Early Diagnosis of Cerebral Palsy in Low- and Middle-Income Countries
Abstract
:1. Introduction
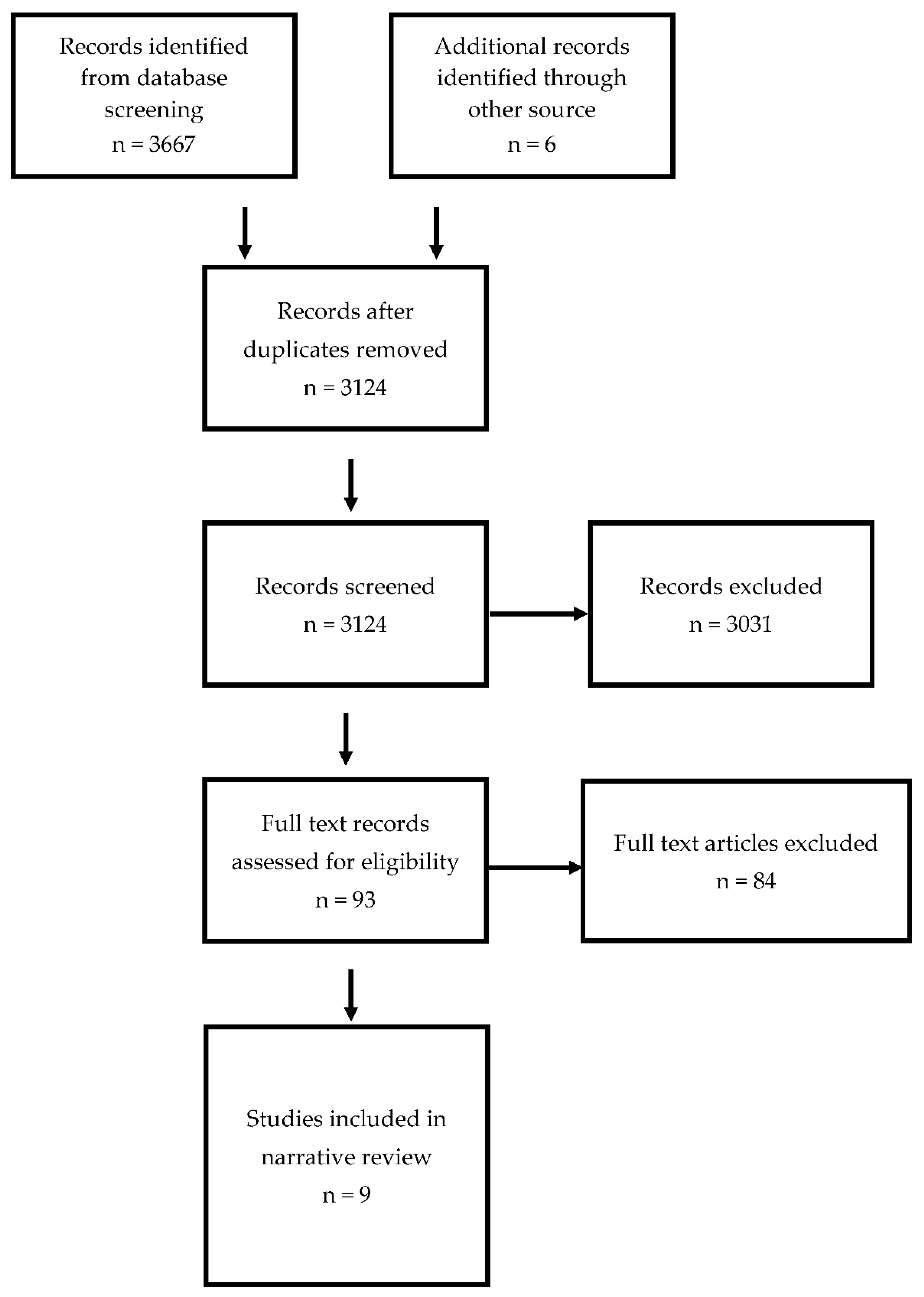
2. Methods
3. Results
4. Discussion
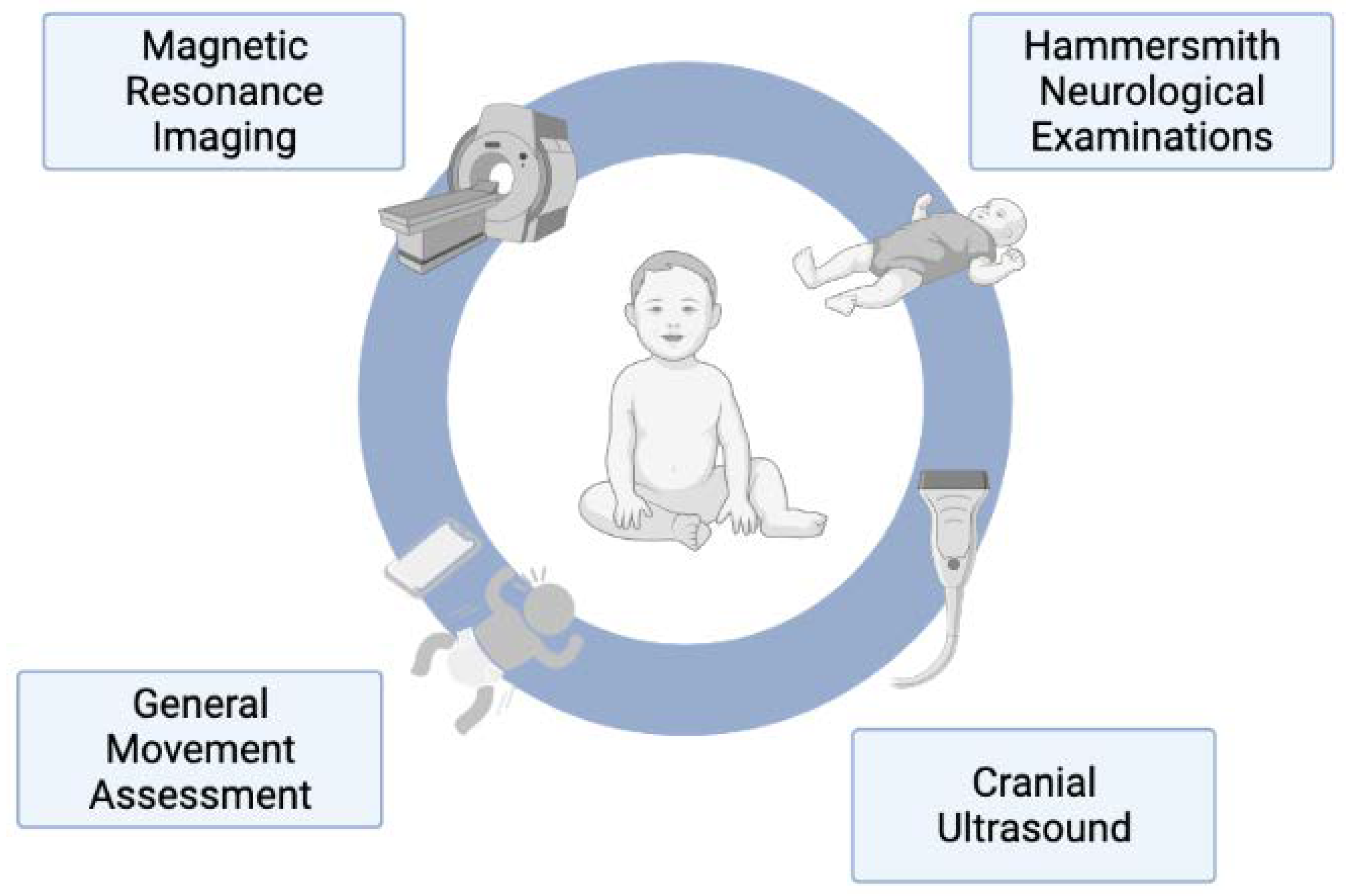
4.1. General Movement Assessment
4.2. Hammersmith Neonatal Neurological Examination and Hammersmith Infant Neurological Examination
4.3. Neuroimaging
4.4. Implementation into Clinical Practice
4.5. Populations Studied
4.6. Developmental Delay
4.7. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
- exp Diagnosis/ 9030218
- exp Early Diagnosis/ 59539
- (early adj5 (diagnos$ or identif$ or detect$ or discover$)).mp. [mp=title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 270347
- 1 or 2 or 3 9125633
- exp Cerebral Palsy/ 22023
- exp Developmental Disabilities/ 21441
- cerebral palsy.mp. 26681
- cp.mp. 48217
- ((development$ or motor$) adj5 (disorder$ or disabilit$ or condition$ or impair$ or deficit$ or delay$)).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 169477
- 5 or 6 or 7 or 8 or 9 234112
- exp Developing Countries/ 78123
- (low adj2 income adj2 countr$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 7949
- (middle adj2 income adj2 countr$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 19670
- LMIC.mp. 2175
- exp Asia/ 939643
- exp Africa/ 293441
- exp South America/ 180680
- asia$.mp. 223443
- africa$.mp. 306178
- (south adj1 america$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 31113
- (low adj2 middle adj2 income adj2 countr$).mp. [mp = title, abstract, original title, name of substance word, subject heading word, floating sub-heading word, keyword heading word, organism supplementary concept word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier, synonyms] 16066
- 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 1685014
- 4 and 10 and 22 3861
- limit 23 to (humans and “all infant (birth to 23 months)”) 1205
- limit 24 to (english language and last 10 years) 643.
References
- Australian Cerebral Palsy Register Group. Report of the Australian Cerebral Palsy Register; Birth Years 1995–2012, November 2018; Cerebral Palsy Alliance: Sydney, Australia, 2018. [Google Scholar]
- Novak, I.; Morgan, C.; Adde, L.; Blackman, J.; Boyd, R.N.; Brunstrom-Hernandez, J.; Cioni, G.; Damiano, D.; Darrah, J.; Eliasson, A.C.; et al. Early, Accurate Diagnosis and Early Intervention in Cerebral Palsy: Advances in Diagnosis and Treatment. JAMA Pediatr. 