPACm to Differentiate between HIEmild and HIEcooled
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
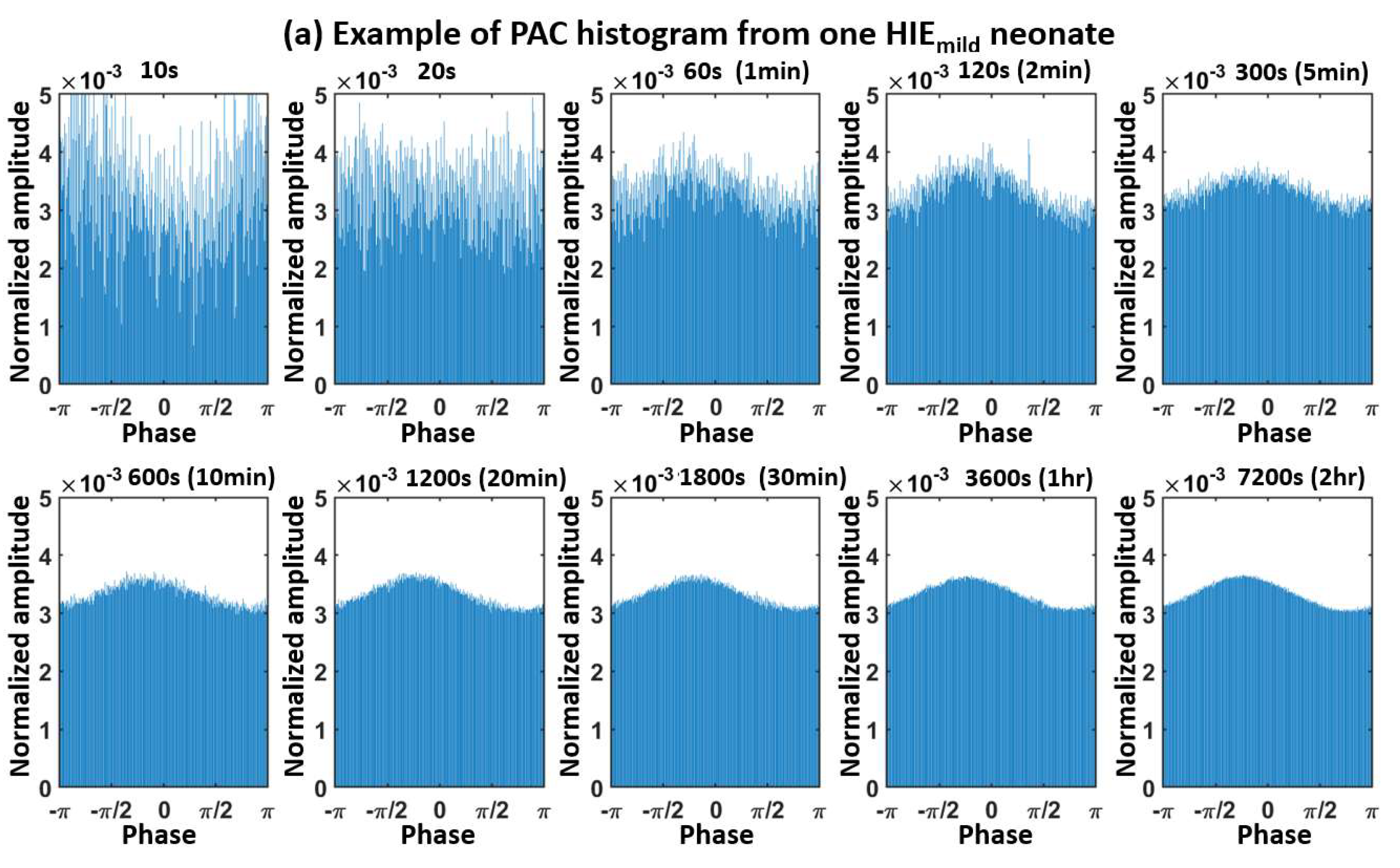
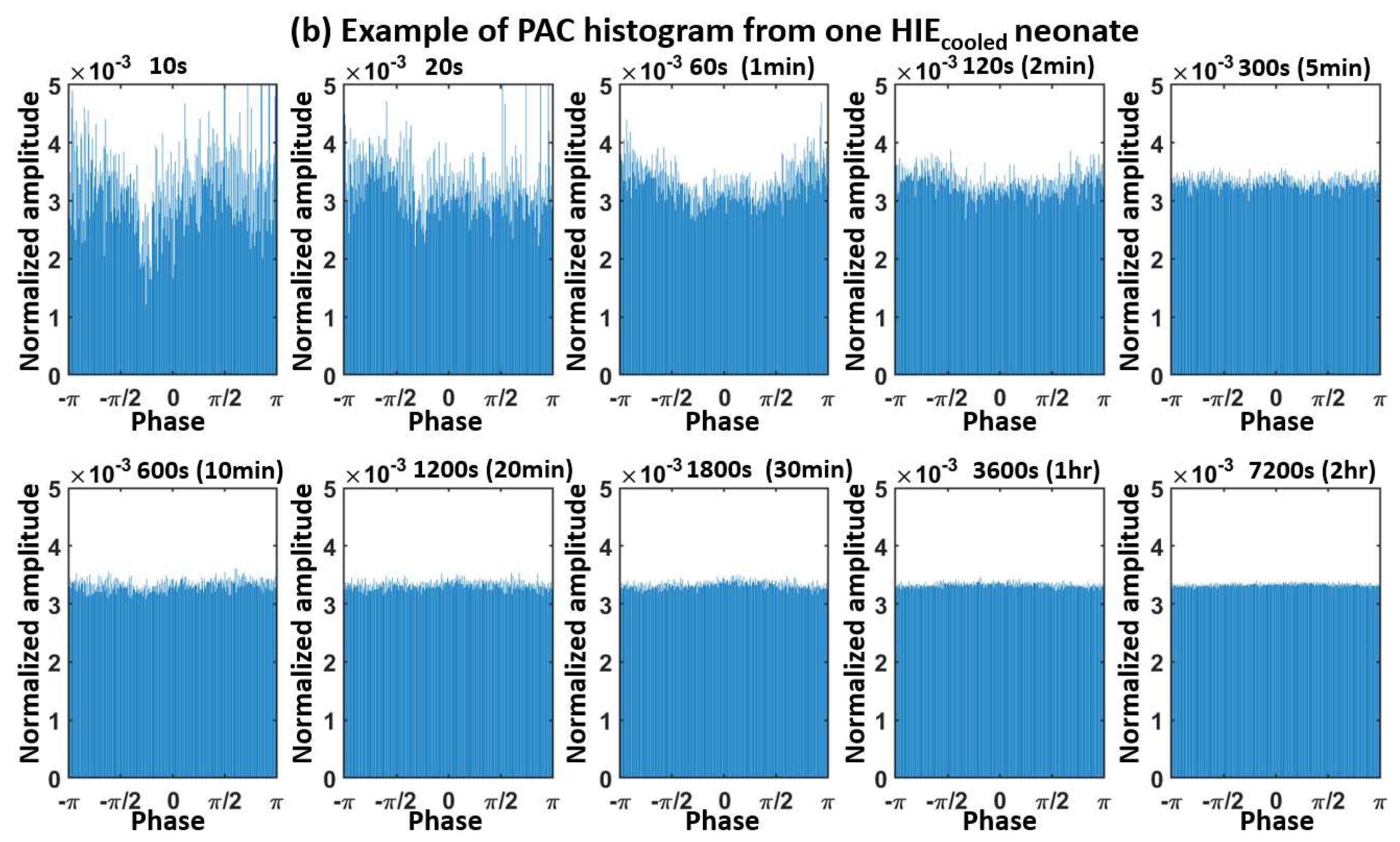
Appendix A. Determination of the Moving-Window Length
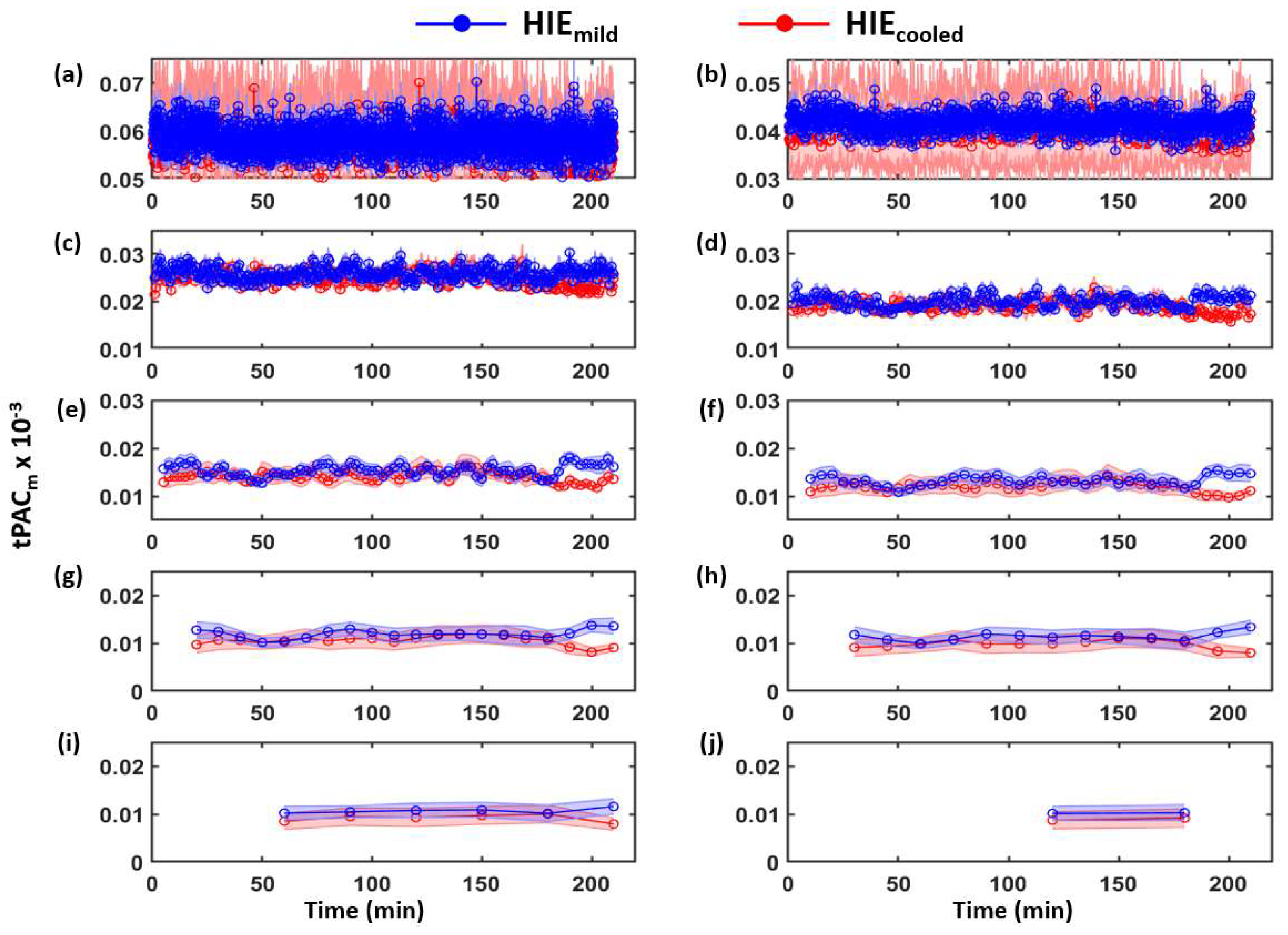

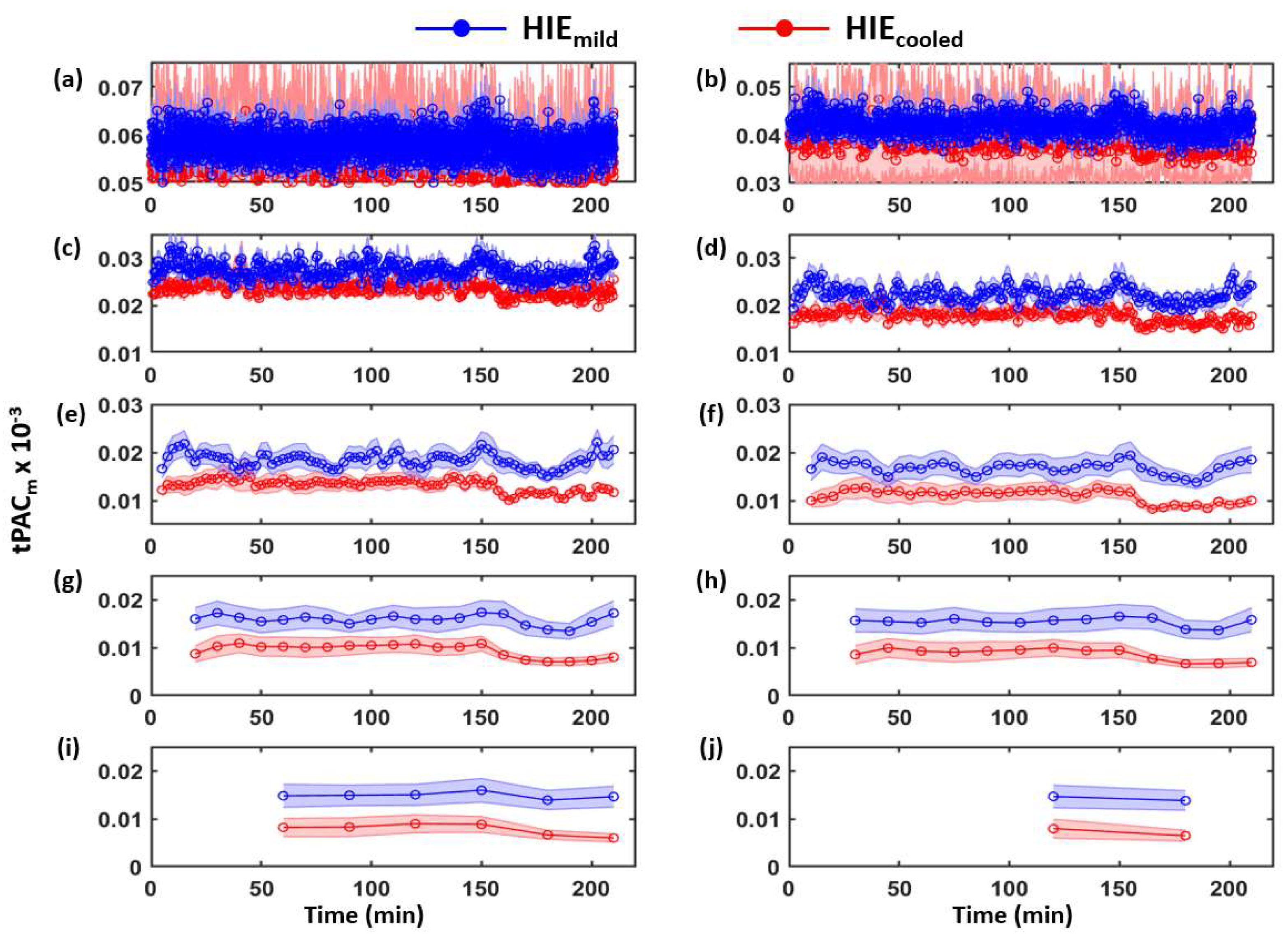


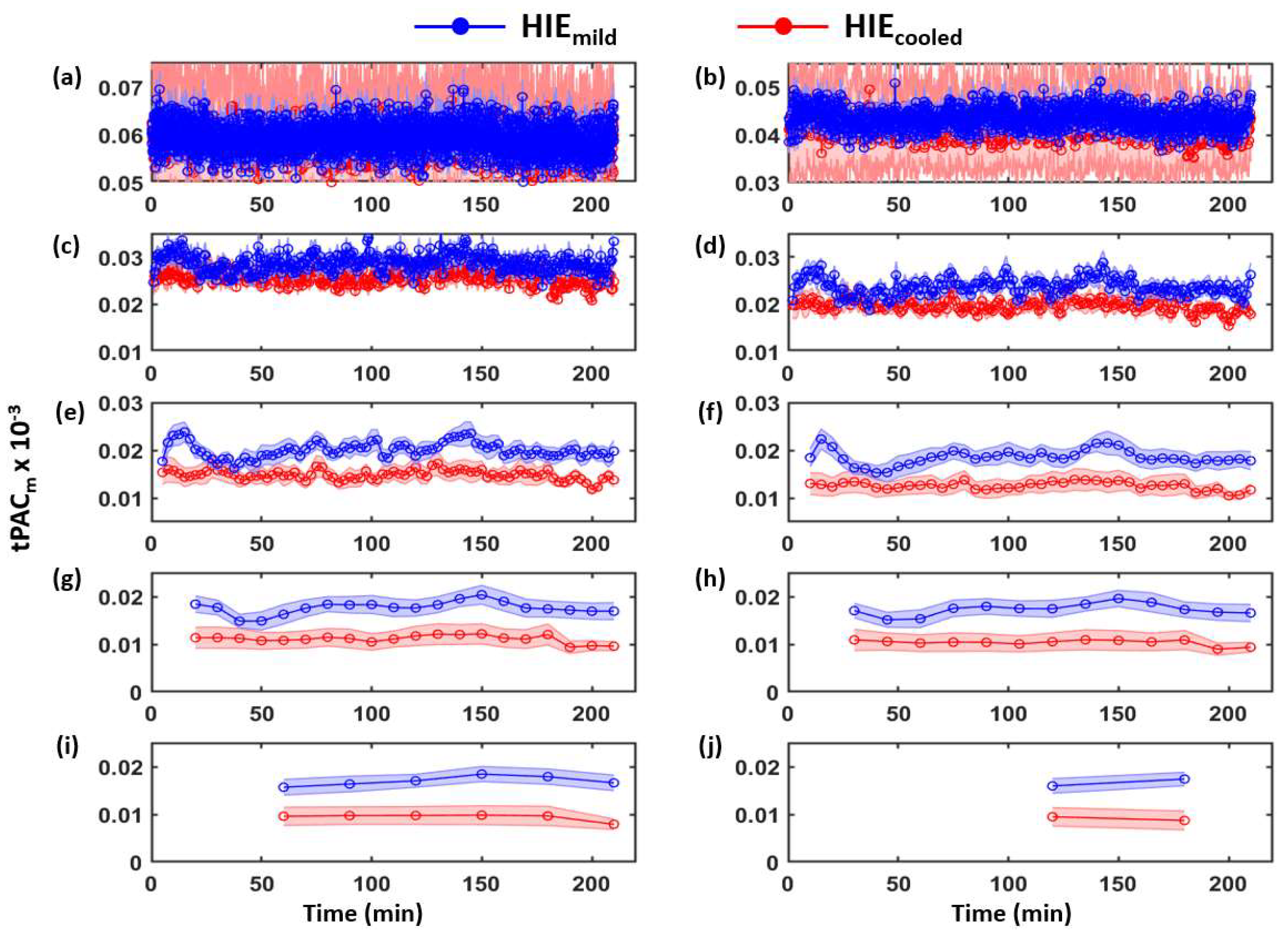
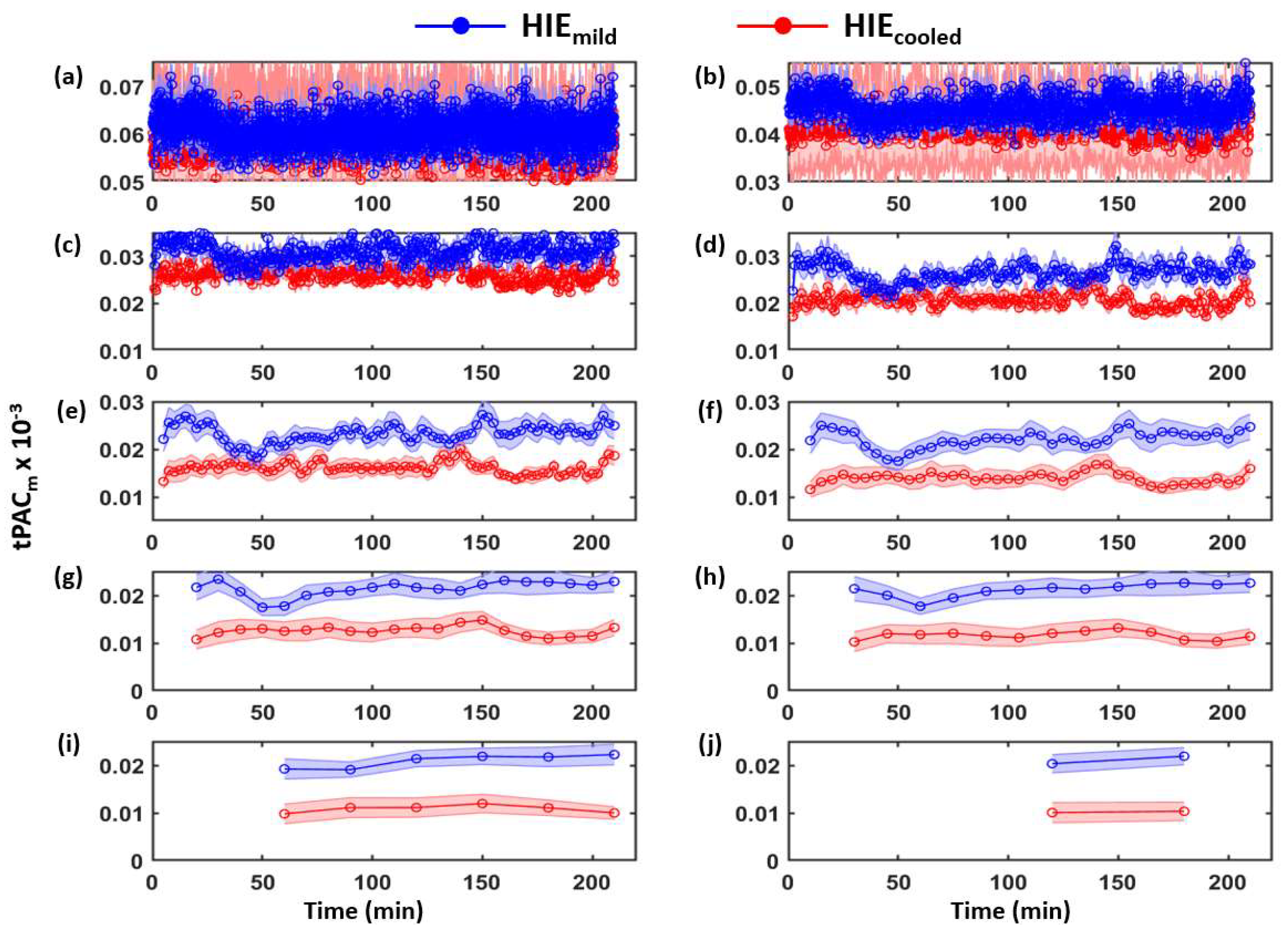
| Category | Signs of HIE | ||
|---|---|---|---|
| Normal/Mild HIE | Moderate HIE | Severe HIE | |
| 1. Level of consciousness | 1 | 2 = Lethargic | 3 = Stupor/coma |
| 2. Spontaneous activity | 1 | 2 = Decreased activity | 3 = No activity |
| 3. Posture | 1 | 2 = Distal flexion, complete extension | 3 = Decerebrate |
| 4. Tone | 1 | 2a = Hypotonia (focal or general) | 3a = Flaccid |
| 2b = Hypertonia | 3b = Rigid | ||
| 5. Primitive reflexes | |||
| Suck | 1 | 2 = Weak or has bite | 3 = Absent |
| Moro | 1 | 2 = Incomplete | 3 = Absent |
| 6. Autonomic system | |||