2017, 171, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Maitre, N.L.; Burton, V.J.; Duncan, A.F.; Iyer, S.; Ostrander, B.; Winter, S.; Ayala, L.; Burkhardt, S.; Gerner, G.; Getachew, R.; et al. Network Implementation of Guideline for Early Detection Decreases Age at Cerebral Palsy Diagnosis. Pediatrics 2020, 145, e20192126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, R.; Noritz, G.; Maitre, N.L. Implementation of Early Diagnosis and Intervention Guidelines for Cerebral Palsy in a High-Risk Infant Follow-Up Clinic. Pediatr. Neurol. 2017, 76, 66–71. [Google Scholar] [CrossRef] [Green Version]
- King, A.; Machipisa, C.; Finlayson, F.; Fahey, M.; Novak, I.; Malhotra, A. Early detection of cerebral palsy in high-risk infants: Translation of evidence into practice in an Australian hospital. J. Paediatr. Child. 2020, 57, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Al Imam, M.H.; Jahan, I.; Das, M.C.; Muhit, M.; Smithers-Sheedy, H.; McIntyre, S.; Badawi, N.; Khandaker, G. Population-based surveillance of children with cerebral palsy enables early diagnosis and intervention. Dev. Med. Child. Neurol. 2021, 63, 883–884. [Google Scholar] [CrossRef]
- Khandaker, G.; Muhit, M.; Karim, T.; Smithers-Sheedy, H.; Novak, I.; Jones, C.; Badawi, N. Epidemiology of cerebral palsy in Bangladesh: A population-based surveillance study. Dev. Med. Child. Neurol. 2019, 61, 601–609. [Google Scholar] [CrossRef] [Green Version]
- Kakooza-Mwesige, A.; Andrews, C.; Peterson, S.; Wabwire Mangen, F.; Eliasson, A.C.; Forssberg, H. Prevalence of cerebral palsy in Uganda: A population-based study. Lancet Glob. Health 2017, 5, e1275–e1282. [Google Scholar] [CrossRef] [Green Version]
- Chauhan, A.; Singh, M.; Jaiswal, N.; Agarwal, A.; Sahu, J.K.; Singh, M. Prevalence of Cerebral Palsy in Indian Children: A Systematic Review and Meta-Analysis. Indian J. Pediatr. 2019, 86, 1124–1130. [Google Scholar] [CrossRef] [PubMed]
- Oskoui, M.; Coutinho, F.; Dykeman, J.; Jetté, N.; Pringsheim, T. An update on the prevalence of cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2013, 55, 509–519. [Google Scholar] [CrossRef]
- Langlois, É.V.; Miszkurka, M.; Zunzunegui, M.V.; Ghaffar, A.; Ziegler, D.; Karp, I. Inequities in postnatal care in low- and middle-income countries: A systematic review and meta-analysis. Bull. World Health Organ. 2015, 93, 259–270G. [Google Scholar] [CrossRef]
- Faruk, T.; King, C.; Muhit, M.; Islam, M.K.; Jahan, I.; Baset, K.u.; Badawi, N.; Khandaker, G. Screening tools for early identification of children with developmental delay in low- and middle-income countries: A systematic review. BMJ Open 2020, 10, e038182. [Google Scholar] [CrossRef] [PubMed]
- Marlow, M.; Servili, C.; Tomlinson, M. A review of screening tools for the identification of autism spectrum disorders and developmental delay in infants and young children: Recommendations for use in low- and middle-income countries. Autism Res. 2019, 12, 176–199. [Google Scholar] [CrossRef]
- Tricco, A.; Lillie, E.; Zarin, W.; O’Brien, K.; Colquhoun, J.; Levac, D.; Moher, D.; Peters, M.; Horsely, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Bank Group. World Bank Country and Lending Groups. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (accessed on 14 September 2021).
- Apaydın, U.; Erol, E.; Yıldız, A.; Yıldız, R.; Acar, Ş.S.; Gücüyener, K.; Elbasan, B. The use of neuroimaging, Prechtl’s general movement assessment and the Hammersmith infant neurological examination in determining the prognosis in 2-year-old infants with hypoxic ischemic encephalopathy who were treated with hypothermia. Early Hum. Dev. 2021, 163, 105487. [Google Scholar] [CrossRef] [PubMed]
- Aker, K.; Thomas, N.; Adde, L.; Koshy, B.; Martinez-Biarge, M.; Nakken, I.; Padankatti, C.S.; Støen, R. Prediction of outcome from MRI and general movements assessment after hypoxic-ischaemic encephalopathy in low-income and middle-income countries: Data from a randomised controlled trial. Arch. Dis. Child. Fetal Neonatal. Ed. 2021, 107, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Einspieler, C.; Utsch, F.; Brasil, P.; Panvequio Aizawa, C.Y.; Peyton, C.; Hydee Hasue, R.; Françoso Genovesi, F.; Damasceno, L.; Moreira, M.E.; Adachi, K.; et al. Association of Infants Exposed to Prenatal Zika Virus Infection with Their Clinical, Neurologic, and Developmental Status Evaluated via the General Movement Assessment Tool. JAMA Netw. Open 2019, 2, e187235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venkata, S.; Pournami, F.; Prabhakar, J.; Nandakumar, A.; Jain, N. Disability Prediction by Early Hammersmith Neonatal Neurological Examination: A Diagnostic Study. J. Child. Neurol. 2020, 35, 731–736. [Google Scholar] [CrossRef]
- Medina-Alva, P.; Duque, K.R.; Zea-Vera, A.; Bellomo, S.; Cárcamo, C.; Guillen-Pinto, D.; Rivas, M.; Tori, A.; Zegarra, J.; Cam, L.; et al. Combined predictors of neurodevelopment in very low birth weight preterm infants. Early Hum. Dev. 2019, 130, 109–115. [Google Scholar] [CrossRef]
- Dimitrijević, L.; Bjelaković, B.; Čolović, H.; Mikov, A.; Živković, V.; Kocić, M.; Lukić, S. Assessment of general movements and heart rate variability in prediction of neurodevelopmental outcome in preterm infants. Early Hum. Dev. 2016, 99, 7–12. [Google Scholar] [CrossRef]
- Soleimani, F.; Badv, R.S.; Momayezi, A.; Biglarian, A.; Marzban, A. General movements as a predictive tool of the neurological outcome in term born infants with hypoxic ischemic encephalopathy. Early Hum. Dev. 2015, 91, 479–482. [Google Scholar] [CrossRef]