| Pupils | 1 | 2 = Constricted | 3 = Deviation/dilated/non-reactive to light |
| Heart rate | 1 | 2 = Bradycardia | 3 = Variable heart rate |
| Respiration | 1 | 2 = Periodic breathing | 3 = Apnea or requires ventilator |
References
- Azzopardi, D.; The TOBY Study Group; Brocklehurst, P.; Edwards, D.; Halliday, H.; Levene, M.; Thoresen, M.; Whitelaw, A. The TOBY Study. Whole body hypothermia for the treatment of perinatal asphyxial encephalopathy: A randomised controlled trial. BMC Pediatr. 2008, 8, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shankaran, S.; Laptook, A.R.; Ehrenkranz, R.A.; Tyson, J.E.; McDonald, S.A.; Donovan, E.F.; Fanaroff, A.A.; Poole, W.K.; Wright, L.L.; Higgins, R.D.; et al. Whole-Body Hypothermia for Neonates with Hypoxic–Ischemic Encephalopathy. N. Engl. J. Med. 2005, 353, 1574–1584. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.E.; Morley, C.J.; Inder, T.E.; Stewart, M.J.; Smith, K.R.; McNamara, P.J.; Wright, I.M.R.; Kirpalani, H.M.; Darlow, B.A.; Doyle, L.W. Whole-Body