- Lally, P.J.; Price, D.L.; Pauliah, S.S.; Bainbridge, A.; Kurien, J.; Sivasamy, N.; Cowan, F.M.; Balraj, G.; Ayer, M.; Satheesan, K.; et al. Neonatal Encephalopathic Cerebral Injury in South India Assessed by Perinatal Magnetic Resonance Biomarkers and Early Childhood Neurodevelopmental Outcome. PLoS ONE 2014, 9, e87874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burger, M.; Frieg, A.; Louw, Q.A. General movements as a predictive tool of the neurological outcome in very low and extremely low birth weight infants—A South African perspective. Early Hum. Dev. 2011, 87, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Romeo, D.M.; Ricci, D.; Brogna, C.; Mercuri, E. Use of the Hammersmith Infant Neurological Examination in infants with cerebral palsy: A critical review of the literature. Dev. Med. Child. Neurol. 2016, 58, 240–245. [Google Scholar] [CrossRef]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A systematic review of tests to predict cerebral palsy in young children. Dev. Med. Child. Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Tomantschger, I.; Herrero, D.; Einspieler, C.; Hamamura, C.; Voos, M.C.; Marschik, P.B. The general movement assessment in non-European low- and middle-income countries. Rev. Saude Publica 2018, 52, 6. [Google Scholar] [CrossRef]
- Darsaklis, V.; Snider, L.M.; Majnemer, A.; Mazer, B. Predictive validity of Prechtl’s Method on the Qualitative Assessment of General Movements: A systematic review of the evidence. Dev. Med. Child. Neurol. 2011, 53, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Crowle, C.; Goyen, T.-A.; Hardman, C.; Jackman, M.; Novak, I.; Badawi, N. Sensitivity and specificity of General Movements Assessment for diagnostic accuracy of detecting cerebral palsy early in an Australian context. J. Paediatr. Child. Health 2016, 52, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Øberg, G.K.; Jacobsen, B.K.; Jørgensen, L. Predictive Value of General Movement Assessment for Cerebral Palsy in Routine Clinical Practice. Phys. Ther. 2015, 95, 1489–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwong, A.K.L.; Fitzgerald, T.L.; Doyle, L.W.; Cheong, J.L.Y.; Spittle, A.J. Predictive validity of spontaneous early infant movement for later cerebral palsy: A systematic review. Dev. Med. Child. Neurol. 2018, 60, 480–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubowitz, L.M.; Dubowitz, V.; Mercuri, E. The Neurological Assessment of the Preterm and Full-Term Newborn Infant; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Haataja, L.; Mercuri, E.; Regev, R.; Cowan, F.; Rutherford, M.; Dubowitz, V.; Dubowitz, L. Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J. Paediatr. 1999, 135, 153–161. [Google Scholar] [CrossRef]