Hypothermia for Term and Near-Term Newborns with Hypoxic-Ischemic Encephalopathy: A Randomized Controlled Trial. Obstet. Gynecol. Surv. 2011, 66, 743–744. [Google Scholar] [CrossRef]
- Azzopardi, D.; Robertson, N.J.; Bainbridge, A.; Cady, E.; Charles-Edwards, G.; Deierl, A.; Fagiolo, G.; Franks, N.P.; Griffiths, J.; Hajnal, J.; et al. Moderate hypothermia within 6 h of birth plus inhaled xenon versus moderate hypothermia alone after birth asphyxia (TOBY-Xe): A proof-of-concept, open-label, randomised controlled trial. Lancet Neurol. 2015, 15, 145–153. [Google Scholar] [CrossRef] [Green Version]
- Maiwald, C.A.; for the ALBINO Study Group; Annink, K.V.; Rüdiger, M.; Benders, M.J.N.L.; Van Bel, F.; Allegaert, K.; Naulaers, G.; Bassler, D.; Klebermaß-Schrehof, K.; et al. Effect of allopurinol in addition to hypothermia treatment in neonates for hypoxic-ischemic brain injury on neurocognitive outcome (ALBINO): Study protocol of a blinded randomized placebo-controlled parallel group multicenter trial for superiority (phase III). BMC Pediatr. 2019, 19, 210. [Google Scholar] [CrossRef]
- Thayyil, S.; for the HELIX Trial Group; Oliveira, V.; Lally, P.J.; Swamy, R.; Bassett, P.; Chandrasekaran, M.; Mondkar, J.; Mangalabharathi, S.; Benkappa, N.; et al. Hypothermia for encephalopathy in low and middle-income countries (HELIX): Study protocol for a randomised controlled trial. Trials 2017, 18, 432. [Google Scholar] [CrossRef] [Green Version]
- Charon, V.; Proisy, M.; Bretaudeau, G.; Bruneau, B.; Pladys, P.; Beuchée, A.; Burnouf-Rose, G.; Ferré, J.-C.; Rozel, C. Early MRI in neonatal hypoxic-ischaemic encephalopathy treated with hypothermia: Prognostic role at 2-year follow-up. Eur. J. Radiol. 2016, 85, 1366–1374. [Google Scholar] [CrossRef]
- Fries, P. A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends Cogn. Sci. 2005, 9, 474–480. [Google Scholar] [CrossRef]
- Sirota, A.; Buzsáki, G. Interaction between neocortical and hippocampal networks via slow oscillations. Thalamus Relat. Syst. 2005, 3, 245–259. [Google Scholar] [CrossRef] [Green Version]
- Sotero, R.C. Modeling the Generation of Phase-Amplitude Coupling in Cortical Circuits: From Detailed Networks to Neural Mass Models. BioMed Res. Int. 2015, 2015, 915606. [Google Scholar] [CrossRef] [Green Version]
- Penny, W.; Duzel, E.; Miller, K.; Ojemann, J. Testing for nested oscillation. J. Neurosci. Methods 2008, 174, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Canolty, R.T.; Knight, R.T. The functional role of cross-frequency coupling. Trends Cogn. Sci. 2010, 14, 506–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tort, A.B.L.; Komorowski, R.; Eichenbaum, H.; Kopell, N. Measuring Phase-Amplitude Coupling between Neuronal Oscillations of Different Frequencies. J. Neurophysiol. 2010, 104, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.E.; Jensen, O. The Theta-Gamma Neural Code. Neuron 2013, 77, 1002–1016. [Google Scholar] [CrossRef] [Green Version]
- Lisman, J.; Buzsaki, G. A Neural Coding Scheme Formed by the Combined Function of Gamma and Theta Oscillations. Schizophr. Bull. 2008, 34, 974–980. [Google Scholar] [CrossRef] [Green Version]
- Soto, J.L.P.; Jerbi, K. Investigation of cross-frequency phase-amplitude coupling in visuomotor networks using magnetoencephalography. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; Volume 2012, pp. 1550–1553. [Google Scholar] [CrossRef]
- Staresina, B.P.; Bergmann, T.O.; Bonnefond, M.; Van Der Meij, R.; Jensen, O.; Deuker, L.; Elger, C.E.; Axmacher, N.; Fell, J. Hierarchical nesting of slow oscillations, spindles and ripples in the human hippocampus during sleep. Nat. Neurosci. 2015, 18, 1679–1686. [Google Scholar] [CrossRef] [Green Version]
- Nayak, C.S.; Anilkumar, A.C.; Neonatal, E.E.G. StatPearls; Neonatal EEG: Treasure Island, FL, USA, 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536953/ (accessed on 20 March 2022).