- Spittle, A.J.; Walsh, J.M.; Potter, C.; McInnes, E.; Olsen, J.E.; Lee, K.J.; Anderson, P.J.; Doyle, L.W.; Cheong, J.L. Neurobehaviour at term-equivalent age and neurodevelopmental outcomes at 2 years in infants born moderate-to-late preterm. Dev. Med. Child. Neurol. 2017, 59, 207–215. [Google Scholar] [CrossRef]
- Lawford, H.L.S.; Nuamah, M.A.; Liley, H.G.; Lee, A.C.; Kumar, S.; Adjei, A.A.; Bora, S. Neonatal neurological examination in a resource-limited setting: What defines normal? Eur. J. Paediatr. Neurol. 2020, 29, 71–80. [Google Scholar] [CrossRef]
- Hagmann, C.F.; Chan, D.; Robertson, N.J.; Acolet, D.; Nyombi, N.; Nakakeeto, M.; Cowan, F.M. Neonatal neurological examination in well newborn term Ugandan infants. Early Hum. Dev. 2015, 91, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Bax, M.; Tydeman, C.; Flodmark, O. Clinical and MRI Correlates of Cerebral PalsyThe European Cerebral Palsy Study. JAMA 2006, 296, 1602–1608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirmiran, M.; Barnes, P.D.; Keller, K.; Constantinou, J.C.; Fleisher, B.E.; Hintz, S.R.; Ariagno, R.L. Neonatal brain magnetic resonance imaging before discharge is better than serial cranial ultrasound in predicting cerebral palsy in very low birth weight preterm infants. Pediatrics 2004, 114, 992–998. [Google Scholar] [CrossRef]
- Benfer, K.A.; Novak, I.; Morgan, C.; Whittingham, K.; Khan, N.Z.; Ware, R.S.; Bell, K.L.; Bandaranayake, S.; Salt, A.; Ghosh, A.K.; et al. Community-based parent-delivered early detection and intervention programme for infants at high risk of cerebral palsy in a low-resource country (Learning through Everyday Activities with Parents (LEAP-CP): Protocol for a randomised controlled trial. BMJ Open 2018, 8, e021186. [Google Scholar] [CrossRef] [PubMed]
- Toldo, M.; Varishthananda, S.; Einspieler, C.; Tripathi, N.; Singh, A.; Verma, S.K.; Vishwakarma, K.; Zhang, D.; Dwivedi, A.; Gupta, R.; et al. Enhancing early detection of neurological and developmental disorders and provision of intervention in low-resource settings in Uttar Pradesh, India: Study protocol of the G.A.N.E.S.H. programme. BMJ Open 2020, 10, e037335. [Google Scholar] [CrossRef]
- Jahan, I.; Muhit, M.; Hardianto, D.; Laryea, F.; Chhetri, A.B.; Smithers-Sheedy, H.; McIntyre, S.; Badawi, N.; Khandaker, G. Epidemiology of cerebral palsy in low- and middle-income countries: Preliminary findings from an international multi-centre cerebral palsy register. Dev. Med. Child. Neurol. 2021, 63, 1327–1336. [Google Scholar] [CrossRef]