- Shibata, T.; Otsubo, H. Phase-amplitude coupling of delta brush unveiling neuronal modulation development in the neonatal brain. Neurosci. Lett. 2020, 735, 135211. [Google Scholar] [CrossRef]
- Whitehead, K.; Pressler, R.; Fabrizi, L. Characteristics and clinical significance of delta brushes in the EEG of premature infants. Clin. Neurophysiol. Pract. 2017, 2, 12–18. [Google Scholar] [CrossRef]
- Wang, X.; Liu, H.; Kota, S.; Das, Y.; Liu, Y.; Zhang, R.; Chalak, L. EEG phase-amplitude coupling to stratify encephalopathy severity in the developing brain. Comput. Methods Prog. Biomed. 2021, 214, 106593. [Google Scholar] [CrossRef]
- Verbeke, G. Linear mixed models for longitudinal data. In Linear Mixed Models in Practice; Springer: Berlin/Heidelberg, Germany, 1997; pp. 63–153. [Google Scholar]
- Lacan, L.; Betrouni, N.; Lamblin, M.-D.; Chaton, L.; Delval, A.; Bourriez, J.-L.; Storme, L.; Derambure, P.; Tich, S.N. Quantitative approach to early neonatal EEG visual analysis in hypoxic-ischemic encephalopathy severity: Bridging the gap between eyes and machine. Neurophysiol. Clin. 2021, 51, 121–131. [Google Scholar] [CrossRef]
- Jacobs, S.E.; Berg, M.; Hunt, R.; Tarnow-Mordi, W.O.; Inder, T.E.; Davis, P.G. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst. Rev. 2013, 2013, CD003311. [Google Scholar] [CrossRef] [PubMed]
- Shankaran, S.; Pappas, A.; McDonald, S.A.; Vohr, B.R.; Hintz, S.R.; Yolton, K.; Gustafson, K.E.; Leach, T.M.; Green, C.; Bara, R.; et al. Childhood Outcomes after Hypothermia for Neonatal Encephalopathy. N. Engl. J. Med. 2012, 366, 2085–2092. [Google Scholar] [CrossRef] [PubMed]
- Hülsemann, M.; Naumann, E.; Rasch, B. Quantification of Phase-Amplitude Coupling in Neuronal Oscillations: Comparison of Phase-Locking Value, Mean Vector Length, Modulation Index, and Generalized-Linear-Modeling-Cross-Frequency-Coupling. Front. Neurosci. 2019, 13, 573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hamelin, S.; Delnard, N.; Cneude, F.; Debillon, T.; Vercueil, L. Influence of hypothermia on the prognostic value of early EEG in full-term neonates with hypoxic ischemic encephalopathy. Neurophysiol. Clin. Neurophysiol. 2011, 41, 19–27. [Google Scholar] [CrossRef]
- Murray, D.M.; Boylan, G.B.; Ryan, C.A.; Connolly, S. Early EEG Findings in Hypoxic-Ischemic Encephalopathy Predict Outcomes at 2 Years. Pediatrics 2009, 124, e459–e467. [Google Scholar] [CrossRef] [PubMed]
- Pressler, R.; Boylan, G.; Morton, M.; Binnie, C.; Rennie, J. Early serial EEG in hypoxic ischaemic encephalopathy. Clin. Neurophysiol. 2001, 112, 31–37. [Google Scholar] [CrossRef]
- Kota, S.; Jasti, K.; Liu, Y.; Liu, H.; Zhang, R.; Chalak, L. EEG Spectral Power: A Proposed Physiological Biomarker to Classify the Hypoxic-Ischemic Encephalopathy Severity in Real Time. Pediatr. Neurol. 2021, 122, 7–14. [Google Scholar] [CrossRef]
- Del Rio, R.; Ochoa, C.; Alarcon, A.; Arnaez, J.; Blanco, D.; Garcia-Alix, A. Amplitude Integrated Electroencephalogram as a Prognostic Tool in Neonates with Hypoxic-Ischemic Encephalopathy: A Systematic Review. PLoS ONE 2016, 11, e0165744. [Google Scholar] [CrossRef]
- Das, Y.; Liu, H.; Tian, F.; Kota, S.; Zhang, R.; Chalak, L.F. Rigor of Neurovascular Coupling (NVC) Assessment in Newborns Using Different Amplitude EEG Algorithms. Sci. Rep. 2020, 10, 9183. [Google Scholar] [CrossRef]
- Das, Y.; Wang, X.; Kota, S.; Zhang, R.; Liu, H.; Chalak, L.F. Neurovascular coupling (NVC) in newborns using processed EEG versus amplitude-EEG. Sci. Rep. 2021, 11, 9426. [Google Scholar] [CrossRef]
| Neonatal Characteristics | Overall | Encephalopathy Grade | |
|---|---|---|---|
| HIEmild | HIEcooled | ||
| Total N | 33 | 15 | 18 |
| Male: N (%) | 19 (58%) | 10 (67%) | 9 (50%) |
| Gestational Age (weeks), mean (SD) | 39 (1.3) | 39 (1.1) | 39 (1.4) |
| Birth Weight (kg), mean (SD) | 3.3 (0.7) | 3.3 (0.5) | 3.3 (0.8) |
| Apgar 1 min *, median (IQR) | 2 (1 3) | 3 (2 4) | 1 (1 2) |
| Apgar 5 min *, median (IQR) | 6 (4 7) | 7 (6 8) | 4 (2 6) |
| Umbilical Cord Gas pH, mean (SD) | 7.0 (0.1) | 7.0 (0.1) | 7.0 (0.2) |
| Base Deficit, mean (SD) | 16.6 (6.2) | 17.6 (3.8) | 15.6 (7.6) |