| Year | Author | Population | NICU Follow Up | Country (City/State) Income Level | Tools Used | Age of Assessment | Main Findings |
|---|---|---|---|---|---|---|---|
| 2021 | Aker [17] | Term/near term infants with moderate to severe hypoxic ischaemic encephalopathy | Yes | India (Vellore/Tamil Nadu) Lower-middle | MRI GMA | MRI at 5 days of life GMA at 10 to 15 weeks corrected age | MRI brain had a sensitivity of 62% and specificity of 90% for predicting adverse outcomes including cerebral palsy when compared to Bayley-III. Absent fidgety movements on GMA had a sensitivity of 60% and specificity of 89%. |
| 2021 | Apaydin [16] | Term infants with moderate to severe hypoxic ischaemic encephalopathy treated with hypothermia | No | Turkey (Ankara) Upper-middle | MRI GMA HINE | MRI 7 to 14 days after cooling GMA 12 weeks of age HINE 12 to 42 weeks | MRI had a sensitivity of 83% and specificity of 95% for predicting cerebral palsy diagnosis at 2 years of age when compared to the Bayley-II. Absent fidgety movements had a sensitivity of 83% and specificity of 100%, HINE had a sensitivity of 83% and specificity of 88%. |
| 2020 | Venkata [19] | Preterm infants and term infants admitted to the NICU with significant risk factors for cerebral palsy | Yes | India (Kerala) Lower- middle | HNNE | Early assessment performed after NICU care completed. Recommended assessment performed at 2 weeks of age (term) or 40 weeks corrected (preterm). | HNNE performed early had sensitivity of 64% and specificity of 73% for predicting neurodevelopmental disability, including cerebral palsy when compared to Development Assessment Scale for Indian Infants. HNNE performed at the recommended age had sensitivity of 50% and specificity of 77%. |
| 2019 | Einspieler [18] | Infants exposed to acute maternal zika infection | No | Brazil (Rio de Janeiro) Upper-middle | GMA | 9 to 20 weeks corrected age | 100% of infants with maternal zika-infection and microcephaly had abnormal or absent fidgety movements observed in infancy and developed bilateral spastic cerebral palsy at 12 months of age. |
| 2019 | Medina-Alva [20] | Preterm infants with birth weights between 500 and 2000 g | Yes | Peru (Lima) Upper-middle | Cranial ultrasound Head circumference HNNE | 38 to 42 weeks corrected age | Cranial ultrasound had a sensitivity of 31% and specificity of 96% for detecting neurodevelopmental delay, including cerebral palsy at 24 months of age when compared to Mullen Scales of Early Learning. Abnormal HNNE score had a sensitivity of 69% and specificity of 54%. Microcephaly had sensitivity of 34% and specificity 97%. |
| 2016 | Dimitrijevic [21] | Preterm infants born < 37 weeks gestation | No | Serbia (Novi Sad) Upper-middle | GMA | 4 to 12 weeks corrected age | Cramped synchronised movements had a sensitivity of 82% and specificity of 99% for predicting cerebral palsy at 24 months of age, when compared to paediatric examination. Absent fidgety movements had a sensitivity of 100% and specificity of 96%. |
| 2015 | Soleimani [22] | Infants with hypoxic ischaemic encephalopathy | Yes | Iran (Zanjan) Lower-middle | GMA | 12 to 20 weeks corrected age | Absent fidgety movements had a sensitivity of 80% and specificity of 100% for detecting abnormal neurodevelopment including cerebral palsy, when compared to the Infant Neurological International Battery at 12 to 18 months of age. |
| 2014 | Lally [23] | Infants with hypoxic ischaemic encephalopathy | Yes | India (Kerala) Lower-middle | MRI | 1 to 3 weeks chronological age | Abnormality on MRI imaging had a sensitivity 57% and specificity of 79% for diagnosing cerebral palsy or low Bayley III scores at 3 years of age, when compared to Bayley examination. |
| 2011 | Burger [24] | Preterm infants weighing </= 1250 g | Yes | South Africa (Western Cape) Upper-middle | GMA | 12 weeks corrected age | Absent fidgety movements had a sensitivity of 89% and specificity of 89% for diagnosis neurodevelopmental delay and cerebral palsy at 12 months of age when compared to Pea Body Developmental Motor Scale and Alberta Infant Motor Scale. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
King, A.R.; Al Imam, M.H.; McIntyre, S.; Morgan, C.; Khandaker, G.; Badawi, N.; Malhotra, A. Early Diagnosis of Cerebral Palsy in Low- and Middle-Income Countries. Brain Sci. 2022, 12, 539. https://doi.org/10.3390/brainsci12050539
King AR, Al Imam MH, McIntyre S, Morgan C, Khandaker G, Badawi N, Malhotra A. Early Diagnosis of Cerebral Palsy in Low- and Middle-Income Countries. Brain Sciences. 2022; 12(5):539. https://doi.org/10.3390/brainsci12050539
Chicago/Turabian StyleKing, Arrabella R., Mahmudul Hassan Al Imam, Sarah McIntyre, Catherine Morgan, Gulam Khandaker, Nadia Badawi, and Atul Malhotra. 2022. "Early Diagnosis of Cerebral Palsy in Low- and Middle-Income Countries" Brain Sciences 12, no. 5: 539. https://doi.org/10.3390/brainsci12050539
APA StyleKing, A. R., Al Imam, M. H., McIntyre, S., Morgan, C., Khandaker, G., Badawi, N., & Malhotra, A. (2022). Early Diagnosis of Cerebral Palsy in Low- and Middle-Income Countries. Brain Sciences, 12(5), 539. https://doi.org/10.3390/brainsci12050539