| Maternal Race/Ethnicity: N (%) | |||
| Caucasian non-Hispanic | 2 (6%) | 1 (7%) | 1 (6%) |
| Black non-Hispanic | 8 (24%) | 4 (27%) | 4 (22%) |
| Hispanic | 21 (64%) | 9 (60%) | 12 (67%) |
| Other non-Hispanic | 2 (6%) | 1 (7%) | 1 (6%) |
| Delivery Mode: N (%) | |||
| Caesarean | 20 (61%) | 8 (53%) | 12 (67%) |
| Vaginal | 13 (39%) | 7 (47%) | 6 (33%) |
| Maternal Risk Factors: N (%) | |||
| Hypertension | 8 (24%) | 4 (27%) | 4 (22%) |
| Diabetes | 2 (6%) | 1 (7%) | 1 (6%) |
| Pre-eclampsia | 9 (27%) | 3 (20%) | 6 (33%) |
| Labor Complications: N (%) | |||
| Meconium | 9 (27%) | 2 (13%) | 7 (39%) |
| Placental Abruption | 2 (6%) | 1 (7%) | 1 (6%) |
| Uterine Rupture | 2 (6%) | 1 (7%) | 1 (6%) |
| Maternal Chorioamnionitis | 9 (27%) | 5 (33%) | 4 (22%) |
| Placental Chorioamnionitis | 19 (58%) | 9 (60%) | 10 (56%) |
| Disposition: | |||
| DOL at discharge *, median (IQR) | 9 (6 16) | 6 (5 7) | 14 (9 20) |
| Death prior to discharge | 1 (3%) | 0 (0%) | 1 (6%) |
| Mixed Effect Models | |||
|---|---|---|---|
| Time-Window | Variable | Coefficient Estimates (95% CI) | p-Value |
| 10 s | Group (HIEmild vs. HIEcooled) | −3.62465 (−6.00060, −1.24869) | 0.004 * |
| Time | −0.00043 (−0.00059, −0.00027) | <0.001 * | |
| × Time | −0.00006 (−0.00029, 0.00016) | 0.594 | |
| 20 s | Group (HIEmild vs. HIEcooled) | −3.90037 (−6.16340, −1.63734) | 0.001 * |
| Time | −0.00063 (−0.00099, −0.00028) | <0.001 * | |
| × Time | −0.00005 (−0.00055, 0.00045) | 0.842 | |
| 1 min | Group (HIEmild vs. HIEcooled) | −4.92412 (−5.42233, −4.42591) | <0.001 * |
| Time | −0.00013 (−0.00170, 0.00143) | 0.868 | |
| × Time | 0.00051 (−0.00166, 0.00269) | 0.644 | |
| 2 min | Group (HIEmild vs. HIEcooled) | −5.91112 (−8.69999, −3.12225) | <0.001 * |
| Time | −0.00148 (−0.00512, 0.00216) | 0.425 | |
| × Time | −0.00154 (−0.00666, 0.00358) | 0.556 | |
| 5 min | Group (HIEmild vs. HIEcooled) | −7.17580 (−10.44403, −3.90756) | <0.001 * |
| Time | −0.00362 (−0.01689, 0.00964) | 0.592 | |
| × Time | −0.00089 (−0.01953, 0.01776) | 0.926 | |
| 10 min | Group (HIEmild vs. HIEcooled) | −7.81816 (−11.43931, −4.19699) | <0.001 * |
| Time | −0.00206 (−0.03730, 0.03318) | 0.909 | |
| × Time | −0.00630 (−0.05579, 0.04318) | 0.803 | |
| 20 min | Group (HIEmild vs. HIEcooled) | −8.29617 (−12.21319, −4.37910) | <0.001 * |
| Time | 0.00953 (−0.07861, 0.09766) | 0.833 | |
| × Time | −0.02562 (−0.14949, 0.09826) | 0.686 | |
| 30 min | Group (HIEmild vs. HIEcooled) | −8.15252 (−12.17035, −4.13469) | <0.001 * |
| Time | 0.06239 (−0.07834, 0.20311]) | 0.386 | |
| × Time | −0.10171 (−0.29620, 0.09278) | 0.307 | |
| 60 min | Group (HIEmild vs. HIEcooled) | −8.59229 (−12.89037, −4.29420) | <0.001 * |
| Time | 0.17269 (−0.16906, 0.51445) | 0.325 | |
| × Time | −0.27497 (−0.74849, 0.19853) | 0.258 | |
| 120 min | Group (HIEmild vs. HIEcooled) | −9.24454 (−14.32783, −4.16125) | 0.001* |
| Time | 0.27341 (−0.91995, 1.46678) | 0.652 | |
| × Time | 0.08464 (−1.569787, 1.73906) | 0.920 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Liu, H.; Ortigoza, E.B.; Kota, S.; Liu, Y.; Zhang, R.; Chalak, L.F. Feasibility of EEG Phase-Amplitude Coupling to Stratify Encephalopathy Severity in Neonatal HIE Using Short Time Window. Brain Sci. 2022, 12, 854. https://doi.org/10.3390/brainsci12070854
Wang X, Liu H, Ortigoza EB, Kota S, Liu Y, Zhang R, Chalak LF. Feasibility of EEG Phase-Amplitude Coupling to Stratify Encephalopathy Severity in Neonatal HIE Using Short Time Window. Brain Sciences. 2022; 12(7):854. https://doi.org/10.3390/brainsci12070854
Chicago/Turabian StyleWang, Xinlong, Hanli Liu, Eric B. Ortigoza, Srinivas Kota, Yulun Liu, Rong Zhang, and Lina F. Chalak. 2022. "Feasibility of EEG Phase-Amplitude Coupling to Stratify Encephalopathy Severity in Neonatal HIE Using Short Time Window" Brain Sciences 12, no. 7: 854. https://doi.org/10.3390/brainsci12070854
APA StyleWang, X., Liu, H., Ortigoza, E. B., Kota, S., Liu, Y., Zhang, R., & Chalak, L. F. (2022). Feasibility of EEG Phase-Amplitude Coupling to Stratify Encephalopathy Severity in Neonatal HIE Using Short Time Window. Brain Sciences, 12(7), 854. https://doi.org/10.3390/brainsci12070854






